Fingolimod Associated Bilateral Cystoid Macular Edema—Wait and See?
Abstract
:1. Introduction
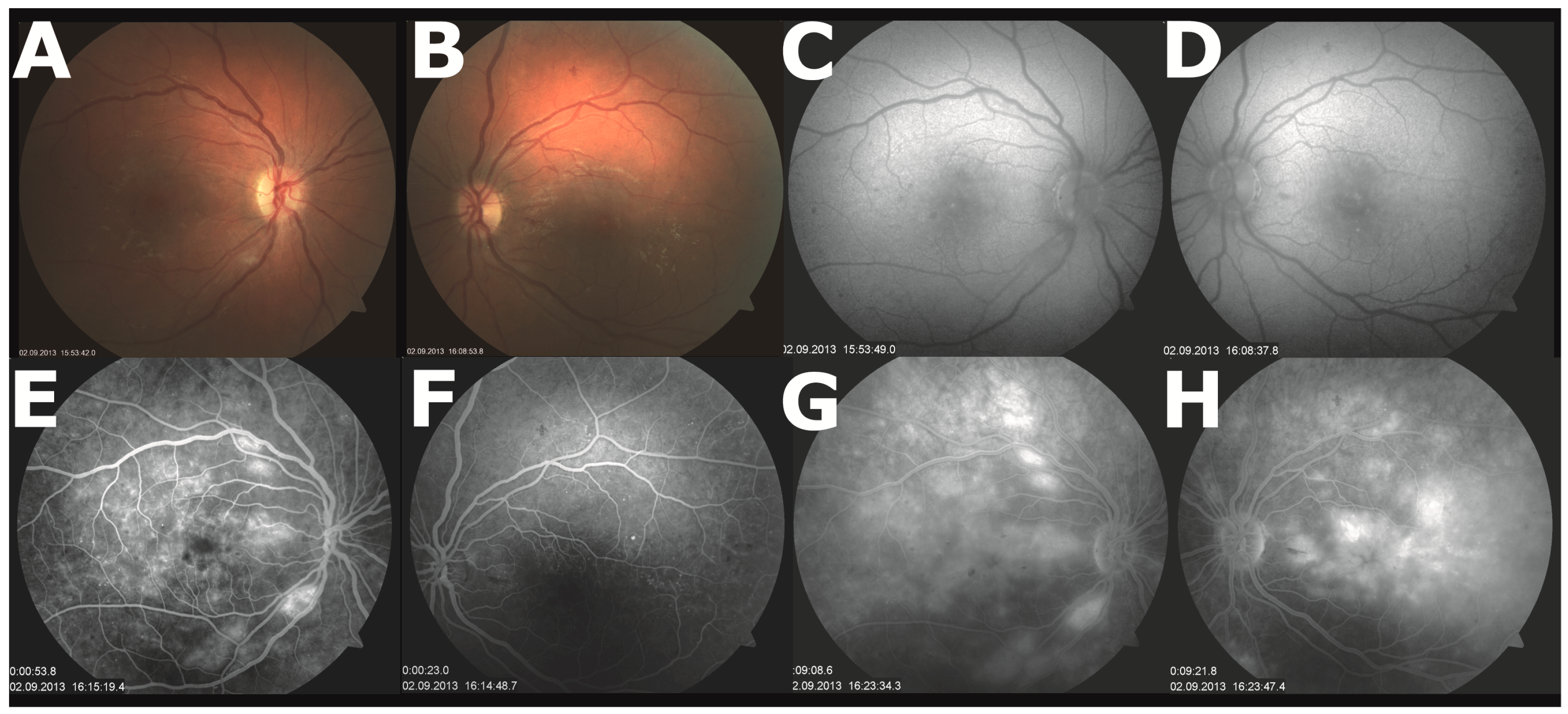
2. Case Presentation
3. Discussion
Author Contributions
Conflicts of Interest
References
- European Medicines Agency. Annex I. Summary of Product Characteristics. Gilenya (fingolimod). 2011. (last updated 07.03.2016). Available online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002202/WC500104528.pdf (accessed on 20 July 2016).
- Brinkmann, V.; Davis, M.D.; Heise, C.E.; Albert, R.; Cottens, S.; Hof, R.; Bruns, C.; Prieschl, E.; Baumruker, T.; Hiestand, P.; et al. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J. Biol. Chem. 2002, 277, 21453–21457. [Google Scholar] [CrossRef] [PubMed]
- Tedesco-Silva, H.; Pescovitz, M.D.; Cibrik, D.; Rees, M.A.; Mulgaonkar, S.; Kahan, B.D.; Gugliuzza, K.K.; Rajagopalan, P.R.; Esmeraldo Rde, M.; Lord, H.; et al. Randomized controlled trial of FTY720 versus MMF in de novo renal transplantation. Transplantation 2006, 82, 1689–1697. [Google Scholar] [CrossRef] [PubMed]
- Tedesco-Silva, H.; Szakaly, P.; Shoker, A.; Sommerer, C.; Yoshimura, N.; Schena, F.P.; Cremer, M.; Hmissi, A.; Mayer, H.; Lang, P. FTY720 versus mycophenolate mofetil in de novo renal transplantation: Six-month results of a double-blind study. Transplantation 2007, 84, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Salvadori, M.; Budde, K.; Charpentier, B.; Klempnauer, J.; Nashan, B.; Pallardo, L.M.; Eris, J.; Schena, F.P.; Eisenberger, U.; Rostaing, L.; et al. FTY720 versus MMF with cyclosporine in de novo renal transplantation: A 1-year, randomized controlled trial in Europe and Australasia. Am. J. Transplant. 2006, 6, 2912–2921. [Google Scholar] [CrossRef] [PubMed]
- Hoitsma, A.J.; Woodle, E.S.; Abramowicz, D.; Proot, P.; Vanrenterghem, Y. FTY720 combined with tacrolimus in de novo renal transplantation: 1-year, multicenter, open-label randomized study. Nephrol. Dial. Transplant. 2011, 26, 3802–3805. [Google Scholar] [CrossRef] [PubMed]
- Kappos, L.; Antel, J.; Comi, G.; Montalban, X.; O’Connor, P.; Polman, C.H.; Haas, T.; Korn, A.A.; Karlsson, G.; Radue, E.W. Oral fingolimod (FTY720) for relapsing multiple sclerosis. N. Engl. J. Med. 2006, 355, 1124–1140. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, P.; Comi, G.; Montalban, X.; Antel, J.; Radue, E.W.; de Vera, A.; Pohlmann, H.; Kappos, L. Oral fingolimod (FTY720) in multiple sclerosis: Two-year results of a phase II extension study. Neurology 2009, 72, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Comi, G.; O’Connor, P.; Montalban, X.; Antel, J.; Radue, E.W.; Karlsson, G.; Pohlmann, H.; Aradhye, S.; Kappos, L. Phase II study of oral fingolimod (FTY720) in multiple sclerosis: 3-year results. Mult. Scler. 2010, 16, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, G.; O’Connor, P.; Montalban, X.; von Rosenstiel, P.; Cremer, M.; de Vera, A.; Sfikas, N.; Francis, G.; Radue, E.; Kappos, L. Five-year results from a phase 2 study of oral fingolimod in relapsing multiple sclerosis. Mult. Scler. 2013, 20, 877–881. [Google Scholar] [CrossRef] [PubMed]
- Calabresi, P.A.; Radue, E.W.; Goodin, D.; Jeffery, D.; Rammohan, K.W.; Reder, A.T.; Vollmer, T.; Agius, M.A.; Kappos, L.; Stites, T.; et al. Safety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 2014, 13, 545–556. [Google Scholar] [CrossRef]
- Cohen, J.A.; Barkhof, F.; Comi, G.; Hartung, H.P.; Khatri, B.O.; Montalban, X.; Pelletier, J.; Capra, R.; Gallo, P.; Izquierdo, G.; et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N. Engl. J. Med. 2010, 362, 402–415. [Google Scholar] [CrossRef] [PubMed]
- Kappos, L.; Radue, E.W.; O’Connor, P.; Polman, C.; Hohlfeld, R.; Calabresi, P.; Selmaj, K.; Agoropoulou, C.; Leyk, M.; Zhang-Auberson, L.; et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N. Engl. J. Med. 2010, 362, 387–401. [Google Scholar] [CrossRef] [PubMed]
- Polman, C.H.; Reingold, S.C.; Banwell, B.; Clanet, M.; Cohen, J.A.; Filippi, M.; Fujihara, K.; Havrdova, E.; Hutchinson, M.; Kappos, L.; et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann. Neurol. 2011, 69, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Makri, O.E.; Georgalas, I.; Georgakopoulos, C.D. Drug-induced macular edema. Drugs 2013, 73, 789–802. [Google Scholar] [CrossRef] [PubMed]
- Tedesco-Silva, H.; Mourad, G.; Kahan, B.D.; Boira, J.G.; Weimar, W.; Mulgaonkar, S.; Nashan, B.; Madsen, S.; Charpentier, B.; Pellet, P.; et al. FTY720, a novel immunomodulator: Efficacy and safety results from the first phase 2A study in de novo renal transplantation. Transplantation 2004, 77, 1826–1833. [Google Scholar] [CrossRef] [PubMed]
- Mulgaonkar, S.; Tedesco, H.; Oppenheimer, F.; Walker, R.; Kunzendorf, U.; Russ, G.; Knoflach, A.; Patel, Y.; Ferguson, R. FTY720/cyclosporine regimens in de novo renal transplantation: A 1-year dose-finding study. Am. J. Transplant. 2006, 6, 1848–1857. [Google Scholar] [CrossRef] [PubMed]
- Ontaneda, D.; Hara-Cleaver, C.; Rudick, R.A.; Cohen, J.A.; Bermel, R.A. Early tolerability and safety of fingolimod in clinical practice. J. Neurol. Sci. 2012, 323, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Zarbin, M.A.; Jampol, L.M.; Jager, R.D.; Reder, A.T.; Francis, G.; Collins, W.; Tang, D.; Zhang, X. Ophthalmic evaluations in clinical studies of fingolimod (FTY720) in multiple sclerosis. Ophthalmology 2013, 120, 1432–1439. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Bhatti, M.T. Fingolimod-associated macular edema: Incidence, detection, and management. Neurology 2012, 78, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Coppes, O.J.; Gutierrez, I.; Reder, A.T.; Ksiazek, S.; Bernard, J. Severe early bilateral macular edema following fingolimod therapy. Mult. Scler. Relat. Disord. 2013, 2, 256–258. [Google Scholar] [CrossRef] [PubMed]
- Li, V.; Kane, J.; Chan, H.H.; Hall, A.J.; Butzkueven, H. Continuing fingolimod after development of macular edema: A case report. Neurol. Neuroimmunol. Neuroinflamm. 2014, 1, e13. [Google Scholar] [CrossRef] [PubMed]
- Saida, T.; Kikuchi, S.; Itoyama, Y.; Hao, Q.; Kurosawa, T.; Nagato, K.; Tang, D.; Zhang-Auberson, L.; Kira, J. A randomized, controlled trial of fingolimod (FTY720) in Japanese patients with multiple sclerosis. Mult. Scler. 2012, 18, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Gold, R.; Comi, G.; Palace, J.; Siever, A.; Gottschalk, R.; Bijarnia, M.; von Rosenstiel, P.; Tomic, D.; Kappos, L. Assessment of cardiac safety during fingolimod treatment initiation in a real-world relapsing multiple sclerosis population: A phase 3b, open-label study. J. Neurol. 2014, 261, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Correia, I.; Batista, S.; Marques, I.B.; Sousa, M.; Ferreira, R.; Nunes, C.; Macario, M.C.; Sousa, L. The effectiveness of fingolimod in a Portuguese real-world population. Mult. Scler. Relat. Disord. 2016, 6, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Ordonez-Boschetti, L.; Rey, R.; Cruz, A.; Sinha, A.; Reynolds, T.; Frider, N.; Alvarenga, R. Safety and Tolerability of Fingolimod in Latin American Patients with Relapsing-Remitting Multiple Sclerosis: The Open-Label FIRST LATAM Study. Adv. Ther. 2015, 32, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Al-Hashel, J.; Ahmed, S.F.; Behbehani, R.; Alroughani, R. Real-world use of fingolimod in patients with relapsing remitting multiple sclerosis: A retrospective study using the national multiple sclerosis registry in Kuwait. CNS Drugs 2014, 28, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Kappos, L.; O’Connor, P.; Radue, E.W.; Polman, C.; Hohlfeld, R.; Selmaj, K.; Ritter, S.; Schlosshauer, R.; von Rosenstiel, P.; Zhang-Auberson, L.; et al. Long-term effects of fingolimod in multiple sclerosis: The randomized FREEDOMS extension trial. Neurology 2015, 84, 1582–1591. [Google Scholar] [CrossRef] [PubMed]
- Khatri, B.; Barkhof, F.; Comi, G.; Hartung, H.P.; Kappos, L.; Montalban, X.; Pelletier, J.; Stites, T.; Wu, S.; Holdbrook, F.; et al. Comparison of fingolimod with interferon β-1α in relapsing-remitting multiple sclerosis: A randomised extension of the TRANSFORMS study. Lancet Neurol. 2011, 10, 520–529. [Google Scholar] [CrossRef]
- Cohen, J.A.; Khatri, B.; Barkhof, F.; Comi, G.; Hartung, H.P.; Montalban, X.; Pelletier, J.; Stites, T.; Ritter, S.; von Rosenstiel, P.; et al. Long-term (up to 4.5 years) treatment with fingolimod in multiple sclerosis: Results from the extension of the randomised TRANSFORMS study. J. Neurol. Neurosurg. Psychiatry 2016, 87, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Kira, J.; Itoyama, Y.; Kikuchi, S.; Hao, Q.; Kurosawa, T.; Nagato, K.; Tsumiyama, I.; von Rosenstiel, P.; Zhang-Auberson, L.; Saida, T. Fingolimod (FTY720) therapy in Japanese patients with relapsing multiple sclerosis over 12 months: Results of a phase 2 observational extension. BMC Neurol. 2014, 14, 21. [Google Scholar] [CrossRef] [PubMed]
- Lublin, F.; Miller, D.H.; Freedman, M.S.; Cree, B.A.; Wolinsky, J.S.; Weiner, H.; Lubetzki, C.; Hartung, H.P.; Montalban, X.; Uitdehaag, B.M.; et al. Oral fingolimod in primary progressive multiple sclerosis (INFORMS): A phase 3, randomised, double-blind, placebo-controlled trial. Lancet 2016, 387, 1075–1084. [Google Scholar] [CrossRef]
- Yau, J.W.; Rogers, S.L.; Kawasaki, R.; Lamoureux, E.L.; Kowalski, J.W.; Bek, T.; Chen, S.J.; Dekker, J.M.; Fletcher, A.; Grauslund, J.; et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012, 35, 556–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hietala, K.; Forsblom, C.; Summanen, P.; Groop, P.H. Higher age at onset of type 1 diabetes increases risk of macular oedema. Acta Ophthalmol. 2013, 91, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Knudtson, M.D.; Lee, K.E.; Gangnon, R.; Klein, B.E. The Wisconsin Epidemiologic Study of Diabetic Retinopathy XXIII: The twenty-five-year incidence of macular edema in persons with type 1 diabetes. Ophthalmology 2009, 116, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, M.L.; Broe, R.; Frydkjaer-Olsen, U.; Olsen, B.S.; Mortensen, H.B.; Peto, T.; Grauslund, J. Microaneurysm count as a predictor of long-term progression in diabetic retinopathy in young patients with type 1 diabetes: The Danish Cohort of Pediatric Diabetes 1987 (DCPD1987). Graefes Arch. Clin. Exp. Ophthalmol. 2015, 253, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.W.; Park, C.Y.; Ham, D.I. The correlation between fluorescein angiographic and optical coherence tomographic features in clinically significant diabetic macular edema. Am. J. Ophthalmol. 2004, 137, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.; Bandello, F.; Schmidt-Erfurth, U.; Lang, G.E.; Massin, P.; Schlingemann, R.O.; Sutter, F.; Simader, C.; Burian, G.; Gerstner, O.; et al. The RESTORE study: Ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology 2011, 118, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Massin, P.; Bandello, F.; Garweg, J.G.; Hansen, L.L.; Harding, S.P.; Larsen, M.; Mitchell, P.; Sharp, D.; Wolf-Schnurrbusch, U.E.; Gekkieva, M.; et al. Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE Study): A 12-month, randomized, controlled, double-masked, multicenter phase II study. Diabetes Care 2010, 33, 2399–2405. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.; Wong, T.Y.; Sabanayagam, C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis. 2015, 2, 17. [Google Scholar] [CrossRef] [PubMed]
- Sykes, D.A.; Riddy, D.M.; Stamp, C.; Bradley, M.E.; McGuiness, N.; Sattikar, A.; Guerini, D.; Rodrigues, I.; Glaenzel, A.; Dowling, M.R.; et al. Investigating the molecular mechanisms through which FTY720-P causes persistent S1P1 receptor internalization. Br. J. Pharmacol. 2014, 171, 4797–4807. [Google Scholar] [CrossRef] [PubMed]
- Camm, J.; Hla, T.; Bakshi, R.; Brinkmann, V. Cardiac and vascular effects of fingolimod: Mechanistic basis and clinical implications. Am. Heart J. 2014, 168, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Oo, M.L.; Thangada, S.; Wu, M.T.; Liu, C.H.; Macdonald, T.L.; Lynch, K.R.; Lin, C.Y.; Hla, T. Immunosuppressive and anti-angiogenic sphingosine 1-phosphate receptor-1 agonists induce ubiquitinylation and proteasomal degradation of the receptor. J. Biol. Chem. 2007, 282, 9082–9089. [Google Scholar] [CrossRef] [PubMed]
- Graler, M.H.; Goetzl, E.J. The immunosuppressant FTY720 down-regulates sphingosine 1-phosphate G-protein-coupled receptors. FASEB J. 2004, 18, 551–553. [Google Scholar] [CrossRef] [PubMed]
- McVerry, B.J.; Peng, X.; Hassoun, P.M.; Sammani, S.; Simon, B.A.; Garcia, J.G. Sphingosine 1-phosphate reduces vascular leak in murine and canine models of acute lung injury. Am. J. Respir. Crit. Care Med. 2004, 170, 987–993. [Google Scholar] [CrossRef] [PubMed]
- Saab, G.; Almony, A.; Blinder, K.J.; Schuessler, R.; Brennan, D.C. Reversible cystoid macular edema secondary to fingolimod in a renal transplant recipient. Arch. Ophthalmol. 2008, 126, 140–141. [Google Scholar] [CrossRef] [PubMed]
- Turaka, K.; Bryan, J.S. Does fingolimod in multiple sclerosis patients cause macular edema? J. Neurol. 2012, 259, 386–388. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Cuthbertson, F. Early bilateral cystoid macular oedema secondary to fingolimod in multiple sclerosis. Case Rep. Med. 2012, 2012, 134636. [Google Scholar] [CrossRef] [PubMed]
- Afshar, A.R.; Fernandes, J.K.; Patel, R.D.; Ksiazek, S.M.; Sheth, V.S.; Reder, A.T.; Hariprasad, S.M. Cystoid macular edema associated with fingolimod use for multiple sclerosis. JAMA Ophthalmol. 2013, 131, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Chui, J.; Herkes, G.K.; Chang, A. Management of fingolimod-associated macular edema. JAMA Ophthalmol. 2013, 131, 694–696. [Google Scholar] [CrossRef] [PubMed]
- Minuk, A.; Belliveau, M.J.; Almeida, D.R.; Dorrepaal, S.J.; Gale, J.S. Fingolimod-associated macular edema: Resolution by sub-tenon injection of triamcinolone with continued fingolimod use. JAMA Ophthalmol. 2013, 131, 802–804. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Bhatti, M.T.; Costello, F. Famous. Surv. Ophthalmol. 2016, 61, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Ueda, N.; Saida, K. Retinal hemorrhages following fingolimod treatment for multiple sclerosis; a case report. BMC Ophthalmol. 2015, 15, 135. [Google Scholar] [CrossRef] [PubMed]
- Schroder, K.; Finis, D.; Harmel, J.; Ringelstein, M.; Hartung, H.P.; Geerling, G.; Aktas, O.; Guthoff, R. Acetazolamide therapy in a case of fingolimod-associated macular edema: Early benefits and long-term limitations. Mult. Scler. Relat. Disord. 2015, 4, 406–408. [Google Scholar] [CrossRef] [PubMed]
- Thoo, S.; Cugati, S.; Lee, A.; Chen, C. Successful treatment of fingolimod-associated macular edema with intravitreal triamcinolone with continued fingolimod use. Mult. Scler. 2015, 21, 249–251. [Google Scholar] [CrossRef] [PubMed]
- Tauseef, M.; Kini, V.; Knezevic, N.; Brannan, M.; Ramchandaran, R.; Fyrst, H.; Saba, J.; Vogel, S.M.; Malik, A.B.; Mehta, D. Activation of sphingosine kinase-1 reverses the increase in lung vascular permeability through sphingosine-1-phosphate receptor signaling in endothelial cells. Circ. Res. 2008, 103, 1164–1172. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Thangada, S.; Claffey, K.P.; Ancellin, N.; Liu, C.H.; Kluk, M.; Volpi, M.; Sha’afi, R.I.; Hla, T. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell 1999, 99, 301–312. [Google Scholar] [CrossRef]
- Nishihara, H.; Shimizu, F.; Sano, Y.; Takeshita, Y.; Maeda, T.; Abe, M.; Koga, M.; Kanda, T. Fingolimod prevents blood-brain barrier disruption induced by the sera from patients with multiple sclerosis. PLoS ONE 2015, 10, e0121488. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Hassoun, P.M.; Sammani, S.; McVerry, B.J.; Burne, M.J.; Rabb, H.; Pearse, D.; Tuder, R.M.; Garcia, J.G. Protective effects of sphingosine 1-phosphate in murine endotoxin-induced inflammatory lung injury. Am. J. Respir. Crit. Care Med. 2004, 169, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chiang, E.T.; Simmons, J.T.; Garcia, J.G.; Dudek, S.M. FTY720-induced human pulmonary endothelial barrier enhancement is mediated by c-Abl. Eur. Respir. J. 2011, 38, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Foster, C.A.; Mechtcheriakova, D.; Storch, M.K.; Balatoni, B.; Howard, L.M.; Bornancin, F.; Wlachos, A.; Sobanov, J.; Kinnunen, A.; Baumruker, T. FTY720 rescue therapy in the dark agouti rat model of experimental autoimmune encephalomyelitis: Expression of central nervous system genes and reversal of blood-brain-barrier damage. Brain Pathol. 2009, 19, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.F.; Gordon, S.; Estrada, R.; Wang, L.; Siow, D.L.; Wattenberg, B.W.; Lominadze, D.; Lee, M.J. Balance of S1P1 and S1P2 signaling regulates peripheral microvascular permeability in rat cremaster muscle vasculature. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H33–H42. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Yan, H. FTY720 Attenuates Retinal Inflammation and Protects Blood-Retinal Barrier in Diabetic Rats. Investig. Ophthalmol. Vis. Sci. 2016, 57, 1254–1263. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.; Chen, J.; Pan, M.; Zhang, M.; Zhang, J.; Chen, P.; Liu, B. FTY720 prevents progression of renal fibrosis by inhibiting renal microvasculature endothelial dysfunction in a rat model of chronic kidney disease. J. Mol. Histol. 2013, 44, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Fan, L.; Wei, L.; Gao, H.; Zhang, R.; Tao, L.; Cao, F.; Wang, H. FTY720 protects cardiac microvessels of diabetes: A critical role of S1P1/3 in diabetic heart disease. PLoS ONE 2012, 7, e42900. [Google Scholar] [CrossRef] [PubMed]
- Mullershausen, F.; Zecri, F.; Cetin, C.; Billich, A.; Guerini, D.; Seuwen, K. Persistent signaling induced by FTY720-phosphate is mediated by internalized S1P1 receptors. Nat. Chem. Biol. 2009, 5, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Dudek, S.M.; Camp, S.M.; Chiang, E.T.; Singleton, P.A.; Usatyuk, P.V.; Zhao, Y.; Natarajan, V.; Garcia, J.G. Pulmonary endothelial cell barrier enhancement by FTY720 does not require the S1P1 receptor. Cell Signal. 2007, 19, 1754–1764. [Google Scholar] [CrossRef] [PubMed]
- Shea, B.S.; Brooks, S.F.; Fontaine, B.A.; Chun, J.; Luster, A.D.; Tager, A.M. Prolonged exposure to sphingosine 1-phosphate receptor-1 agonists exacerbates vascular leak, fibrosis, and mortality after lung injury. Am. J. Respir. Cell Mol. Biol. 2010, 43, 662–673. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Bao, S.; Hambly, B.D.; Gillies, M.C. Vascular endothelial growth factor-A: A multifunctional molecular player in diabetic retinopathy. Int. J. Biochem. Cell Biol. 2009, 41, 2368–2371. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Wang, J.; Zheng, T.; Liang, Y.; Yin, D.; Song, R.; Pei, T.; Pan, S.; Jiang, H.; Liu, L. FTY720 inhibits proliferation and epithelial-mesenchymal transition in cholangiocarcinoma by inactivating STAT3 signaling. BMC Cancer 2014, 14, 783. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Dunk, C.E.; Lye, S.J. Sphingosine signalling regulates decidual NK cell angiogenic phenotype and trophoblast migration. Hum. Reprod. 2013, 28, 3026–3037. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Ye, Y.; Lv, L.; Zhu, C.; Ye, S. FTY720 attenuates paraquat-induced lung injury in mice. Int. Immunopharmacol. 2014, 21, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Masuko, K.; Murata, M.; Beppu, M.; Nakamura, H.; Kato, T.; Yudoh, K. Sphingosine-1-phosphate modulates expression of vascular endothelial growth factor in human articular chondrocytes: A possible new role in arthritis. Int. J. Rheum. Dis. 2012, 15, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Brunkhorst, R.; Kanaan, N.; Koch, A.; Ferreiros, N.; Mirceska, A.; Zeiner, P.; Mittelbronn, M.; Derouiche, A.; Steinmetz, H.; Foerch, C.; et al. FTY720 treatment in the convalescence period improves functional recovery and reduces reactive astrogliosis in photothrombotic stroke. PLoS ONE 2013, 8, e70124. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Tan, W.; Guo, D.; Zhu, X.; Qian, K.; He, S. Altered expression of signaling genes in Jurkat cells upon FTY720 induced apoptosis. Int. J. Mol. Sci. 2010, 11, 3087–3105. [Google Scholar] [CrossRef] [PubMed]

| Study | Phase | Fingolimod 2.5 mg % (n) | Fingolimod 5 mg % (n) | Placebo or Active Comparator | Combination | Exposition Time |
|---|---|---|---|---|---|---|
| Tedesco-Silva et al. 2004 [16] * | II | 0% (0/41) | - | 0% (0/41) | Cyclosporine + Steroids | 3 months |
| Tedesco-Silva et al. 2006 [3] | III | 1.8% (4/224) | 3.4% (8/234) | 1.3% (3/229) | Cyclosporine + Steroids | 12 months |
| Salvadori et al. 2006 [5] | III | 1.3% (3/219) | 2.2% (5/224) | 3% (6/226) | Cyclosporine + Steroids | 12 months |
| Mulgaonkar et al. 2006 [17] | II | 0% (0/150) | 0% (0/72) | 0% (0/39) | Cyclosporine + Steroids | 12 months |
| Tedesco-Silva et al. 2007 [4] | II | 1.1% (1/89) | 0% (0/87) | 0% (0/94) | Cyclosporine + Steroids | 12 months |
| Hoitsma et al. 2011 [6] | III | 12.2% (6/48) | - | 9.3% (5/54) | Tacrolimus + Steroids | 12 months |
| Summary | 1.8% (14/771) | 2.1% (13/617) | 2.0% (14/683) | - | - | |
| 1.9% (27/1388) | - | - | - | |||
| Study | Phase | Acronym | Description | Fingolimod 0.5 mg % (n) | Fingolimod 1.25 mg % (n) | Placebo or Active Comparator | Exposition Time |
|---|---|---|---|---|---|---|---|
| Kappos et al. 2010 [13] Kappos et al. 2015 [28] | III Extension | FREEDOMS | Core study Placebo-fingolimod Continuous group | 0% (0/425) 0.6% (1/155) 0.3% (1/331) | 1.6% (7/429) 0% (0/145) 0.3% (1/289) | 0% (0/428) n.a. n.a. | 24 months up to 52 months |
| Calabresi et al. 2014 [11] | III | FREEDOMSII | Core study | 0.8% (3/358) | 1.1% (4/370) | 1.2% (6/487) | 24 months |
| Cohen et al. 2010 [12] Khatri et al. 2011 [29] Cohen et al. 2016 [30] | III Extension Extension | TRANSFORMS | Core study Placebo-fingolimod Continuous group Continuous group | 0.5% (2/429) 0.6% (1/167) *% (*/356) 0% (0/404) | 1% (4/420) 1.1% (2/174) *% (*/330) 0% (0/368) | 0% (0/431) n.a. n.a. n.a. | 12 months 24 months up to 54 months |
| Saida et al. 2012 [23] Kira et al. 2014 [31] | II Extension | - | Core study Placebo-fingolimod Continuous group | 0% (0/57) 0% (0/27) 0% (0/47) | 0% (0/57) 0% (0/27) 0% (0/46) | 0% (0/57) n.a. n.a. | 6 months 12 months |
| Lublin et al. 2016 [32] | III | INFORMS | Core study | 2.1% (7/336) | switch to 0.5 mg due to protocol amendment on 19 November 2009 | 1.2% (6/487) | 36–60 months |
| Summary | 0.8% (15/1954) | 1.1% (18/1622) | 0.6% (12/1890) | - | |||
| 0.9% (33/3576) | - | ||||||
| Study | n | Study Type | Study Duration/Follow-up Time | ME Cases (n) | n Diabetic MS Patients | n ME in Diabetic MS Patients |
|---|---|---|---|---|---|---|
| Ontenada et al. 2012 [18] | 317 | Retrospective | 3 months | 3 | 12 | 1 |
| Gold et al. 2014 [24] | 2417 | Open-label | 4 months | 19 | 26 | 1 |
| Al-Hashel et al. 2014 [27] | 175 | Retrospective | Up to 22 months | 0 | n.m. | n.a. |
| Ordonẽz-Boschetti et al. 2015 [26] | 138 | Open-label | 4 months | 0 | n.m. | n.a. |
| Correia et al. 2016 [25] | 104 | Retrospective | Up to 21 months | 0 | n.m. | n.a. |
| Study | Age | Gender | Diabetes/Uveitis | Onset (Months) | Time of Resolution (Months) | Uni- or Bilateral | Fingolimod | Therapy (Outcome) |
|---|---|---|---|---|---|---|---|---|
| Saab et al. 2008 * [46] | 58 | F | No | 3.0 | 2.0 | U | Discontinued | - |
| Turaka and Bryan 2012 [47] | 52 | M | No | 3.0 | 3.0 | U | Discontinued | Prednisolone |
| Liu and Cuthbertson 2012 [48] | 34 | F | No | 0.2 | NR. | B | Discontinued | Ketorolac and prednisolone (persistence of minimal ME in the right eye after 5 months) |
| Afshar et al. 2013 [49] | 52 | M | No | 0.2 | 1.0 | U | Continued | Nepafenac and prednisolone |
| 60 | F | Diabetes | 0.3 | 1.0 | B | Discontinued | - | |
| 57 | M | No | 1.0 | NR | U | Discontinued | Bromfenac (epiretinal membrane and still increased foveal thickness after 3 months) | |
| Chui et al. 2013 [50] | 67 | F | No | 6.0 | 1.2 | U | Discontinued | Ketorolac and dexamethasone |
| Minuk et al. 2013 [51] | 58 | F | Uveitis | 2.0 | 1.0 | B | Discontinued | Ketorolac, prednisolone, ST triamcinolone injection |
| Coppes et al. 2013 [21] | 60 | F | Diabetes | 0.3 | 1.6 | B | Discontinued | - |
| Li et al. 2014 [22] | 37 | F | No | 4.0 | NR | U | Continued | - (not resolved after 25 months) |
| Kim et al. 2015 [52] | 62 | F | No | 2.5 | 1.0 | B | Discontinued | Ketorolac and prednisolone |
| Ueda and Saida 2015 [53] | 31 | M | No | 1.0 | 1.0 | U | Discontinued | Betamethasone (resolved in 1 month but persistence of decreased visual acuity) |
| Schröder et al. 2015 [54] | 24 | F | No | 1.0 | NM | B | Discontinued | Acetazolamide (visual acuity returned to normal after 2 weeks while it remains unknown whether ME resolved) |
| Thoo et al. 2015 [55] | 59 | F | No | 0.7 | 1.0 | B | Continued | Prednisolone and IV triamcinolone |
| 66 | F | No | 12.0 | 2.0 | U | Continued | Keterolac and prednisolone IV triamcinolone (resolved in 1 week after IV triamcinolone) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pul, R.; Osmanovic, A.; Schmalstieg, H.; Pielen, A.; Pars, K.; Schwenkenbecher, P.; Sühs, K.W.; Yildiz, Ö.; Frank, B.; Stangel, M.; et al. Fingolimod Associated Bilateral Cystoid Macular Edema—Wait and See? Int. J. Mol. Sci. 2016, 17, 2106. https://doi.org/10.3390/ijms17122106
Pul R, Osmanovic A, Schmalstieg H, Pielen A, Pars K, Schwenkenbecher P, Sühs KW, Yildiz Ö, Frank B, Stangel M, et al. Fingolimod Associated Bilateral Cystoid Macular Edema—Wait and See? International Journal of Molecular Sciences. 2016; 17(12):2106. https://doi.org/10.3390/ijms17122106
Chicago/Turabian StylePul, Refik, Alma Osmanovic, Holger Schmalstieg, Amelie Pielen, Kaweh Pars, Philipp Schwenkenbecher, Kurt Wolfram Sühs, Özlem Yildiz, Benedikt Frank, Martin Stangel, and et al. 2016. "Fingolimod Associated Bilateral Cystoid Macular Edema—Wait and See?" International Journal of Molecular Sciences 17, no. 12: 2106. https://doi.org/10.3390/ijms17122106







