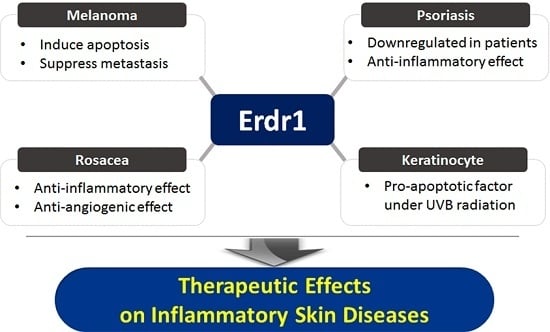
Roles of Erythroid Differentiation Regulator 1 (Erdr1) on Inflammatory Skin Diseases
Abstract
:1. Introduction
2. Roles of Erythroid Differentiation Regulator 1 (Erdr1) on Inflammatory Skin Diseases
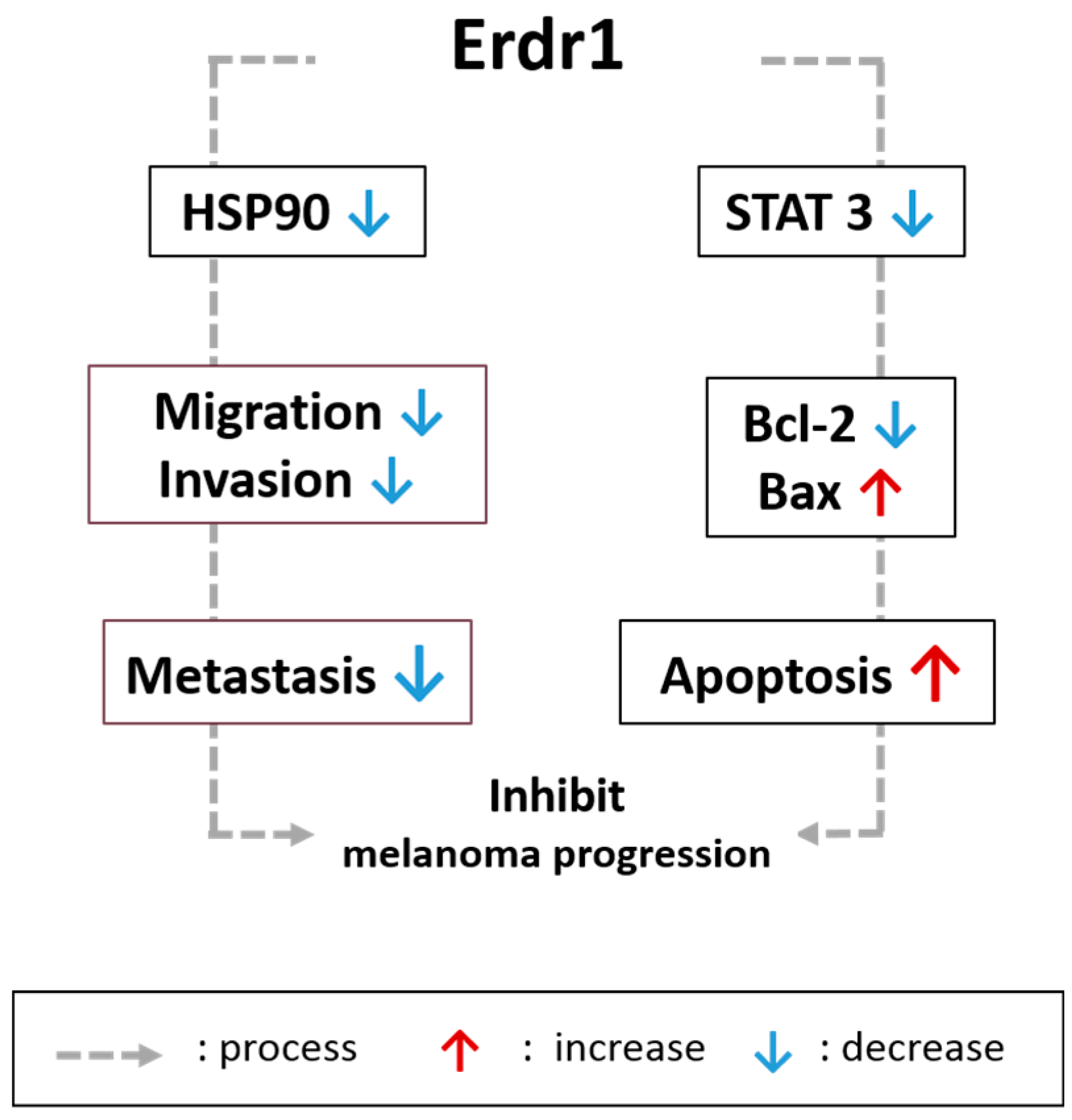
2.1. Effects of Erdr1 on Malignant Skin Cancer and Melanoma
2.2. Roles of Erdr1 on Psoriasis
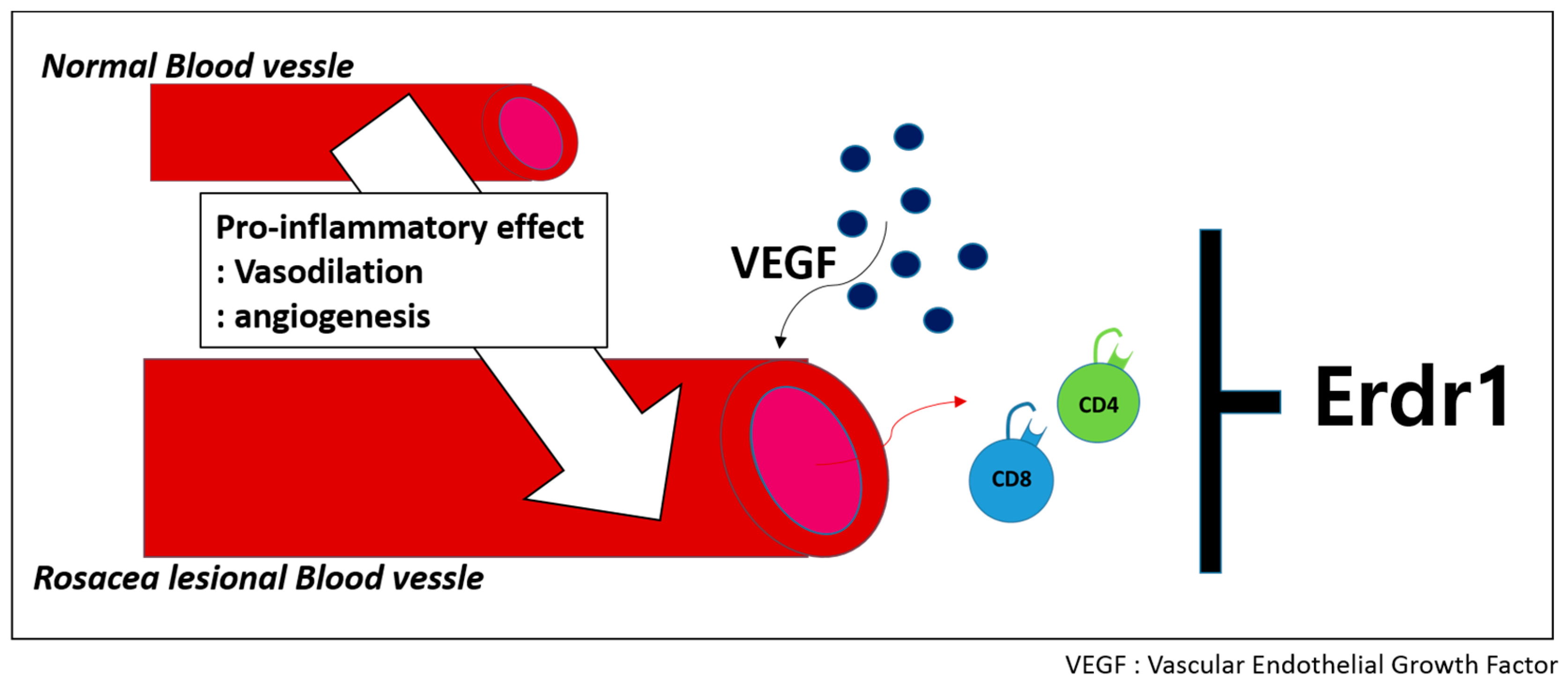
2.3. Therapeutinc Effect of Erdr1 on Rosacea
3. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dormer, P.; Spitzer, E.; Frankenberger, M.; Kremmer, E. Erythroid differentiation regulator (Edr), a novel, highly conserved factor I. Induction of haemoglobin synthesis in erythroleukaemic cells. Cytokine 2004, 26, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Dormer, P.; Spitzer, E.; Moller, W. Edr is a stress-related survival factor from stroma and other tissues acting on early haematopoietic progenitors (E-Mix). Cytokine 2004, 27, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.K.; Park, Y.; Song, S.B.; Cheon, S.Y.; Park, S.; Houh, Y.; Ha, S.; Kim, H.J.; Park, J.M.; Kim, T.S.; et al. Erythroid differentiation regulator 1, an interleukin 18-regulated gene, acts as a metastasis suppressor in melanoma. J. Investig. Dermatol. 2011, 131, 2096–2104. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; El Hajj, N.; Sittler, S.; Lammert, N.; Barnes, R.; Meloni-Ehrig, A. Gastric cancer: Classification, histology and application of molecular pathology. J. Gastrointest. Oncol. 2012, 3, 251–261. [Google Scholar] [PubMed]
- Jung, M.K.; Houh, Y.K.; Ha, S.; Yang, Y.; Kim, D.; Kim, T.S.; Yoon, S.R.; Bang, S.I.; Cho, B.J.; Lee, W.J.; et al. Recombinant Erdr1 suppresses the migration and invasion ability of human gastric cancer cells, SNU-216, through the JNK pathway. Immunol. Lett. 2013, 150, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.R.; Huh, S.Y.; Hur, D.Y.; Jeong, H.; Kim, T.S.; Kim, S.Y.; Park, S.B.; Yang, Y.; Bang, S.I.; Park, H.; et al. Erdr1 enhances human NK cell cytotoxicity through an actin-regulated degranulation-dependent pathway. Cell. Immunol. 2014, 292, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Roda-Navarro, P.; Reyburn, H.T. Intercellular protein transfer at the NK cell immune synapse: Mechanisms and physiological significance. FASEB J. 2007, 21, 1636–1646. [Google Scholar] [CrossRef] [PubMed]
- Davis, D.M.; Chiu, I.; Fassett, M.; Cohen, G.B.; Mandelboim, O.; Strominger, J.L. The human natural killer cell immune synapse. Proc. Natl. Acad. Sci. USA 1999, 96, 15062–15067. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Vanaclocha, F.; Fantuzzi, G.; Mendoza, L.; Fuentes, A.M.; Anasagasti, M.J.; Martin, J.; Carrascal, T.; Walsh, P.; Reznikov, L.L.; Kim, S.H.; et al. IL-18 regulates IL-1β-dependent hepatic melanoma metastasis via vascular cell adhesion molecule-1. Proc. Natl. Acad. Sci. USA 2000, 97, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.S.; Bae, S.Y.; Kim, H.R.; Kim, Y.S.; Kim, D.J.; Cho, B.J.; Yang, H.K.; Hwang, Y.I.; Kim, K.J.; Park, H.S.; et al. Interleukin-18 increases metastasis and immune escape of stomach cancer via the downregulation of CD70 and maintenance of CD44. Carcinogenesis 2009, 30, 1987–1996. [Google Scholar] [CrossRef] [PubMed]
- Keyel, P.A. How is inflammation initiated? Individual influences of IL-1, IL-18 and HMGB1. Cytokine 2014, 69, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Sedimbi, S.K.; Hagglof, T.; Karlsson, M.C. IL-18 in inflammatory and autoimmune disease. Cell. Mol. Life Sci. 2013, 70, 4795–4808. [Google Scholar] [CrossRef] [PubMed]
- Okamura, H.; Tsutsi, H.; Komatsu, T.; Yutsudo, M.; Hakura, A.; Tanimoto, T.; Torigoe, K.; Okura, T.; Nukada, Y.; Hattori, K.; et al. Cloning of a new cytokine that induces IFN-γ production by T cells. Nature 1995, 378, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Interleukin-18, a proinflammatory cytokine. Eur. Cytokine Netw. 2000, 11, 483–486. [Google Scholar] [PubMed]
- Pasparakis, M.; Haase, I.; Nestle, F.O. Mechanisms regulating skin immunity and inflammation. Nat. Rev. Immunol. 2014, 14, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Di Meglio, P.; Perera, G.K.; Nestle, F.O. The multitasking organ: Recent insights into skin immune function. Immunity 2011, 35, 857–869. [Google Scholar] [CrossRef] [PubMed]
- Nestle, F.O.; di Meglio, P.; Qin, J.Z.; Nickoloff, B.J. Skin immune sentinels in health and disease. Nat. Rev. Immunol. 2009, 9, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Shishodia, S.; Sandur, S.K.; Pandey, M.K.; Sethi, G. Inflammation and cancer: How hot is the link? Biochem. Pharmacol. 2006, 72, 1605–1621. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Cho, D.H.; Park, H.J. IL-18 and cutaneous inflammatory diseases. Int. J. Mol. Sci. 2015, 16, 29357–29369. [Google Scholar] [CrossRef] [PubMed]
- Nedoszytko, B.; Sokolowska-Wojdylo, M.; Ruckemann-Dziurdzinska, K.; Roszkiewicz, J.; Nowicki, R.J. Chemokines and cytokines network in the pathogenesis of the inflammatory skin diseases: Atopic dermatitis, psoriasis and skin mastocytosis. Postep. Derm. Alergol. 2014, 31, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Sauder, D.N. The role of epidermal cytokines in inflammatory skin diseases. J. Investig. Dermatol. 1990, 95, 27S–28S. [Google Scholar] [CrossRef] [PubMed]
- Boraschi, D.; Dinarello, C.A. IL-18 in autoimmunity: Review. Eur. Cytokine Netw. 2006, 17, 224–252. [Google Scholar] [PubMed]
- Pietrzak, A.; Janowski, K.; Chodorowska, G.; Michalak-Stoma, A.; Rolinski, J.; Zalewska, A.; Jastrzebska, I.; Tabarkiewicz, J.; Paszkowski, T.; Kapec, E.; et al. Plasma interleukin-18 and dendritic cells in males with psoriasis vulgaris. Mediators Inflamm. 2007, 2007, 61254. [Google Scholar] [CrossRef] [PubMed]
- Flisiak, I.; Klepacki, A.; Chodynicka, B. Plasma and scales levels of interleukin 18 in comparison with other possible clinical and laboratory biomarkers of psoriasis activity. Biomarkers 2006, 11, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Hon, K.L.; Leung, T.F.; Ma, K.C.; Wong, C.K.; Wan, H.; Lam, C.W. Serum concentration of IL-18 correlates with disease extent in young children with atopic dermatitis. Pediatr. Dermatol. 2004, 21, 619–622. [Google Scholar] [CrossRef] [PubMed]
- Puxeddu, I.; Italiani, P.; Giungato, P.; Pratesi, F.; Panza, F.; Bartaloni, D.; Rocchi, V.; del Corso, I.; Boraschi, D.; Migliorini, P. Free IL-18 and IL-33 cytokines in chronic spontaneous urticaria. Cytokine 2013, 61, 741–743. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Song, S.B.; Yang, Y.; Eun, Y.S.; Cho, B.K.; Park, H.J.; Cho, D.H. Erythroid differentiation regulator 1 (Erdr1) is a proapototic factor in human keratinocytes. Exp. Dermatol. 2011, 20, 920–925. [Google Scholar] [CrossRef] [PubMed]
- Mancianti, M.L.; Herlyn, M. Tumor progression in melanoma: The biology of epidermal melanocytes in vitro. Carcinogenesis 1989, 11, 369–386. [Google Scholar]
- Maru, G.B.; Gandhi, K.; Ramchandani, A.; Kumar, G. The role of inflammation in skin cancer. Adv. Exp. Med. Biol. 2014, 816, 437–469. [Google Scholar] [PubMed]
- Kraus, S.; Arber, N. Inflammation and colorectal cancer. Curr. Opin. Pharmacol. 2009, 9, 405–410. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.M.; Pick, E.; Kluger, Y.; Gould-Rothberg, B.; Lazova, R.; Camp, R.L.; Rimm, D.L.; Kluger, H.M. HSP90 as a marker of progression in melanoma. Ann. Oncol. 2008, 19, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Soo, E.T.; Yip, G.W.; Lwin, Z.M.; Kumar, S.D.; Bay, B.H. Heat shock proteins as novel therapeutic targets in cancer. In Vivo 2008, 22, 311–315. [Google Scholar] [PubMed]
- Sevin, M.; Girodon, F.; Garrido, C.; de Thonel, A. HSP90 and HSP70: Implication in inflammation processes and therapeutic approaches for myeloproliferative neoplasms. Mediators Inflamm. 2015, 2015, 970242. [Google Scholar] [CrossRef] [PubMed]
- Madrigal-Matute, J.; Lopez-Franco, O.; Blanco-Colio, L.M.; Munoz-Garcia, B.; Ramos-Mozo, P.; Ortega, L.; Egido, J.; Martin-Ventura, J.L. Heat shock protein 90 inhibitors attenuate inflammatory responses in atherosclerosis. Cardiovasc. Res. 2010, 86, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Stenderup, K.; Rosada, C.; Gavillet, B.; Vuagniaux, G.; Dam, T.N. DEBIO 0932, a new oral HSP90 inhibitor, alleviates psoriasis in a xenograft transplantation model. Acta Derm. Venereol. 2014, 94, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Soengas, M.S.; Lowe, S.W. Apoptosis and melanoma chemoresistance. Oncogene 2003, 22, 3138–3151. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jung, M.K.; Park, H.J.; Kim, K.E.; Cho, D. Erdr1 suppresses murine melanoma growth via regulation of apoptosis. Int. J. Mol. Sci. 2016, 17, 107. [Google Scholar] [CrossRef] [PubMed]
- Helmbach, H.; Rossmann, E.; Kern, M.A.; Schadendorf, D. Drug-resistance in human melanoma. Int. J. Cancer 2001, 93, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Del Bufalo, D.; Trisciuoglio, D.; Scarsella, M.; Zangemeister-Wittke, U.; Zupi, G. Treatment of melanoma cells with a Bcl-2/Bcl-xl antisense oligonucleotide induces antiangiogenic activity. Oncogene 2003, 22, 8441–8447. [Google Scholar] [CrossRef] [PubMed]
- Piro, L.D. Apoptosis, Bcl-2 antisense, and cancer therapy. Oncology 2004, 18, 5–10. [Google Scholar] [PubMed]
- Fofaria, N.M.; Srivastava, S.K. Critical role of STAT3 in melanoma metastasis through anoikis resistance. Oncotarget 2014, 5, 7051–7064. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Pardoll, D.; Jove, R. STATS in cancer inflammation and immunity: A leading role for STAT3. Nat. Rev. Cancer 2009, 9, 798–809. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.K.; Song, H.K.; Kim, K.E.; Hur, D.Y.; Kim, T.; Bang, S.; Park, H.; Cho, D.H. IL-18 enhances the migration ability of murine melanoma cells through the generation of ROI and the MAPK pathway. Immunol. Lett. 2006, 107, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Lissoni, P.; Brivio, F.; Rovelli, F.; Fumagalli, G.; Malugani, F.; Vaghi, M.; Secondino, S.; Bucovec, R.; Gardani, G.S. Serum concentrations of interleukin-18 in early and advanced cancer patients: Enhanced secretion in metastatic disease. J. Biol. Regul. Homeost. Agents 2000, 14, 275–277. [Google Scholar] [PubMed]
- Crende, O.; Sabatino, M.; Valcarcel, M.; Carrascal, T.; Riestra, P.; Lopez-Guerrero, J.A.; Nagore, E.; Mandruzzato, S.; Wang, E.; Marincola, F.M.; et al. Metastatic lesions with and without interleukin-18-dependent genes in advanced-stage melanoma patients. Am. J. Pathol. 2013, 183, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Byun, D.; Kim, T.S.; Kim, Y.I.; Kang, J.S.; Hahm, E.S.; Kim, S.H.; Lee, W.J.; Song, H.K.; Yoon, D.Y.; et al. Enhanced IL-18 expression in common skin tumors. Immunol. Lett. 2001, 79, 215–219. [Google Scholar] [CrossRef]
- Wagner, E.F.; Schonthaler, H.B.; Guinea-Viniegra, J.; Tschachler, E. Psoriasis: What we have learned from mouse models. Nat. Rev. Rheumatol. 2010, 6, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Capon, F.; di Meglio, P.; Szaub, J.; Prescott, N.J.; Dunster, C.; Baumber, L.; Timms, K.; Gutin, A.; Abkevic, V.; Burden, A.D.; et al. Sequence variants in the genes for the interleukin-23 receptor (IL23R) and its ligand (IL12B) confer protection against psoriasis. Hum. Genet. 2007, 122, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Nestle, F.O.; Kaplan, D.H.; Barker, J. Psoriasis. N. Engl. J. Med. 2009, 361, 496–509. [Google Scholar] [CrossRef] [PubMed]
- Nestle, F.O.; Turka, L.A.; Nickoloff, B.J. Characterization of dermal dendritic cells in psoriasis. Autostimulation of T lymphocytes and induction of Th1 type cytokines. J. Clin. Investig. 1994, 94, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Danilenko, D.M.; Valdez, P.; Kasman, I.; Eastham-Anderson, J.; Wu, J.; Ouyang, W. Interleukin-22, a TH17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature 2007, 445, 648–651. [Google Scholar] [CrossRef] [PubMed]
- Cleaver, J.E.; Crowley, E. Uv damage, DNA repair and skin carcinogenesis. Front. Biosci. 2002, 7, d1024–d1043. [Google Scholar] [CrossRef] [PubMed]
- Sarasin, A. The molecular pathways of ultraviolet-induced carcinogenesis. Mutat. Res. 1999, 428, 5–10. [Google Scholar] [CrossRef]
- Albanesi, C.; Scarponi, C.; Giustizieri, M.L.; Girolomoni, G. Keratinocytes in inflammatory skin diseases. Curr. Drug. Targets Inflamm. Allergy 2005, 4, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, C.E.; Barker, J.N. Pathogenesis and clinical features of psoriasis. Lancet 2007, 370, 263–271. [Google Scholar] [CrossRef]
- Bos, J.D.; de Rie, M.A. The pathogenesis of psoriasis: Immunological facts and speculations. Immunol. Today 1999, 20, 40–46. [Google Scholar] [CrossRef]
- Ohta, Y.; Hamada, Y.; Katsuoka, K. Expression of IL-18 in psoriasis. Arch. Dermatol. Res. 2001, 293, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Arican, O.; Aral, M.; Sasmaz, S.; Ciragil, P. Serum levels of TNF-α, IFN-γ, IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005, 2005, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.E.; Houh, Y.; Lee, J.; Kim, S.; Cho, D.; Park, H.J. Downregulation of erythroid differentiation regulator 1 (Erdr1) plays a critical role in psoriasis pathogenesis. Exp. Dermatol. 2016, 25, 570–572. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.E.; Houh, Y.; Park, H.J.; Cho, D. Therapeutic effects of erythroid differentiation regulator 1 on imiquimod-induced psoriasis-like skin inflammation. Int. J. Mol. Sci. 2016, 17, 244. [Google Scholar] [CrossRef] [PubMed]
- Van der Fits, L.; Mourits, S.; Voerman, J.S.; Kant, M.; Boon, L.; Laman, J.D.; Cornelissen, F.; Mus, A.M.; Florencia, E.; Prens, E.P.; et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J. Immunol. 2009, 182, 5836–5845. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, T.; Yang, X.O.; Chung, Y.; Fukunaga, A.; Nurieva, R.; Pappu, B.; Martin-Orozco, N.; Kang, H.S.; Ma, L.; Panopoulos, A.D.; et al. CCR6 regulates the migration of inflammatory and regulatory T cells. J. Immunol. 2008, 181, 8391–8401. [Google Scholar] [CrossRef] [PubMed]
- Steinhoff, M.; Schauber, J.; Leyden, J.J. New insights into rosacea pathophysiology: A review of recent findings. J. Am. Acad. Dermatol. 2013, 69, S15–S26. [Google Scholar] [CrossRef] [PubMed]
- Steinhoff, M.; Buddenkotte, J.; Aubert, J.; Sulk, M.; Novak, P.; Schwab, V.D.; Mess, C.; Cevikbas, F.; Rivier, M.; Carlavan, I.; et al. Clinical, cellular, and molecular aspects in the pathophysiology of rosacea. J. Investig. Dermatol. Symp. Proc. 2011, 15, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Lindow, K.B.; Warren, C. Understanding rosacea. A guide to facilitating care. Am. J. Nurs. 2001, 101, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Detmar, M. Molecular regulation of angiogenesis in the skin. J. Investig. Dermatol. 1996, 106, 207–208. [Google Scholar] [CrossRef] [PubMed]
- Aroni, K.; Tsagroni, E.; Kavantzas, N.; Patsouris, E.; Ioannidis, E. A study of the pathogenesis of rosacea: How angiogenesis and mast cells may participate in a complex multifactorial process. Arch. Dermatol. Res. 2008, 300, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, K.E.; Jung, H.Y.; Jo, H.; Jeong, S.W.; Lee, J.; Kim, C.H.; Kim, H.; Cho, D.; Park, H.J. Recombinant erythroid differentiation regulator 1 inhibits both inflammation and angiogenesis in a mouse model of rosacea. Exp. Dermatol. 2015, 24, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Park, C.C.; Morel, J.C.; Amin, M.A.; Connors, M.A.; Harlow, L.A.; Koch, A.E. Evidence of IL-18 as a novel angiogenic mediator. J. Immunol. 2001, 167, 1644–1653. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.R.; Lanier, V.B.; Braziel, R.M.; Falkenhagen, K.M.; White, C.; Rosenbaum, J.T. Expression of vascular endothelial growth factor and its receptors in rosacea. Br. J. Ophthalmol. 2007, 91, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Chajra, H.; Nadim, M.; Auriol, D.; Schweikert, K.; Lefevre, F. Combination of new multifunctional molecules for erythematotelangiectatic rosacea disorder. Clin. Cosmet. Investig. Dermatol. 2015, 8, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Domingo, D.S.; Camouse, M.M.; Hsia, A.H.; Matsui, M.; Maes, D.; Ward, N.L.; Cooper, K.D.; Baron, E.D. Anti-angiogenic effects of epigallocatechin-3-gallate in human skin. Int. J. Clin. Exp. Pathol. 2010, 3, 705–709. [Google Scholar] [PubMed]
- Miyoshi, K.; Takaishi, M.; Nakajima, K.; Ikeda, M.; Kanda, T.; Tarutani, M.; Iiyama, T.; Asao, N.; DiGiovanni, J.; Sano, S. STAT3 as a therapeutic target for the treatment of psoriasis: A clinical feasibility study with STA-21, a STAT3 inhibitor. J. Investig. Dermatol. 2011, 131, 108–117. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Houh, Y.K.; Kim, K.E.; Park, H.J.; Cho, D. Roles of Erythroid Differentiation Regulator 1 (Erdr1) on Inflammatory Skin Diseases. Int. J. Mol. Sci. 2016, 17, 2059. https://doi.org/10.3390/ijms17122059
Houh YK, Kim KE, Park HJ, Cho D. Roles of Erythroid Differentiation Regulator 1 (Erdr1) on Inflammatory Skin Diseases. International Journal of Molecular Sciences. 2016; 17(12):2059. https://doi.org/10.3390/ijms17122059
Chicago/Turabian StyleHouh, Youn Kyung, Kyung Eun Kim, Hyun Jeong Park, and Daeho Cho. 2016. "Roles of Erythroid Differentiation Regulator 1 (Erdr1) on Inflammatory Skin Diseases" International Journal of Molecular Sciences 17, no. 12: 2059. https://doi.org/10.3390/ijms17122059