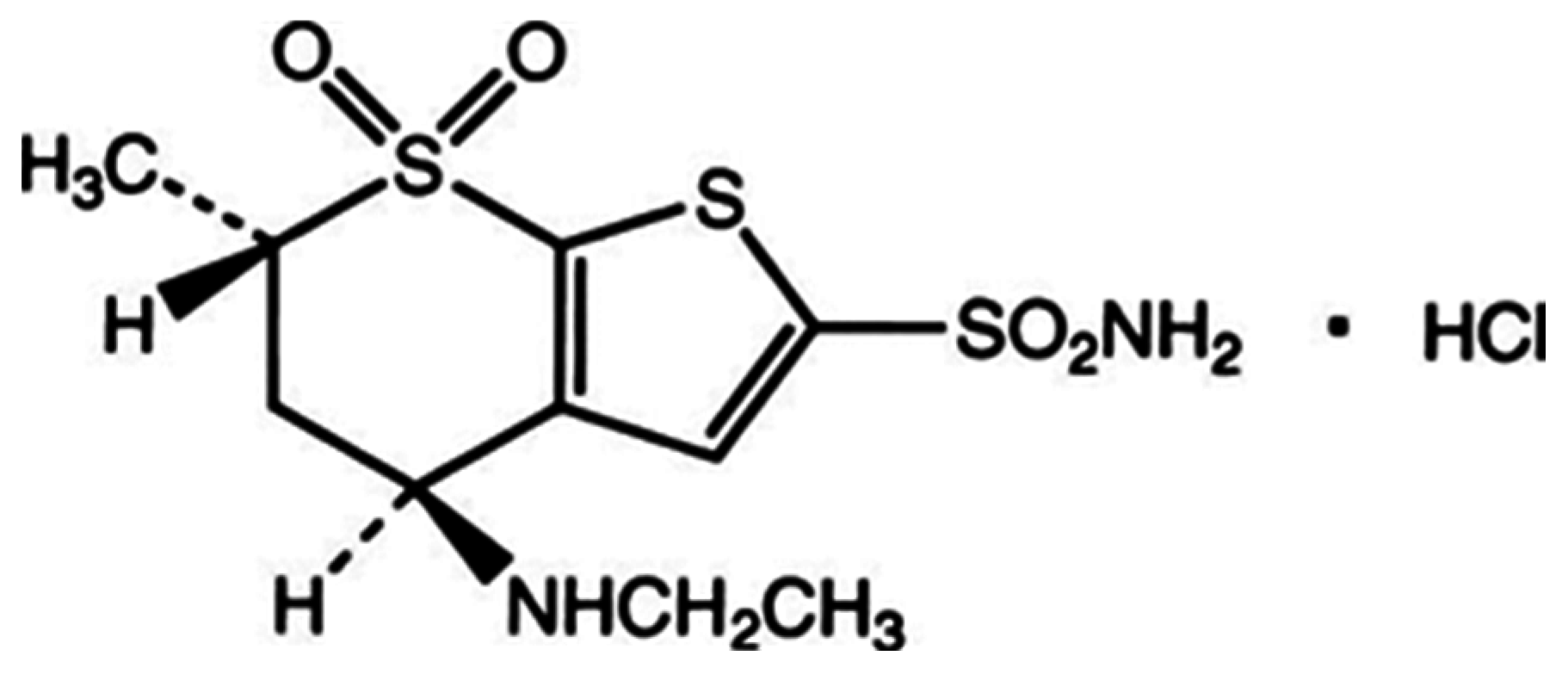
New Electrochemically-Modified Carbon Paste Inclusion β-Cyclodextrin and Carbon Nanotubes Sensors for Quantification of Dorzolamide Hydrochloride
Abstract
:1. Introduction
2. Results and Discussion
2.1. Performance Characteristics of Dorzolamide Carbon Paste Sensors
2.2. Effect of Plasticizers
2.3. Response Time
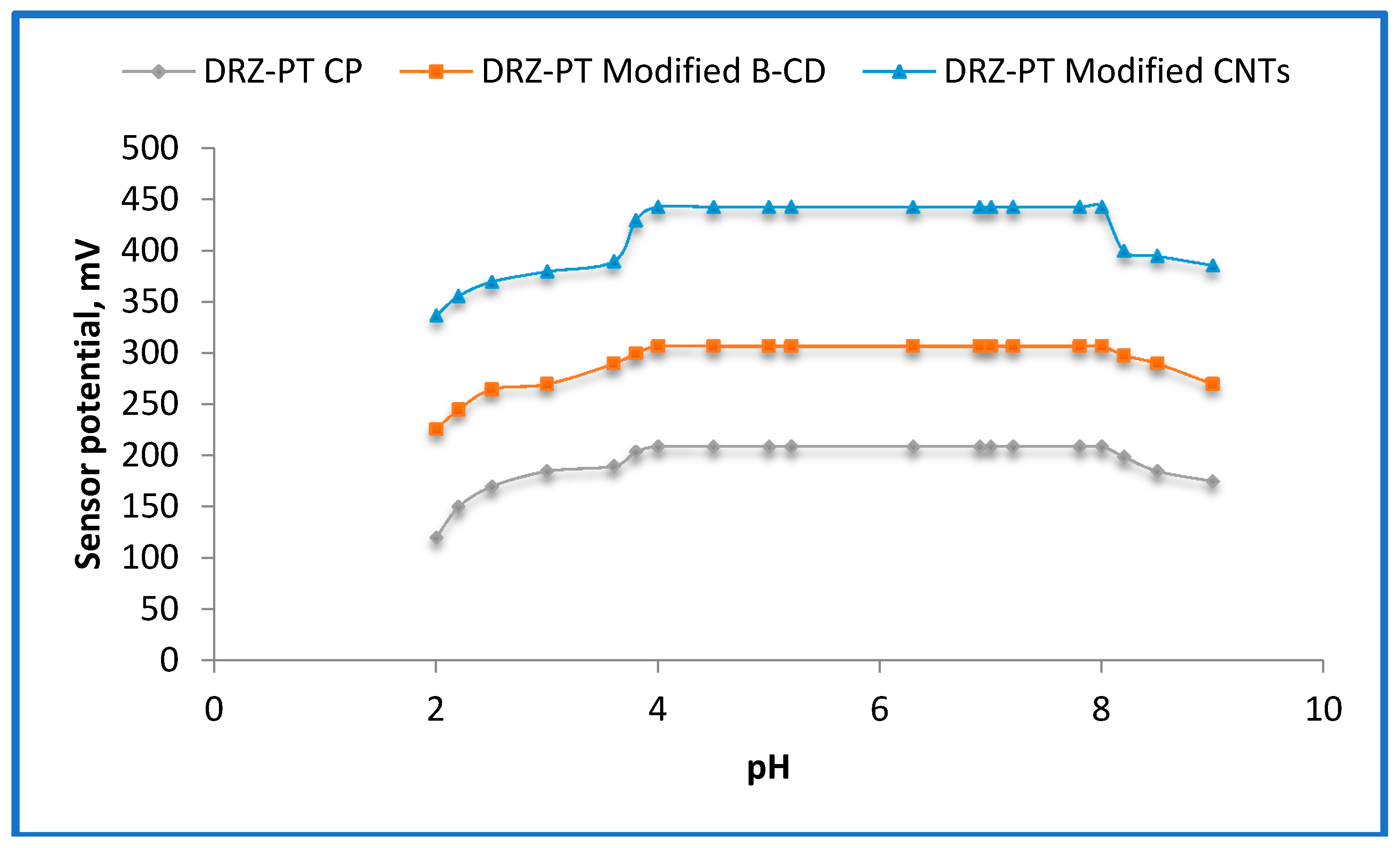
2.4. Effect of pH
2.5. Selectivity of Sensors
2.6. Effect of Temperature
2.7. Effect of Soaking Time
2.8. Analytical Applications
2.8.1. Quantification of Dorzolamide Hydrochloride
2.8.2. Content Uniformity Assay of Dorzolamide Ophthalmic Solution
2.9. Method Validation
2.9.1. Linearity and Lower Limit of Detection
2.9.2. Accuracy and Precision
2.9.3. Robustness and Ruggedness
3. Experimental
3.1. Materials and Reagents
3.2. Instruments
3.3. Preparation of Analytical Solutions
3.3.1. Standard Drug Solution
3.3.2. Preparation of Dorzolamide Hydrochloride Ophthalmic Solution
3.3.3. Preparation of Human Serum and Urine Samples
3.4. Preparation of Dorzolamide-Phosphotungstate Ion Pairs
3.5. Sensor Fabrication
3.5.1. Fabrication of the Carbon Paste Sensor
3.5.2. Preparation of the Modified Carbon Nanotubes Sensor
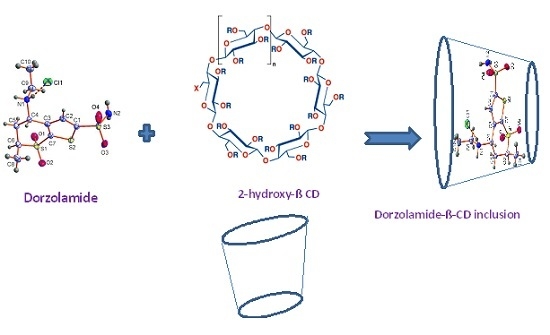
3.5.3. Preparation of the Modified β-Cyclodextrin Carbon Paste Sensor
3.6. Sensor Calibration
3.7. Standard Addition Method
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kubinyi, H. Chance favors the prepared mind from serendipity to rational drug design. J. Recept. Signal Transduct. Res. 1999, 19, 15–39. [Google Scholar] [CrossRef] [PubMed]
- Erk, N. Simultaneous determination of dorzolamide HCl and timolol maleate in eye drops by two different spectroscopic methods. J. Pharm. Biomed. Anal. 2002, 28, 391–397. [Google Scholar] [CrossRef]
- Sharath, H.M.; Jose, G.B.; Channabasavaraj, K.P.; Modiya, J.S. Development and validation of spectrophotometric methods for estimation of dorzolamide HCl in bulk and pharmaceutical dosage forms. Int. J. Pharm. Sci. Res. 2011, 2, 948–953. [Google Scholar]
- Dias, A.N.; Subrahmanyam, E.V.S.; Shabaraya, R.; Misquith, J. New analytical method and its validation for the estimation of dorzolamide hydrochloride in bulk and marketed formulation. Int. J. Chem. Tech. Res. 2013, 5, 1655–1663. [Google Scholar]
- Deshpande, S.V.; Funne, S.M.; Mahaparale, S.P.; Onkar, P.R. Development and validation of UV spectrophotometric method for estimation of timolol maleate and dorzolamide hydrochloride in bulk and eye drop formulation. Int. J. Pharm. Chem. Sci. 2014, 3, 838–844. [Google Scholar]
- Erk, N. Voltammetric and HPLC determination of dorzolamide hydrochloride in eye drops. Pharmazie 2003, 58, 870–873. [Google Scholar] [PubMed]
- Kanchan, R.U.; Shikha, M.N.R.; Rane, R.B. Simultaneous RP-HPLC determination of dorzolamide hydrochloride and timolol maleate in pharmaceutical preparations. Anal. Chem. Ind. J. 2008, 7, 638–641. [Google Scholar]
- Shadoul, W.A.; Gad-Kariem, E.A.; Adam, M.E.; Ibrahim, K.E.E. Simultaneous determination of dorzolamide hydrochloride and timolol maleate in ophthalmic solutions using HPLC. Elixir Pharm. 2011, 38, 4060–4063. [Google Scholar]
- Nagoria, B.P.; Maru, A.; Muysunic, P.; Guptad, S. Method development and its validation for simultaneous estimation of timolol maleate and dorzolamide hydrochloride in as API and in ophthalmic solution dosage form by RPHPLC. J. Chem. Pharm. Res. 2011, 3, 866–874. [Google Scholar]
- Kumar, S.R.; Charumathi, S.; Lavanya, J.; Duganath, N.; Kumar, P.B.R.; Devanna, N. Development and validation of RP-HPLC method for dorzolamide hydrochloride in bulk and pharmaceutical dosage form. Am. J. Pharm. Tech. Res. 2013, 3, 576–585. [Google Scholar]
- Bebawy, L.I. Application of TLC-densitometry, first-derivative UV-spectrophotometry and ratio derivative spectrophotometry for the determination of dorzolamide hydrochloride and timolol maleate. J. Pharm. Biomed. Anal. 2002, 27, 737–746. [Google Scholar] [CrossRef]
- Svancara, I.; Vytras, K.; Kalcher, K.; Walcarius, A.; Wang, J. Carbon paste electrodes in facts, numbers and notes: A review on the occasion of the 50-years jubilee of carbon paste in electrochemistry and electroanalysis. J. Electroanal. 2009, 21, 7–28. [Google Scholar] [CrossRef]
- Sinha, N.; Ma, J.; Yeow, J.T.M. Carbon nanotube-based sensors. J. Nanosci. Nanotechnol. 2006, 6, 573–590. [Google Scholar] [CrossRef] [PubMed]
- Bernholc, J.; Brenner, D.; Nardelli, M.B.; Meunier, V.; Roland, C. Mechanical and electrical properties of nanotubes. Annu. Rev. Mater. Res. 2012, 32, 347–375. [Google Scholar] [CrossRef]
- Jordi Riu, J.; Pingarron, M.; Rius, F.X. Electrochemical sensing based on carbon nanotubes. Trends Anal. Chem. 2010, 29, 939–953. [Google Scholar]
- Issa, Y.M.; Abdel-Fattah, H.M.; Abdel-Moniem, N.B. Chemically modified carbon paste sensor for potentiometric determination of doxycycline hydrochloride in batch and FIA conditions. Int. J. Electrochem. Sci. 2013, 8, 9578–9592. [Google Scholar]
- Dey, M.K.; Satpati, A.K.; Reddy, A.V.R. Electrochemical determination of melamine on static mercury drop electrode and on gold nano particle modified carbon paste electrode. Am. J. Anal. Chem. 2014, 5, 598–603. [Google Scholar] [CrossRef]
- Singh, R.; Bharti, N.; Madan, J.; Hiremath, S.N. Characterization of cyclodextrin inclusion complexes. J. Pharm. Sci. Technol. 2010, 2, 171–183. [Google Scholar]
- Dahab, A.A. Rapid analysis of drug binding to β-cyclodextrin: Part I substituents effect on physicochemical and co-conformational stability of drug/cyclodextrin complex. Res. Adv. 2014, 4, 6624–6637. [Google Scholar] [CrossRef]
- Bricout, H.; Hapiot, F.; Ponchel, A.; Tilloy, S.; Monflier, E. Chemically modified cyclodextrins: An attractive class of supramolecular hosts for the development of aqueous biphasic catalytic processes. Sustainability 2009, 1, 924–945. [Google Scholar] [CrossRef]
- Mangolim, C.S.; Moriwaki, C.; Nogueira, A.C.; Sato, F.; Baesso, M.L.; Neto, A.M.; Matioli, G. Curcumin-β-cyclodextrin inclusion complex: Stability, solubility, characterization by FT-IR, FT-Raman, X-ray diffraction and photoacoustic spectroscopy, and food application. Food. Chem. 2013, 153, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Aykac, A.; Martos-Maldonado, M.C.; Casas-Solvas, J.M.; Quesada-Soriano, I.; Garcia-Maroto, F.; Garcia-Fuentes, L.; Vargas-Berenguel, A. β-Cyclodextrin-bearing gold glyconanoparticles for the development of site specific drug delivery systems. Langmuir 2014, 30, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Khorshid, A.F.; Issa, Y.M. Modified carbon paste sensor for the potentiometric determination of neostigmine bromide in pharmaceutical formulations, human plasma and urine. Biosens. Bioelectron. 2014, 51, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Buck, R.P.; Linder, E. Recommendations for nomenclature of ion selective electrodes. Pure Appl. Chem. 1994, 66, 2527–2536. [Google Scholar] [CrossRef]
- Ma, T.S.; Hassan, S.S.M. Organic Analysis Using Ion Selective Electrodes; Academic Press: London, UK, 1982. [Google Scholar]
- Ali, T.A.; El-didamony, A.M.; Mohamed, G.G.; Abdel-Rahman, M.A. A novel potentiometric sensor for selective determination of dodecyl (2-hydroxyethyl)-dimethylammonium bromide surfactant in environmental polluted samples. Int. J. Electrochem. Sci. 2014, 9, 4158–4171. [Google Scholar]
- Miller, J.C.; Miller, J.N. Statistics for Analytical Chemistry, 3rd ed.; Ellis Horwood-Prentice Hall: West Sussex, Chichester, UK, 1993. [Google Scholar]
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use ICH, Complementary Guidelines on Methodology; Jones & Bartlett: Washington, DC, USA, 1996; p. 13.
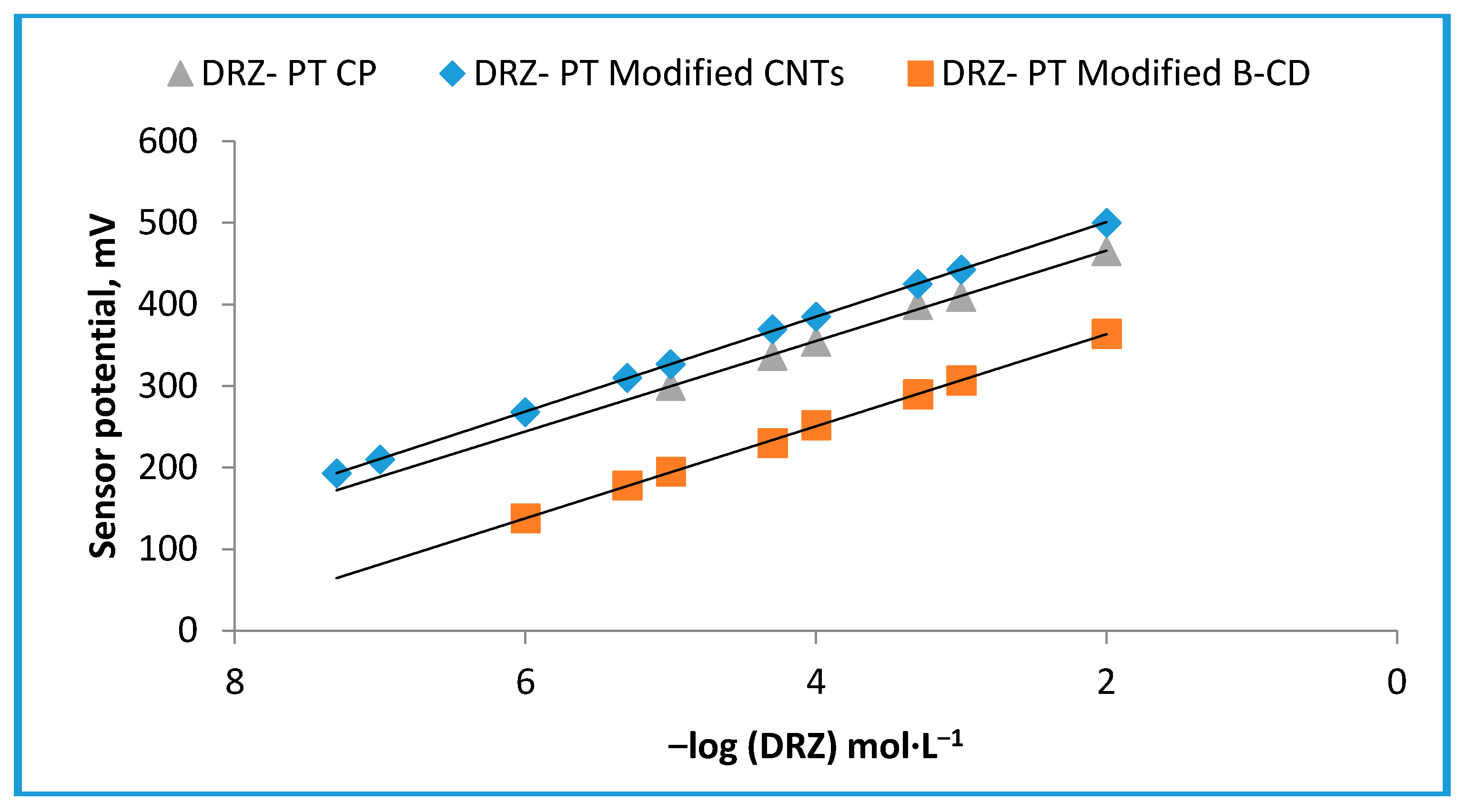
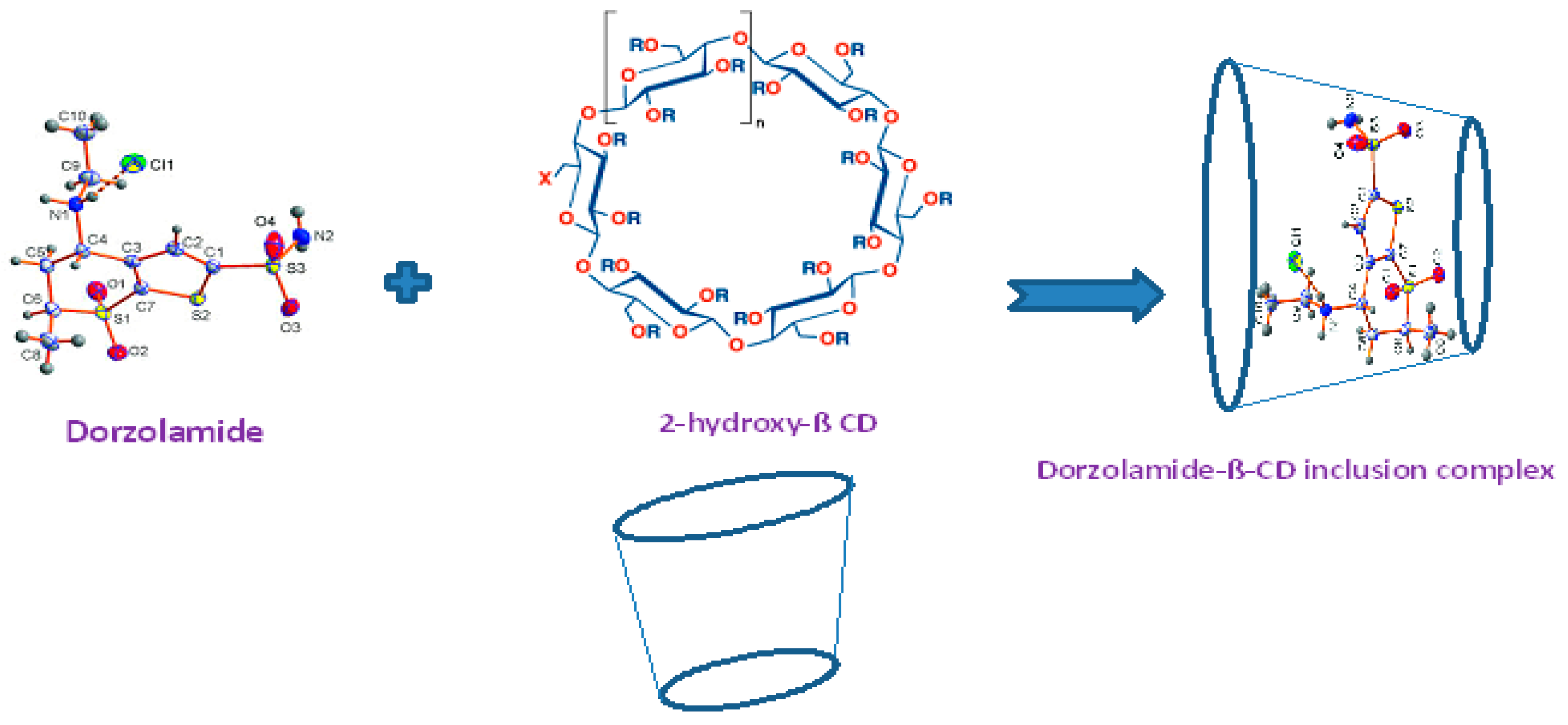

| Parameter a | DRZ-PT Carbon Paste (Sensor I) | DRZ-PT Modified β-CD (Sensor II) | DRZ-PT Modified CNTs (Sensor III) |
|---|---|---|---|
| Slope (mV·decade−1) | 55.4 ± 0.6 | 56.4 ± 0.4 | 58.1 ± 0.2 |
| Intercept | 376.9 | 476.8 | 616.8 |
| Regression equation | EmV = (55.4 ± 0.6) log(DRZ)+ 376.9 | EmV = (56.4 ± 0.4) log(DRZ) + 476.77 | EmV = (58.1 ± 0.2) log(DRZ) + 616.8 |
| Correlation coefficient, r | 0.9991 | 0.9997 | 0.9999 |
| Linear range (mol·L−1) | 1.0 × 10−5–1.0 × 10−2 | 1.0 × 10−6–1.0 × 10−2 | 5.0 × 10−8–1.0 × 10−2 |
| LOD | 5.0 × 10−6 | 5.0 × 10−7 | 2.5 × 10−9 |
| Response time/s | 60 | 45 | 30 |
| Working pH range | 4–8 | 4–8 | 4–8 |
| Lifetime/day | 45 | 65 | 90 |
| Temperature °C | 25 °C | 25 °C | 25 °C |
| Accuracy (%) | 99.2 ± 0.9 | 99.7 ± 0.6 | 99.8 ± 0.1 |
| Robustness b | 98.7 ± 0.5 | 99.2 ± 0.5 | 99.4 ± 0.2 |
| Ruggedness c | 98.9 ± 0.8 | 99.3 ± 0.4 | 99.8 ± 0.1 |
| Plasticizer | DRZ-PT CP (Sensor I) | DRZ-PT Modified β-CD (Sensor II) | DRZ-PT MCNTs (Sensor III) |
|---|---|---|---|
| DOS | 46.3 | 51.4 | 52.9 |
| DBS | 50.9 | 53.7 | 53.1 |
| DBP | 53.5 | 55.9 | 56.7 |
| DOP | 54.9 | 56.0 | 57.9 |
| o-NPOE | 55.2 * | 56.4 * | 58.1 * |
| Interferent | KPotDRZ+ | ||
|---|---|---|---|
| DRZ-PT CP (Sensor I) | DRZ-PT modified β-CD (Sensor II) | DRZ-PT MCNTs (Sensor III) | |
| Na+ | 2.5 × 10−3 | 4.1 × 10−3 | 1.2 × 10−5 |
| K+ | 3.1 × 10−3 | 3.3 × 10−4 | 7.5 × 10−4 |
| NH4+ | 4.5 × 10−3 | 5.5 × 10−4 | 3.5 × 10−5 |
| Ca2+ | 5.9 × 10−4 | 6.2 × 10−4 | 5.6 × 10−6 |
| Mg2+ | 8.2 × 10−4 | 7.2 × 10−5 | 1.6 × 10−5 |
| Zn2+ | 5.6 × 10−4 | 6.3 × 10−4 | 6.9 × 10−4 |
| Cu2+ | 1.8 × 10−3 | 4.2 × 10−4 | 5.8 × 10−4 |
| Fe3+ | 8.3 × 10−4 | 1.2 × 10−4 | 4.9 × 10−5 |
| Al3+ | 4.1 × 10−3 | 6.4 × 10−4 | 7.8 × 10−5 |
| Glucose | 2.6 × 10−5 | 1.0 × 10−4 | 3.6 × 10−5 |
| Lactose | 5.6 × 10−4 | 2.8 × 10−4 | 6.9 × 10−5 |
| Maltose | 5.5 × 10−5 | 1.3 × 10−5 | 5.2 × 10−6 |
| Starch | 1.4 × 10−5 | 7.1 × 10−4 | 6.3 × 10−4 |
| Alanine | 4.9 × 10−5 | 9.9 × 10−5 | 8.6 × 10−5 |
| Glycine | 3.9 × 10−4 | 5.5 × 10−5 | 3.8 × 10−5 |
| Histadine | 9.0 × 10−5 | 2.2 × 10−4 | 2.4 × 10−5 |
| Leucine | 4.8 × 10−4 | 3.8 × 10−4 | 8.9 × 10−6 |
| Ornithine | 5.7 × 10−4 | 6.7 × 10−5 | 5.8 × 10−5 |
| Glutamine | 9.3 × 10−4 | 9.8 × 10−4 | 2.8 × 10−5 |
| Type of Sensors | Temperature (°C) | Slope (mV decade−1) | Usable Concentration Range | E0 (mV) a |
|---|---|---|---|---|
| DRZ-PT carbon paste (Sensor I) | 25 | 55.2 * | 1.0 × 10−5–1.0 × 10−2 | 100 |
| 30 | 56.4 | 5.0 × 10−5–1.0 × 10−3 | 109 | |
| 40 | 57.6 | 1.0 × 10−5–1.0 × 10−3 | 117 | |
| 50 | 57.9 | 1.0 × 10−4–1.0 × 10−4 | 120 | |
| 60 | 59.0 | 5.0 × 10−4–1.0 × 10−4 | 128 | |
| DRZ-PT modified β-CD carbon paste (Sensor II) | 25 | 56.4 * | 1.0 × 10−6–1.0 × 10−2 | 250 |
| 30 | 56.8 | 1.0 × 10−5–1.0 × 10−2 | 264 | |
| 40 | 58.2 | 1.0 × 10−5–1.0 × 10−3 | 273 | |
| 50 | 59.5 | 5.0 × 10−5–1.0 × 10−3 | 280 | |
| 60 | 61.4 | 5.0 × 10−5–1.0 × 10−4 | 291 | |
| DRZ−PT modified CNTs (Sensor III) | 25 | 58.1 * | 5.0 × 10−8–1.0 × 10−2 | 475 |
| 30 | 59.5 | 5.0 × 10−7–1.0 × 10−3 | 481 | |
| 40 | 60.2 | 5.0 × 10−7–1.0 × 10−3 | 487 | |
| 50 | 61.7 | 5.0 × 10−6–1.0 × 10−4 | 493 | |
| 60 | 63.4 | 5.0 × 10−5–1.0 × 10−4 | 501 |
| Samples | DRZ-PT CP (Sensor I) | DRZ-PT Modified β-CD (Sensor II) | DRZ-PT MCNTs (Sensor III) | Recovery (%) (Reference Method [4]) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Taken −log conc. (mol·L−1) | Found –log conc. (mol·L−1) | Recovery (%) | Taken −log conc. (mol·L−1) | Found –log conc. (mol·L−1) | Recovery (%) | Taken −log conc. (mol·L−1) | Found −log conc. (mol·L−1) | Recovery (%) | ||
| Pure drug | 5.0 | 4.97 | 99.4 | 6.0 | 5.99 | 99.8 | 7.3 | 7.28 | 99.7 | |
| 4.3 | 4.25 | 98.8 | 5.3 | 5.28 | 99.6 | 7.0 | 6.99 | 99.9 | ||
| 4.0 | 3.96 | 99.0 | 5.0 | 4.99 | 99.8 | 6.0 | 6.00 | 100.0 | ||
| 3.3 | 3.28 | 99.4 | 4.0 | 3.97 | 99.3 | 5.0 | 5.00 | 100.0 | ||
| 3.0 | 2.99 | 99.7 | 3.0 | 2.95 | 98.3 | 4.0 | 3.99 | 99.8 | ||
| 2.0 | 1.96 | 98.0 | 2.0 | 1.99 | 99.5 | 3.0 | 2.98 | 99.3 | ||
| 2.0 | 2.00 | 100.0 | ||||||||
| Mean ± SD | 99.1 ± 0.6 | 99.3 ± 0.5 | 99.8 ± 0.3 | 99.6 ± 0.4 | ||||||
| n | 6 | 6 | 7 | 6 | ||||||
| Variance | 0.36 | 0.25 | 0.09 | 0.16 | ||||||
| %SE ** | 0.24 | 0.32 | 0.11 | 0.16 | ||||||
| t-test | 1.733 (2.228) * | 0.839 (2.228) * | 1.030 (2.201) * | |||||||
| F-test | 2.25 (5.05) * | 1.56 (5.05) * | 1.78 (4.93) * | |||||||
| Samples | DRZ-PT CP (Sensor I) | DRZ-PT Modified β-CD (Sensor II) | DRZ-PT MCNTs (Sensor III) | Recovery (%) (Reference Method [4]) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Taken −log conc. (mol·L−1) | Found −log conc. (mol·L−1) | Recovery (%) | Taken −log conc. (mol·L−1) | Found −log conc. (mol·L−1) | Recovery (%) | Taken −log conc. (mol·L−1) | Found −log conc. (mol·L−1) | Recovery (%) | ||
| DRZ-solution® 22.3 mg·mL−1 | 5.0 | 4.96 | 99.2 | 6.0 | 6.00 | 100.0 | 7.3 | 7.29 | 99.9 | |
| 4.3 | 4.28 | 99.5 | 5.3 | 5.26 | 99.2 | 7.0 | 7.00 | 100.0 | ||
| 4.0 | 3.99 | 99.8 | 5.0 | 4.96 | 99.2 | 6.0 | 5.99 | 99.8 | ||
| 3.3 | 3.27 | 99.1 | 4.0 | 4.00 | 100.0 | 5.0 | 4.98 | 99.6 | ||
| 3.0 | 2.98 | 99.3 | 3.0 | 2.99 | 99.7 | 4.0 | 4.00 | 100.0 | ||
| 2.0 | 1.97 | 98.5 | 2.0 | 1.99 | 99.5 | 3.0 | 3.00 | 100.0 | ||
| 2.0 | 1.99 | 99.5 | ||||||||
| Mean ± SD | 99.2 ± 0.4 | 99.6 ± 0.3 | 99.8 ± 0.2 | 99.5 ± 0.4 | ||||||
| n | 6 | 6 | 7 | 6 | ||||||
| Variance | 0.16 | 0.09 | 0.04 | 0.16 | ||||||
| %SE ** | 0.16 | 0.12 | 0.08 | 0.16 | ||||||
| t-test | 1.326 (2.228) * | 0.500 (2.228) * | 1.677 (2.201) * | |||||||
| F-test | 1.00 (5.05) * | 1.78 (5.05) * | 4.00 (4.93) * | |||||||
| Samples | DRZ-PT CP (Sensor I) | DRZ-PT Modified β-CD (Sensor II) | DRZ-PT MCNTs (Sensor III) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Taken −log conc. (mol·L−1) | Found −log conc. (mol·L−1) | Recovery (%) | Taken −log conc. (mol·L−1) | Found −log conc. (mol·L−1) | Recovery (%) | Taken −log conc. (mol·L−1) | Found −log conc. (mol·L−1) | Recovery (%) | |
| Human serum | 5.0 | 4.95 | 99.0 | 6.0 | 5.99 | 99.8 | 7.3 | 7.29 | 99.9 |
| 4.3 | 4.25 | 98.8 | 5.3 | 5.26 | 99.2 | 7.0 | 6.96 | 99.4 | |
| 4.0 | 3.96 | 99.0 | 5.0 | 4.97 | 99.4 | 6.0 | 6.00 | 100.0 | |
| 3.3 | 3.26 | 98.8 | 4.0 | 3.98 | 99.5 | 5.0 | 4.99 | 99.8 | |
| 3.0 | 2.99 | 99.7 | 3.0 | 2.92 | 97.3 | 4.0 | 3.98 | 99.5 | |
| 2.0 | 1.97 | 98.5 | 2.0 | 1.99 | 99.5 | 3.0 | 2.95 | 98.3 | |
| 2.0 | 1.99 | 99.5 | |||||||
| Mean ± SD | 98.9 ± 0.4 | 99.1 ± 0.9 | 99.5 ± 0.6 | ||||||
| n | 6 | 6 | 7 | ||||||
| Variance | 0.16 | 0.81 | 0.36 | ||||||
| %SE * | 0.16 | 0.36 | 0.23 | ||||||
| Human urine | 5.0 | 5.00 | 100.0 | 6.0 | 5.96 | 99.3 | 7.3 | 7.30 | 100.0 |
| 4.3 | 4.22 | 98.1 | 5.3 | 5.26 | 99.2 | 7.0 | 7.00 | 100.0 | |
| 4.0 | 3.89 | 97.3 | 5.0 | 4.98 | 99.6 | 6.0 | 5.99 | 99.8 | |
| 3.3 | 3.28 | 99.4 | 4.0 | 3.99 | 99.8 | 5.0 | 4.98 | 99.6 | |
| 3.0 | 2.96 | 98.7 | 3.0 | 2.97 | 99.0 | 4.0 | 3.99 | 99.8 | |
| 2.0 | 1.94 | 97.0 | 2.0 | 1.97 | 98.5 | 3.0 | 2.97 | 99.0 | |
| 2.0 | 1.99 | 99.5 | |||||||
| Mean ± SD | 98.4 ± 1.2 | 99.2 ± 0.5 | 99.7 ± 0.3 | ||||||
| n | 6 | 6 | 7 | ||||||
| Variance | 1.44 | 0.25 | 0.09 | ||||||
| %SE * | 0.48 | 0.20 | 0.11 | ||||||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alarfaj, N.A.; El-Tohamy, M.F. New Electrochemically-Modified Carbon Paste Inclusion β-Cyclodextrin and Carbon Nanotubes Sensors for Quantification of Dorzolamide Hydrochloride. Int. J. Mol. Sci. 2016, 17, 2027. https://doi.org/10.3390/ijms17122027
Alarfaj NA, El-Tohamy MF. New Electrochemically-Modified Carbon Paste Inclusion β-Cyclodextrin and Carbon Nanotubes Sensors for Quantification of Dorzolamide Hydrochloride. International Journal of Molecular Sciences. 2016; 17(12):2027. https://doi.org/10.3390/ijms17122027
Chicago/Turabian StyleAlarfaj, Nawal Ahmad, and Maha Farouk El-Tohamy. 2016. "New Electrochemically-Modified Carbon Paste Inclusion β-Cyclodextrin and Carbon Nanotubes Sensors for Quantification of Dorzolamide Hydrochloride" International Journal of Molecular Sciences 17, no. 12: 2027. https://doi.org/10.3390/ijms17122027