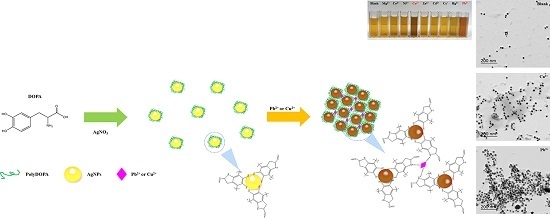
Green Synthesis of Silver Nanoparticles Stabilized with Mussel-Inspired Protein and Colorimetric Sensing of Lead(II) and Copper(II) Ions
Abstract
:1. Introduction
2. Results and Discussion
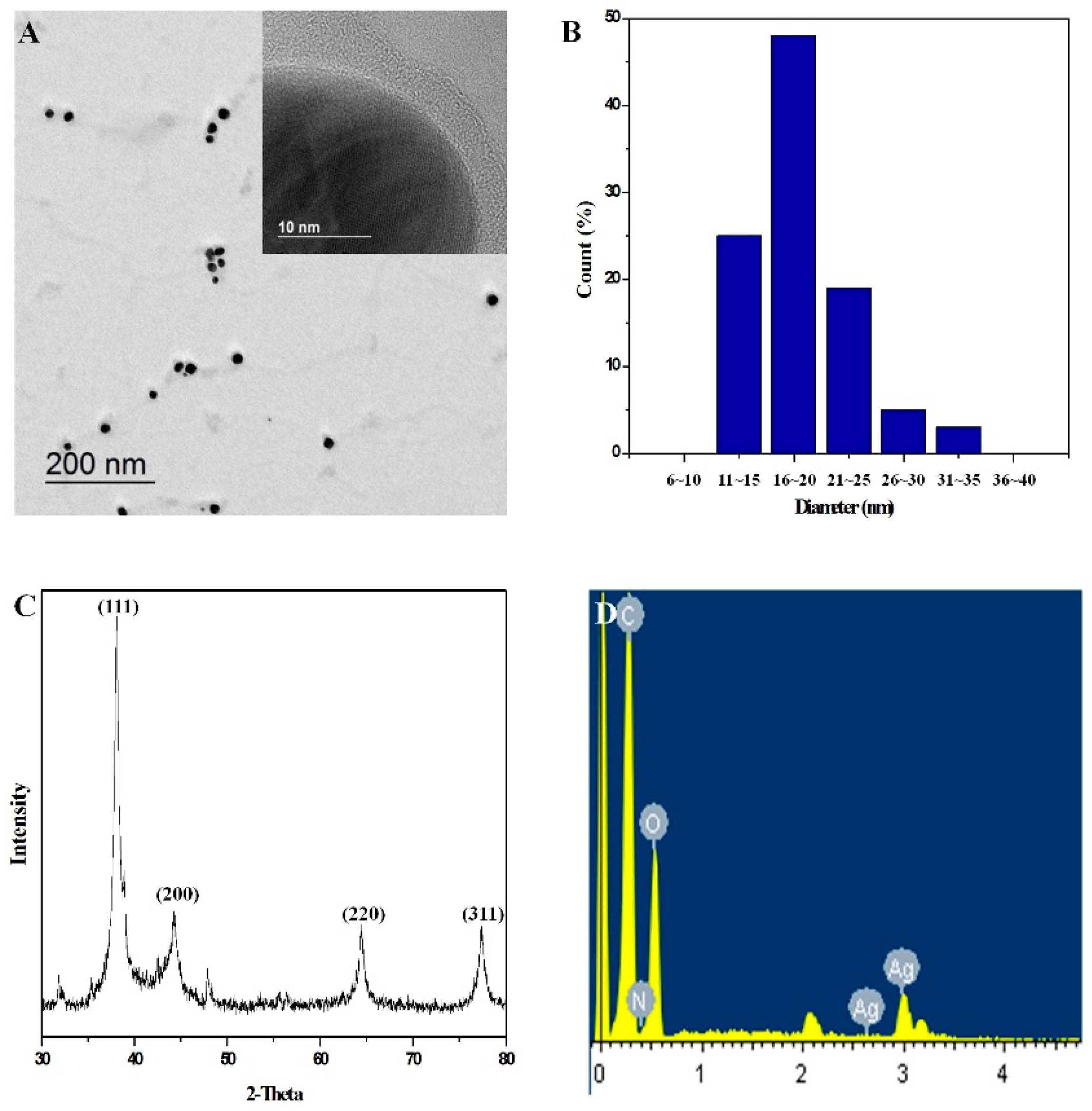
2.1. Characterization of PolyDOPA-AgNPs
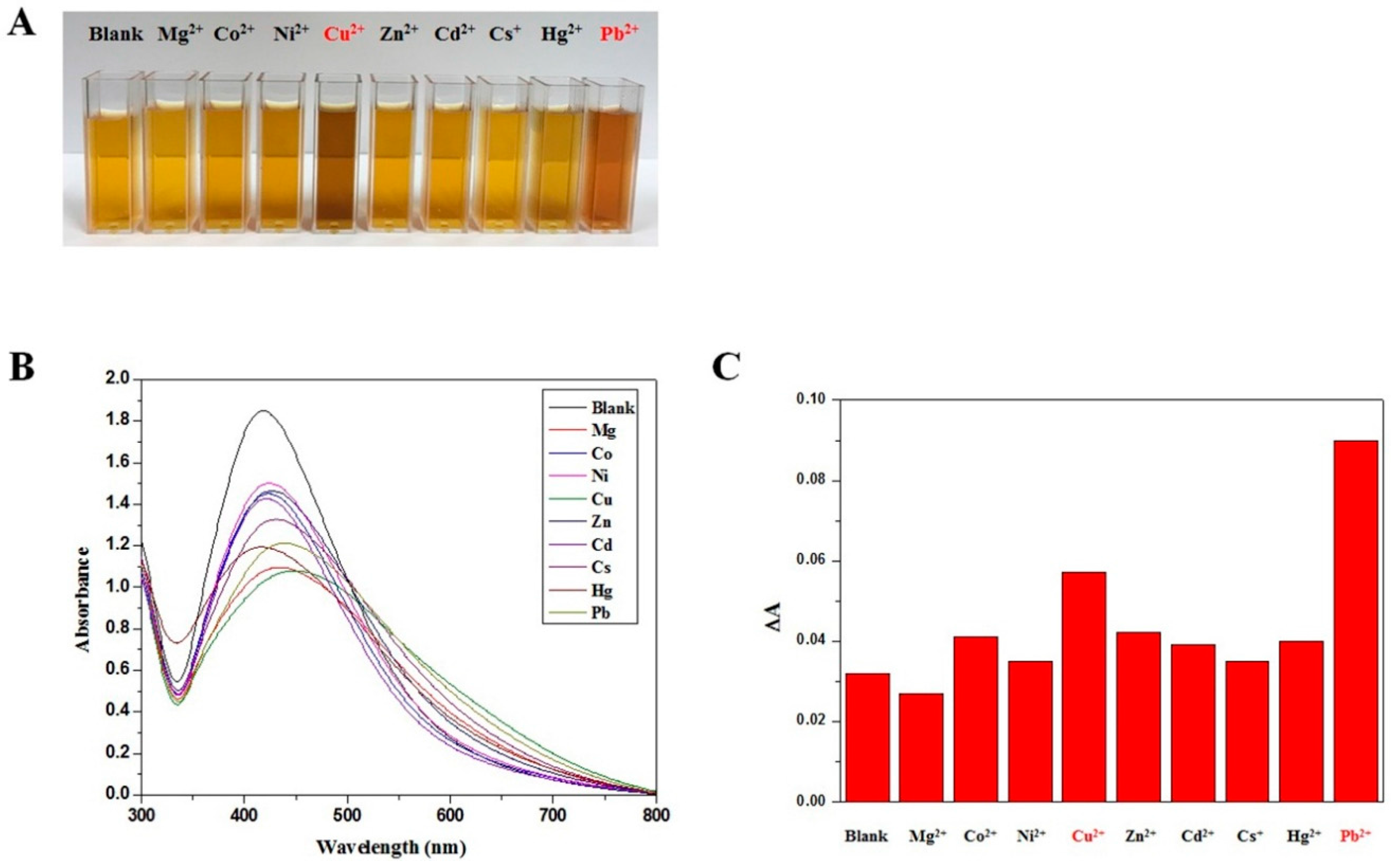
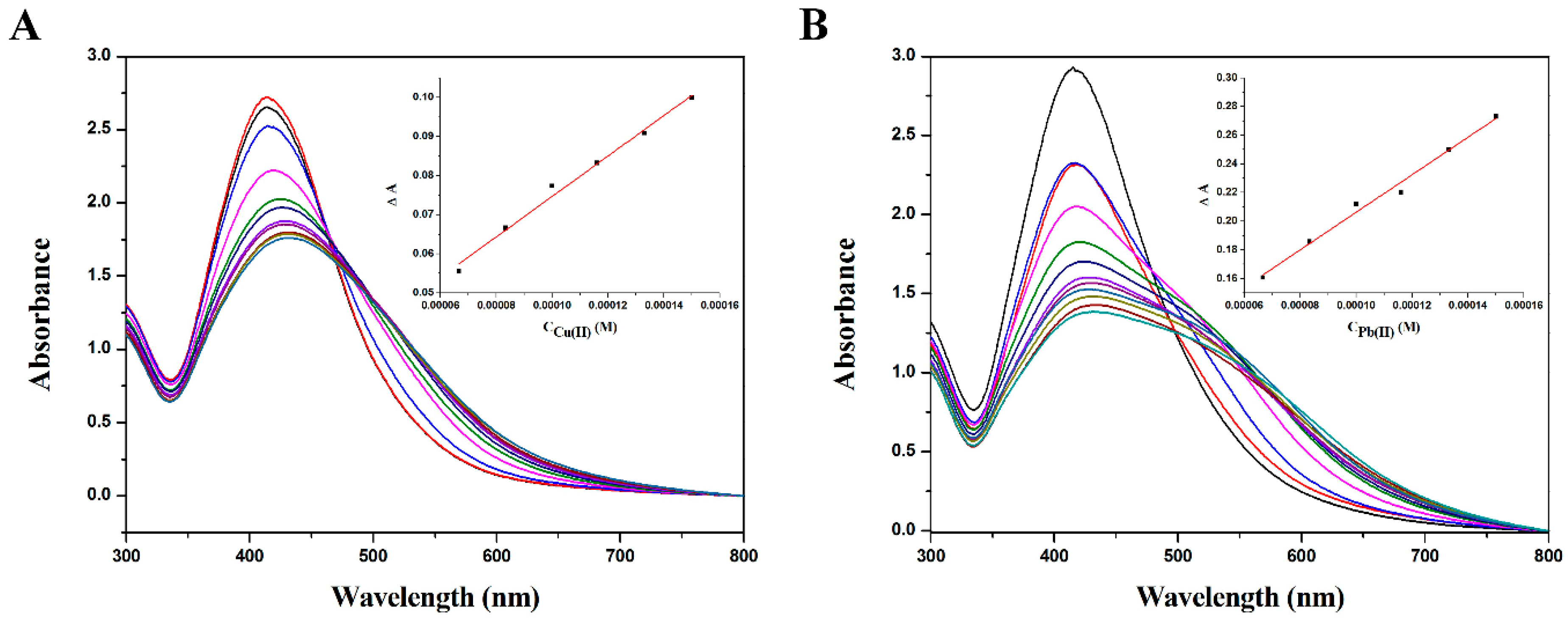
2.2. Detection of Heavy Metal Ions by PolyDOPA-AgNPs
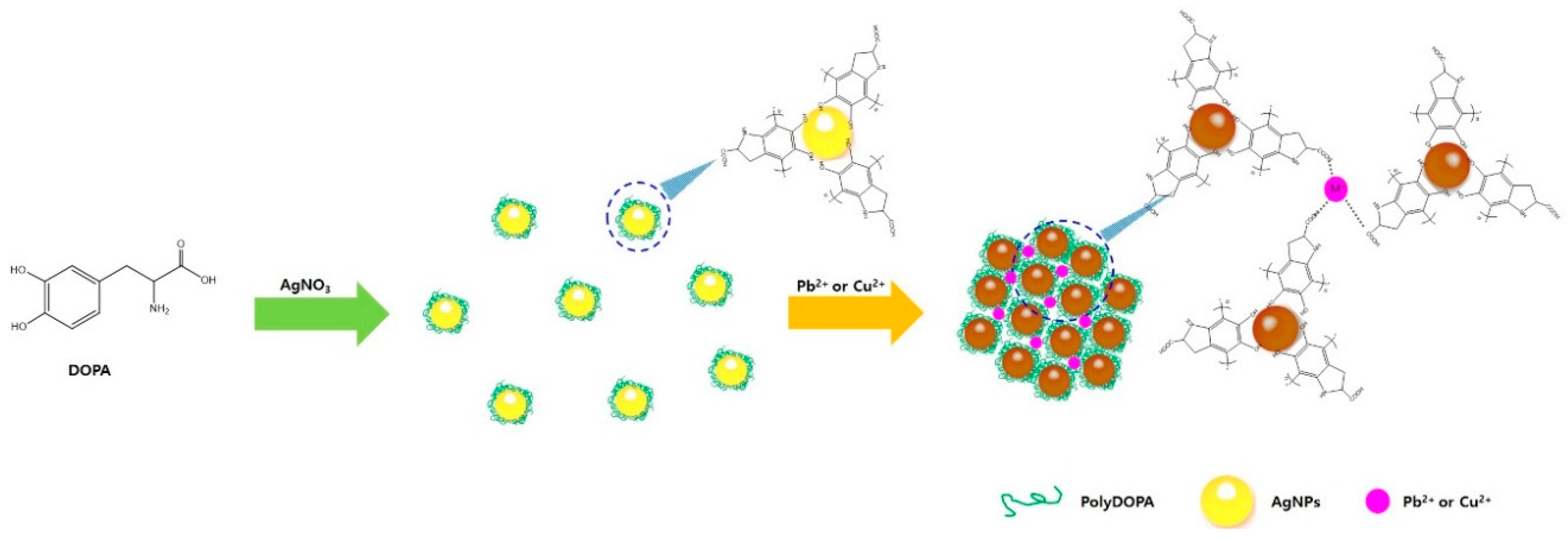
2.3. Sensing Mechanism of PolyDOPA-AgNPs for Cu2+ and Pb2+ Ions
3. Materials and Methods
3.1. Materials
3.2. Preparation of PolyDOPA-AgNPs
3.3. Colorimetric Detection of Heavy Metal Ions
3.4. Analyses
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, M.; Liu, Y.-Q.; Ye, B.-C. Colorimetric assay for parallel detection of Cd2+, Ni2+ and Co2+ using peptide-modified gold nanoparticles. Analyst 2012, 137, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Que, E.L.; Domaille, D.W.; Chang, C.J. Metals in neurobiology: Probing their chemistry and biology with molecular imaging. Chem. Rev. 2008, 108, 1517–1549. [Google Scholar] [CrossRef] [PubMed]
- Mehta, V.N.; Kumar, M.A.; Kailasa, S.K. Colorimetric detection of copper in water samples using dopamine dithiocarbamate-functionalized Au nanoparticles. Ind. Eng. Chem. Res. 2013, 52, 4414–4420. [Google Scholar] [CrossRef]
- Qi, L.; Shang, Y.; Wu, F. Colorimetric detection of lead(II) based on silver nanoparticles capped with iminodiacetic acid. Microchim. Acta 2012, 178, 221–227. [Google Scholar] [CrossRef]
- Kumar, V.V.; Anthony, S.P. Silver nanoparticles based selective colorimetric sensor for Cd2+, Hg2+ and Pb2+ ions: Tuning sensitivity and selectivity using co-stabilizing agents. Sens. Actuator B 2014, 191, 31–36. [Google Scholar] [CrossRef]
- Yoosaf, K.; Ipe, B.I.; Suresh, C.H.; Thomas, K.G. In situ synthesis of metal nanoparticles and selective naked-eye detection of lead ions from aqueous media. J. Phys. Chem. C 2007, 111, 12839–12847. [Google Scholar] [CrossRef]
- Wu, S.-P.; Huang, R.-Y.; Du, K.-J. Colorimetric sensing of Cu(II) by 2-methyl-3-[(pyridin-2-ylmethyl)-amino]-1,4-naphthoquinone: Cu(II) induced deprotonation of NH responsible for color changes. Dalton Trans. 2009, 24, 4735–4740. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.-R.; Niu, H.-Y.; Cai, Y.-Q. Colorimetric detection of copper ions in tap water during the synthesis of silver/dopamine nanoparticles. Chem. Commun. 2011, 47, 12643–12645. [Google Scholar] [CrossRef] [PubMed]
- Jeevika, A.; Shankaran, D.R. Visual colorimetric sensing of copper ions based on reproducible gelatin functionalized silver nanoparticles and gelatin hydrogels. Colloids Surf. A 2014, 461, 240–247. [Google Scholar] [CrossRef]
- Soylak, M.; Cay, R.S. Separation/preconcentration of silver(I) and lead(II) in environmental samples on cellulose nitrate membrane filter prior to their flame atomic absorption spectrometric determinations. J. Hazard. Mater. 2007, 146, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Faghihian, H.; Hajishabani, A.; Dadfarnia, S.; Zamani, H. Use of clinoptilolite loaded with 1-(2-pyridylazo)-2-naphthol as a sorbent for preconcentration of Pb(II), Ni(II), Cd(II) and Cu(II) prior to their determination by flame atomic absorption spectroscopy. Int. J. Environ. Anal. Chem. 2009, 89, 223–231. [Google Scholar] [CrossRef]
- Maria das Graças, A.K.; de Andrade, J.B.; de Jesus, D.S.; Lemos, V.A.; Bandeira, M.L.; dos Santos, W.N.; Bezerra, M.A.; Amorim, F.A.; Souza, A.S.; Ferreira, S.L. Separation and preconcentration procedures for the determination of lead using spectrometric techniques: A review. Talanta 2006, 69, 16–24. [Google Scholar]
- Chen, D.; Hu, B.; Huang, C. Chitosan modified ordered mesoporous silica as micro-column packing materials for on-line flow injection-inductively coupled plasma optical emission spectrometry determination of trace heavy metals in environmental water samples. Talanta 2009, 78, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Willemse, C.M.; Tlhomelang, K.; Jahed, N.; Baker, P.G.; Iwuoha, E.I. Metallo-graphene nanocomposite electrocatalytic platform for the determination of toxic metal ions. Sensors 2011, 11, 3970–3987. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Sun, Q.; Li, H.; Sun, F.; Hu, N.; Wang, P. Screen-printed gold electrode with gold nanoparticles modification for simultaneous electrochemical determination of lead and copper. Sens. Actuators B Chem. 2015, 209, 336–342. [Google Scholar] [CrossRef]
- Wan, H.; Sun, Q.; Li, H.; Sun, F.; Hu, N.; Wang, P. Design of a miniaturized multisensor chip with nanoband electrode array and light addressable potentiometric sensor for ion sensing. Anal. Methods 2015, 7, 9190–9197. [Google Scholar] [CrossRef]
- Vanatta, L.; Vanatta, J.; Riviello, J. Ion-chromatographic study of the possible absorption of copper and zinc by the skin of Rana pipiens. J. Chromatogr. A 2000, 884, 143–150. [Google Scholar] [CrossRef]
- Silva, M.; Tomal, A.; Perez, C.; Ribeiro-Silva, A.; Poletti, M. Determination of Ca, Fe, Cu and Zn and their correlations in breast cancer and normal adjacent tissues. X-ray Spectrom. 2009, 38, 103–111. [Google Scholar] [CrossRef]
- Annadhasan, M.; Muthukumarasamyvel, T.; Sankar Babu, V.; Rajendiran, N. Green synthesized silver and gold nanoparticles for colorimetric detection of Hg2+, Pb2+, and Mn2+ in aqueous medium. ACS Sustain. Chem. Eng. 2014, 2, 887–896. [Google Scholar] [CrossRef]
- Aragay, G.; Pons, J.; Merkoçi, A. Recent trends in macro-, micro-, and nanomaterial-based tools and strategies for heavy-metal detection. Chem. Rev. 2011, 111, 3433–3458. [Google Scholar] [CrossRef] [PubMed]
- Nolan, E.M.; Lippard, S.J. Tools and tactics for the optical detection of mercuric ion. Chem. Rev. 2008, 108, 3443–3480. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.C. Size and shape dependent second order nonlinear optical properties of nanomaterials and their application in biological and chemical sensing. Chem. Rev. 2010, 110, 5332–5365. [Google Scholar] [CrossRef] [PubMed]
- Farhadi, K.; Forough, M.; Molaei, R.; Hajizadeh, S.; Rafipour, A. Highly selective Hg2+ colorimetric sensor using green synthesized and unmodified silver nanoparticles. Sens. Actuator B 2012, 161, 880–885. [Google Scholar] [CrossRef]
- Pandey, S.; Goswami, G.K.; Nanda, K.K. Green synthesis of biopolymer–silver nanoparticle nanocomposite: An optical sensor for ammonia detection. Int. J. Biol. Macromol. 2012, 51, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Malinsky, M.D.; Kelly, K.L.; Schatz, G.C.; van Duyne, R.P. Chain length dependence and sensing capabilities of the localized surface plasmon resonance of silver nanoparticles chemically modified with alkanethiol self-assembled monolayers. J. Am. Chem. Soc. 2001, 123, 1471–1482. [Google Scholar] [CrossRef]
- Han, C.; Zhang, L.; Li, H. Highly selective and sensitive colorimetric probes for Yb3+ ions based on supramolecular aggregates assembled from β-cyclodextrin–4,4′-dipyridine inclusion complex modified silver nanoparticles. Chem. Commun. 2009, 24, 3545–3547. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.K.; Pal, T. Interparticle coupling effect on the surface plasmon resonance of gold nanoparticles: From theory to applications. Chem. Rev. 2007, 107, 4797–4862. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhao, H.; Li, C.; He, P.; Peng, W.; Yuan, L.; Zeng, L.; He, Y. Colorimetric detection of Mn2+ using silver nanoparticles cofunctionalized with 4-mercaptobenzoic acid and melamine as a probe. Talanta 2012, 97, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Zhou, Y.; Yu, P.; Su, L.; Mao, L.; Zhang, D.; Zhu, D. An electrochemical sensor for 3,4-dihydroxyphenylacetic acid with carbon nanotubes as electronic transducer and synthetic cyclophane as recognition element. Chem. Commun. 2008, 36, 4330–4332. [Google Scholar] [CrossRef] [PubMed]
- Pessiglione, M.; Seymour, B.; Flandin, G.; Dolan, R.J.; Frith, C.D. Dopamine-dependent prediction errors underpin reward-seeking behavior in humans. Nature 2006, 442, 1042–1045. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.A.; Mahal, H.S.; Biswas, N.; Mukherjee, T.; Kapoor, S. Role of phenol derivatives in the formation of silver nanoparticles. Langmuir 2008, 24, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Shen, S.; Luo, J.; Wang, X.; Sun, R. Green synthesis of silver nanoparticles in xylan solution via Tollens reaction and their detection for Hg2+. Nanoscale 2015, 7, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Qu, W.-G.; Wang, S.-M.; Hu, Z.-J.; Cheang, T.-Y.; Xing, Z.-H.; Zhang, X.-J.; Xu, A.-W. In situ synthesis of gold@ 3,4-dihydroxy-l-phenylalanine core−shell nanospheres used for cell imaging. J. Phys. Chem. C 2010, 114, 13010–13016. [Google Scholar] [CrossRef]
- Ye, W.; Wang, D.; Zhang, H.; Zhou, F.; Liu, W. Electrochemical growth of flowerlike gold nanoparticles on polydopamine modified ITO glass for SERS application. Electrochim. Acta 2010, 55, 2004–2009. [Google Scholar] [CrossRef]
- Lee, N.; Hummer, D.R.; Sverjensky, D.A.; Rajh, T.; Hazen, R.M.; Steele, A.; Cody, G.D. Speciation of l-DOPA on nanorutile as a function of pH and surface coverage using Surface-Enhanced Raman Spectroscopy (SERS). Langmuir 2012, 28, 17322–17330. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.-J.; Zhang, P.-P.; Wang, A.-J.; Liao, Q.-C.; Xi, J.-L.; Chen, J.-R. One-step synthesis of monodisperse polydopamine-coated silver core–shell nanostructures for enhanced photocatalysis. New J. Chem. 2012, 36, 148–154. [Google Scholar] [CrossRef]
- Shanmuganathan, K.; Cho, J.H.; Iyer, P.; Baranowitz, S.; Ellison, C.J. Thermooxidative stabilization of polymers using natural and synthetic melanins. Macromolecules 2011, 44, 9499–9507. [Google Scholar] [CrossRef]
- Xiong, S.; Wang, Y.; Zhu, J.; Yu, J.; Hu, Z. Mussel-Adhesive-Inspired fabrication of multifunctional silver nanoparticle assemblies. Langmuir 2015, 31, 5504–5512. [Google Scholar] [CrossRef] [PubMed]
- Asoro, M.; Damiano, J.; Ferreira, P. Size effects on the melting temperature of silver nanoparticles: In-situ TEM observations. Microsc. Microanal. 2009, 15, 706–707. [Google Scholar] [CrossRef]
- Asoro, M.; Kovar, D.; Damiano, J.; Ferreira, P. Scale effects on the melting behavior of silver nanoparticles. Microsc. Microanal. 2010, 16, 1802–1803. [Google Scholar] [CrossRef]
- Zangmeister, R.A.; Morris, T.A.; Tarlov, M.J. Characterization of polydopamine thin films deposited at short times by autoxidation of dopamine. Langmuir 2013, 29, 8619–8628. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Wang, Y.; Yu, J.; Chen, L.; Zhu, J.; Hu, Z. Polydopamine particles for next-generation multifunctional biocomposites. J. Mater. Chem. A 2014, 2, 7578–7587. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Emanuelaá Errico, M. A melanin-inspired pro-oxidant system for dopa(mine) polymerization: Mimicking the natural casing process. Chem. Commun. 2011, 47, 10308–10310. [Google Scholar]
- D’Ischia, M.; Napolitano, A.; Pezzella, A.; Land, E.J.; Ramsden, C.A.; Riley, P.A. 5,6-Dihydroxyindoles and Indole-5,6-diones. Adv. Heterocycl. Chem. 2005, 89, 1–63. [Google Scholar]

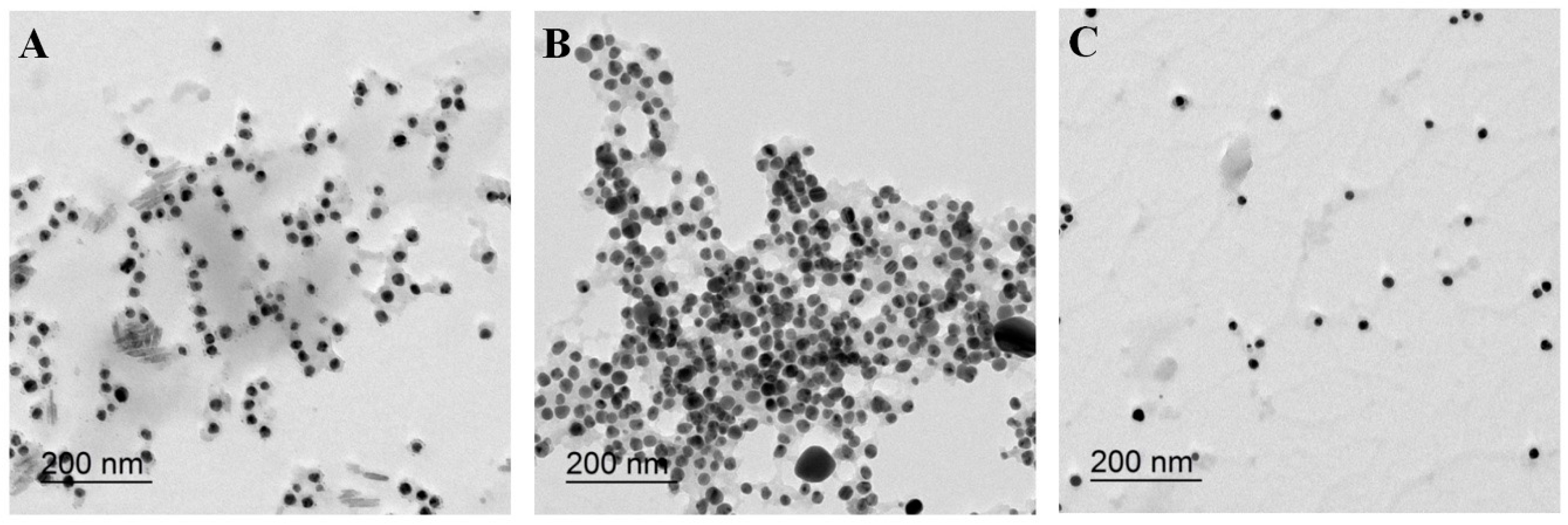
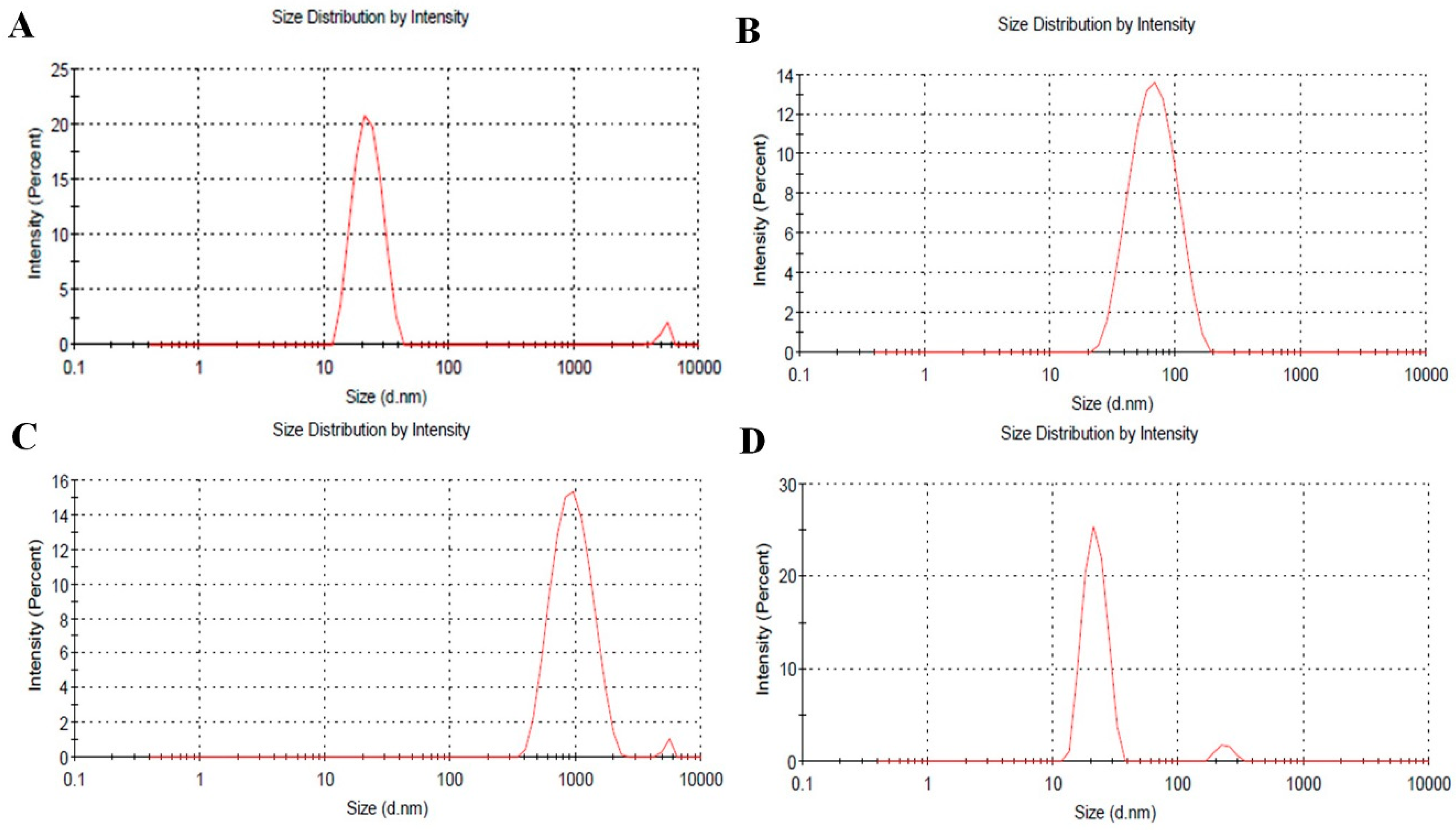
| Additive | None | Cu2+ | Pb2+ | Cd2+ |
|---|---|---|---|---|
| Z-potential value | −18.3 mV | −14.3 mV | −12.0 mV | −17.3 mV |
| Water Samples | Pb2+ | Cu2+ | ||
|---|---|---|---|---|
| LOD (M) | R2 | LOD (M) | R2 | |
| Tap water | 1.6 × 10−4 | 0.9933 | 1.5 × 10−4 | 0.9811 |
| Drinking water | 1.1 × 10−4 | 0.9923 | 9.3 × 10−5 | 0.9868 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheon, J.Y.; Park, W.H. Green Synthesis of Silver Nanoparticles Stabilized with Mussel-Inspired Protein and Colorimetric Sensing of Lead(II) and Copper(II) Ions. Int. J. Mol. Sci. 2016, 17, 2006. https://doi.org/10.3390/ijms17122006
Cheon JY, Park WH. Green Synthesis of Silver Nanoparticles Stabilized with Mussel-Inspired Protein and Colorimetric Sensing of Lead(II) and Copper(II) Ions. International Journal of Molecular Sciences. 2016; 17(12):2006. https://doi.org/10.3390/ijms17122006
Chicago/Turabian StyleCheon, Ja Young, and Won Ho Park. 2016. "Green Synthesis of Silver Nanoparticles Stabilized with Mussel-Inspired Protein and Colorimetric Sensing of Lead(II) and Copper(II) Ions" International Journal of Molecular Sciences 17, no. 12: 2006. https://doi.org/10.3390/ijms17122006