Dendritic Cell-Based Immunotherapies to Fight HIV: How Far from a Success Story? A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Results
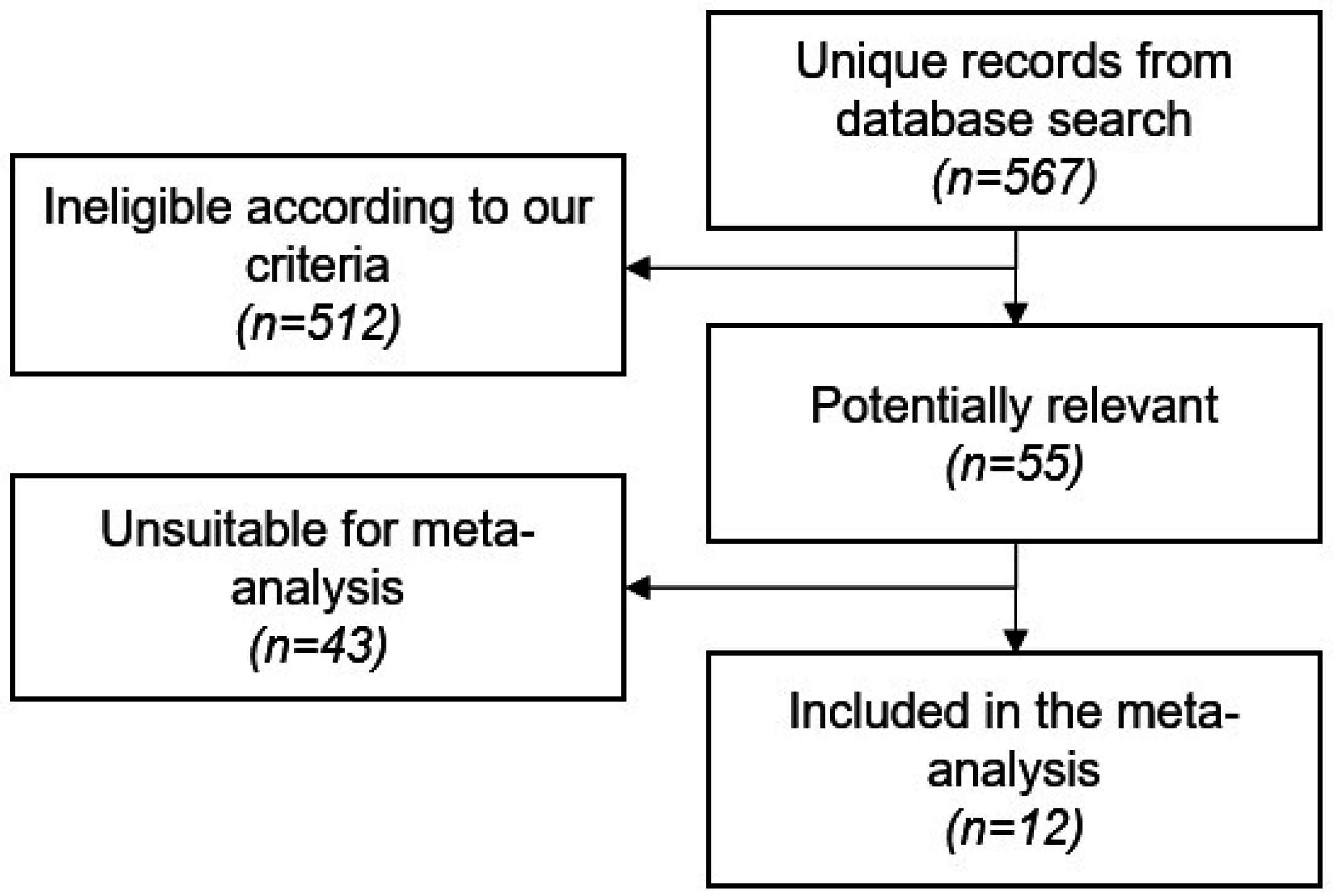
2.1. Study Screening and Characteristics
2.2. Meta-Analysis and Meta-Regression Results
3. Discussion
3.1. Immune Responses Elicited by the Experimental Vaccines
3.2. The Role of Host Genomic and Trascriptomic Background
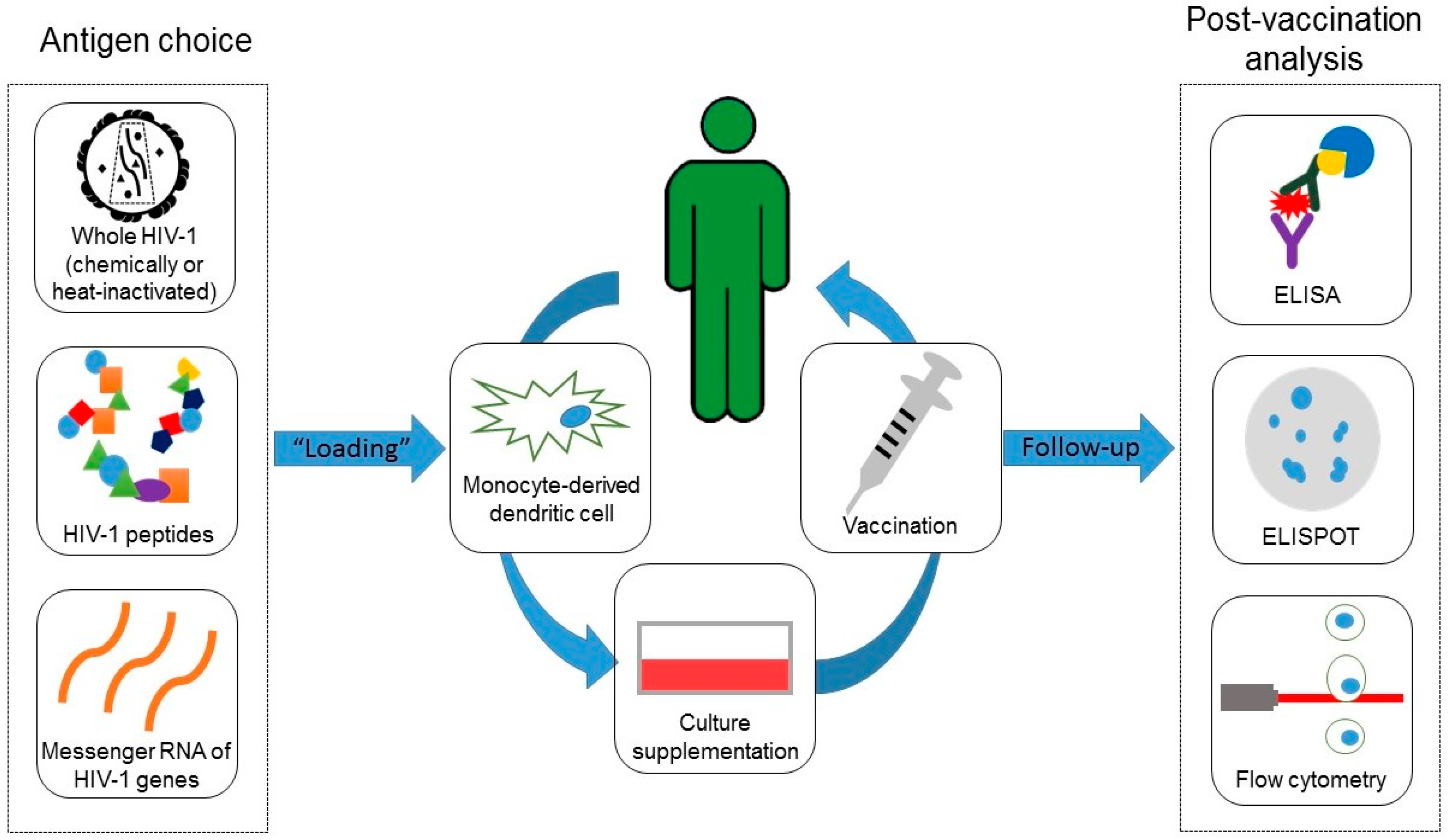
3.3. Recommendations for Future Protocols
4. Material and Methods
4.1. Literature Search Strategy and Study Selection
4.2. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Steinman, R.M. Dendritic cells: Understanding immunogenicity. Eur. J. Immunol. 2007, 3, S53–S60. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Akira, S. Innate immunity to virus infection. Immunol. Rev. 2009, 227, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Buckwalter, M.R.; Albert, M.L. Orchestration of the immune response by dendritic cells. Curr. Biol. 2009, 19, R355–R361. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; KewalRamani, V.N. Dendritic-cell interactions with HIV: Infection and viral dissemination. Nat. Rev. Immunol. 2006, 6, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Ide, F.; Nakamura, T.; Tomizawa, M.; Kawana-Tachikawa, A.; Odawara, T.; Hosoya, N.; Iwamoto, A. Peptide-loaded dendritic-cell vaccination followed by treatment interruption for chronic HIV-1 infection: A phase 1 trial. J. Med. Virol. 2006, 78, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Macatangay, B.J.; Riddler, S.A.; Wheeler, N.D.; Spindler, J.; Lawani, M.; Hong, F.; Buffo, M.J.; Whiteside, T.L.; Kearney, M.F.; Mellors, J.W.; et al. Therapeutic vaccination with dendritic cells loaded with autologous HIV type 1-infected apoptotic cells. J. Infect. Dis. 2016, 213, 1400–1409. [Google Scholar] [CrossRef] [PubMed]
- Kundu, S.K.; Engleman, E.; Benike, C.; Shapero, M.H.; Dupuis, M.; van Schooten, W.C.; Eibl, M.; Merigan, T.C. A pilot clinical trial of HIV antigen-pulsed allogeneic and autologous dendritic cell therapy in HIV-infected patients. AIDS Res. Hum. Retrovir. 1998, 14, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Arraes, L.C.; Ferreira, W.T.; Andrieu, J.M. Therapeutic dendritic-cell vaccine for chronic HIV-1 infection. Nat. Med. 2004, 10, 1359–1365. [Google Scholar] [CrossRef] [PubMed]
- Garcia, F.; Lejeune, M.; Climent, N.; Gil, C.; Alcami, J.; Morente, V.; Alos, L.; Ruiz, A.; Setoain, J.; Fumero, E.; et al. Therapeutic immunization with dendritic cells loaded with heat-inactivated autologous HIV-1 in patients with chronic HIV-1 infection. J. Infect. Dis. 2005, 191, 1680–1685. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, R.T.; O’Neill, D.; Bosch, R.J.; Chan, E.S.; Bucy, R.P.; Shopis, J.; Baglyos, L.; Adams, E.; Fox, L.; Purdue, L.; et al. A randomized therapeutic vaccine trial of canarypox-HIV-pulsed dendritic cells vs. canarypox-HIV alone in HIV-1-infected patients on antiretroviral therapy. Vaccine 2009, 27, 6088–6094. [Google Scholar] [CrossRef] [PubMed]
- Garcia, F.; Climent, N.; Assoumou, L.; Gil, C.; Gonzalez, N.; Alcami, J.; Leon, A.; Romeu, J.; Dalmau, J.; Martinez-Picado, J.; et al. A therapeutic dendritic cell-based vaccine for HIV-1 infection. J. Infect. Dis. 2011, 203, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Garcia, F.; Climent, N.; Guardo, A.C.; Gil, C.; Leon, A.; Autran, B.; Lifson, J.D.; Martinez-Picado, J.; Dalmau, J.; Clotet, B.; et al. A dendritic cell-based vaccine elicits T cell responses associated with control of HIV-1 replication. Sci. Transl. Med. 2013, 5, 166ra2. [Google Scholar] [CrossRef] [PubMed]
- Kloverpris, H.; Karlsson, I.; Bonde, J.; Thorn, M.; Vinner, L.; Pedersen, A.E.; Hentze, J.L.; Andresen, B.S.; Svane, I.M.; Gerstoft, J.; et al. Induction of novel CD8+ T-cell responses during chronic untreated HIV-1 infection by immunization with subdominant cytotoxic T-lymphocyte epitopes. Acquir. Immune Defic. Syndr. 2009, 23, 1329–1340. [Google Scholar] [CrossRef] [PubMed]
- Levy, Y.; Thiebaut, R.; Montes, M.; Lacabaratz, C.; Sloan, L.; King, B.; Perusat, S.; Harrod, C.; Cobb, A.; Roberts, L.K.; et al. Dendritic cell-based therapeutic vaccine elicits polyfunctional HIV-specific T-cell immunity associated with control of viral load. Eur. J. Immunol. 2014, 44, 2802–2810. [Google Scholar] [CrossRef] [PubMed]
- Allard, S.D.; de Keersmaecker, B.; de Goede, A.L.; Verschuren, E.J.; Koetsveld, J.; Reedijk, M.L.; Wylock, C.; de Bel, A.V.; Vandeloo, J.; Pistoor, F.; et al. A phase I/IIa immunotherapy trial of HIV-1-infected patients with Tat, Rev and Nef expressing dendritic cells followed by treatment interruption. Clin. Immunol. 2012, 142, 252–268. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, J.M.; Routy, J.P.; Welles, S.; DeBenedette, M.; Tcherepanova, I.; Angel, J.B.; Asmuth, D.M.; Stein, D.K.; Baril, J.G.; McKellar, M.; et al. Dendritic cell immunotherapy for HIV-1 infection using autologous HIV-1 RNA: A randomized, double-blind, placebo-controlled clinical trial. J. Acquir. Immune Defic. Syndr. 2016. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Huang, X.L.; Kalinski, P.; Young, S.; Rinaldo, C.R., Jr. Dendritic cell function during chronic hepatitis C virus and human immunodeficiency virus type 1 infection. Clin. Vaccine Immunol. 2007, 14, 1127–1137. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.L.; Fan, Z.; Borowski, L.; Rinaldo, C.R. Maturation of dendritic cells for enhanced activation of anti-HIV-1 CD8+ T cell immunity. J. Leukoc. Biol. 2008, 83, 1530–1540. [Google Scholar] [CrossRef] [PubMed]
- Andresen, B.S.; Vinner, L.; Tang, S.; Bragstad, K.; Kronborg, G.; Gerstoft, J.; Corbet, S.; Fomsgaard, A. Characterization of near full-length genomes of HIV type 1 strains in Denmark: Basis for a universal therapeutic vaccine. AIDS Res. Hum. Retrovir. 2007, 23, 1442–1448. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Galarza, F.F.; Takeshita, L.Y.; Santos, E.J.; Kempson, F.; Maia, M.H.; da Silva, A.L.; Teles e Silva, A.L.; Ghattaoraya, G.S.; Alfirevic, A.; Jones, A.R.; et al. Allele frequency net 2015 update: New features for HLA epitopes, KIR and disease and HLA adverse drug reaction associations. Nucleic Acids Res. 2015, 43, D784–D788. [Google Scholar] [CrossRef] [PubMed]
- Corbet, S.; Nielsen, H.V.; Vinner, L.; Lauemoller, S.; Therrien, D.; Tang, S.; Kronborg, G.; Mathiesen, L.; Chaplin, P.; Brunak, S.; et al. Optimization and immune recognition of multiple novel conserved HLA-A2, human immunodeficiency virus type 1-specific CTL epitopes. J. Gen. Virol. 2003, 84, 2409–2421. [Google Scholar] [CrossRef] [PubMed]
- Al Jabri, A.A. HLA and in vitro susceptibility to HIV infection. Mol. Immunol. 2002, 38, 959–967. [Google Scholar] [CrossRef]
- Segat, L.; Brandao, L.A.; Guimaraes, R.L.; Pontillo, A.; Athanasakis, E.; de Oliveira, R.M.; Arraes, L.C.; de Lima Filho, J.L.; Crovella, S. Polymorphisms in innate immunity genes and patients response to dendritic cell-based HIV immuno-treatment. Vaccine 2010, 28, 2201–2206. [Google Scholar] [CrossRef] [PubMed]
- Pontillo, A.; Da Silva, R.C.; Moura, R.; Crovella, S. Host genomic HIV restriction factors modulate the response to dendritic cell-based treatment against HIV-1. Hum. Vaccines Immunother. 2014, 10, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Moura, R.; Pontillo, A.; D'Adamo, P.; Pirastu, N.; Campos Coelho, A.; Crovella, S. Exome analysis of HIV patients submitted to dendritic cells therapeutic vaccine reveals an association of CNOT1 gene with response to the treatment. J. Int. AIDS Soc. 2014, 17, 18938. [Google Scholar] [CrossRef] [PubMed]
- Moura Rodrigues, R.; Plana, M.; Garcia, F.; Zupin, L.; Kuhn, L.; Crovella, S. Genome-wide scan in two groups of HIV-infected patients treated with dendritic cell-based immunotherapy. Immunol. Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- De Goede, A.L.; Andeweg, A.C.; van den Ham, H.J.; Bijl, M.A.; Zaaraoui-Boutahar, F.; van IJcken, W.F.; Wilgenhof, S.; Aerts, J.L.; Gruters, R.A.; Osterhaus, A.D. DC immunotherapy in HIV-1 infection induces a major blood transcriptome shift. Vaccine 2015, 33, 2922–2929. [Google Scholar] [CrossRef] [PubMed]
- Garcia, F.; Routy, J.P. Challenges in dendritic cells-based therapeutic vaccination in HIV-1 infection Workshop in dendritic cell-based vaccine clinical trials in HIV-1. Vaccine 2011, 29, 6454–6463. [Google Scholar] [CrossRef] [PubMed]
- Graziani, G.M.; Angel, J.B. Evaluating the efficacy of therapeutic HIV vaccines through analytical treatment interruptions. J. Int. AIDS Soc. 2015, 18, 20497. [Google Scholar] [CrossRef] [PubMed]
- Strain, M.C.; Little, S.J.; Daar, E.S.; Havlir, D.V.; Gunthard, H.F.; Lam, R.Y.; Daly, O.A.; Nguyen, J.; Ignacio, C.C.; Spina, C.A.; et al. Effect of treatment, during primary infection, on establishment and clearance of cellular reservoirs of HIV-1. J. Infect. Dis. 2005, 191, 1410–1418. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.; Markowitz, M. Should we treat acute HIV infection? Curr. HIV/AIDS Rep. 2012, 9, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Rinaldo, C.R. Dendritic cell-based human immunodeficiency virus vaccine. J. Intern. Med. 2009, 265, 138–158. [Google Scholar] [CrossRef] [PubMed]
- Malyguine, A.M.; Strobl, S.; Dunham, K.; Shurin, M.R.; Sayers, T.J. ELISPOT assay for monitoring cytotoxic T lymphocytes (CTL) activity in cancer vaccine clinical trials. Cells 2012, 1, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Zaritskaya, L.; Shurin, M.R.; Sayers, T.J.; Malyguine, A.M. New flow cytometric assays for monitoring cell-mediated cytotoxicity. Expert Rev. Vaccines 2010, 9, 601–616. [Google Scholar] [CrossRef] [PubMed]
- Andres, C.; Plana, M.; Guardo, A.C.; Alvarez-Fernandez, C.; Climent, N.; Gallart, T.; Leon, A.; Clotet, B.; Autran, B.; Chomont, N.; et al. HIV-1 Reservoir dynamics after vaccination and antiretroviral therapy interruption are associated with dendritic cell vaccine-induced T cell responses. J. Virol. 2015, 89, 9189–9199. [Google Scholar] [CrossRef] [PubMed]
- Leth, S.; Schleimann, M.H.; Nissen, S.K.; Hojen, J.F.; Olesen, R.; Graversen, M.E.; Jorgensen, S.; Kjaer, A.S.; Denton, P.W.; Mork, A.; et al. Combined effect of Vacc-4x, recombinant human granulocyte macrophage colony-stimulating factor vaccination, and romidepsin on the HIV-1 reservoir (REDUC): A single-arm, phase 1B/2A trial. Lancet HIV 2016, 3, e463–e472. [Google Scholar] [CrossRef]
- Imperial College London. Research in Viral Eradication of HIV Reservoirs (RIVER). In: ClinicalTrials.gov [Internet]. 2014-[November 2016]. Available online: http://clinicaltrials.gov/show/NCT00004451 NLM Identifier: NCT00004451 (accessed on 23 July 2016).
- DerSimonian, R.; Kacker, R. Random-effects model for meta-analysis of clinical trials: An update. Contemp. Clin. Trials 2007, 28, 105–114. [Google Scholar] [CrossRef] [PubMed]

| Ref. | Country, City, Year | Inclusion Criteria | Recruited Individuals’ ART Status | Baseline CD4+ T cell count * | Vaccine Doses | Periodicity | Vaccine Administration Form | Anatomical Site (Vaccine Application) | Adverse Effects |
|---|---|---|---|---|---|---|---|---|---|
| [7] | USA, Stanford, 1998 | Asymptomatic HIV-1 infection CD4+ T cell counts >350/mm3 | naive | NR | 6 | Monthly | intravenous injection | NR | None reported |
| [8] | Brazil, Recife, 2004 | Age of ≥18 years; No current pregnancy; HIV-1 asymptomatic seropositivity for ≥1 year; ART-naive for at least 6 months prior to enrollment; Hemoglobin ≥10 g/dL and platelets ≥100,000 | naive | 554 ± 174 | 3 | Biweekly | subcutaneous injection | left and right axillary and inguinal areas | Increase in the size of peripheral lymph nodes |
| [9] | Spain, Barcelona, 2005 | Asymptomatic HIV-1 infection; Baseline and nadir CD4+ T cell counts >500 cells/mL; Baseline pre-ART PVL >5000 copies/mL; PVL <20 copies/mL for at least 104 weeks while on ART | experienced | 754 ± 36 | 5 | Every six weeks | subcutaneous injection | NR | Flu-like reactions |
| [5] | Japan, Tokyo, 2006 | Undetectable viral loads (PVL <50 copies/mL) for 1 year on ART | experienced | 396 (337–504) | 6 | Biweekly | subcutaneous injection | axillary areas | Subcutaneous bleeding or erythema at injection site General malaise |
| [10] | USA, Boston and New York, 2009 | PVL ≤400 copies/mL and CD4+ T cell counts ≥400/mm3 for at least 3 months prior recruitment; PVL <50 copies/mL at screening | experienced | 664 (NR) | 3 | Weeks 3, 7 and 15 | subcutaneous injection | inner aspect of the arm, 6–12 cm from the axilla | Episodes of thrombocytopenia in a patient and neutropenia in another |
| [13] | Denmark, Copenhagen and Hvidovre, 2009 | Asymptomatic HIV-1 infection; CD4+ T cell counts ≥300/mm3; Absence of other chronic diseases; 1000 < PVL < 100,000 copies/mL; Presence of HLA-A * 0201 allele | experienced | 565 (355–982) | 4 | Biweekly, last dose after four weeks | subcutaneous injection | left and right axillary areas | None reported |
| [11] | Spain, Barcelona, 2011 | Asymptomatic HIV-1 infection; ART-naive for at least two years before enrollment; Baseline CD4+ T cell counts >450 cells/mm3; Nadir CD4+ T cell counts >350 cells/mm3; PVL >10,000 HIV-1 copies/mL | naive | 647 (532–776) | 3 | Biweekly | subcutaneous injection | NR | Asymptomatic enlargement of local lymph nodes Flu-like symptoms |
| [15] | Belgium, Brussel and Netherlands, Rotterdam, 2012 | Patients on ART; PVL ≤50 copies/ml and CD4+ T cell counts ≥500/mm3 for a period of at least 3 months prior to enrollment; Nadir CD4+ T-cell count >300/mm3 | experienced | 610 (500–960) | 4 | Monthly | subcutaneous and intradermal injection | antero-median side of an arm or a thigh | Tonsillitis episode |
| [12] | Spain, Barcelona, 2013 | Asymptomatic chronic HIV-1 infection; Baseline CD4+ T cell count >450 cells/mm3; Nadir CD4+ T cell count >350 cells/mm3; Undetectable PVL(<50 copies/mL) on ART | experienced | 702 (568–900) | 3 | Biweekly | subcutaneous or intradermal injection | upper-inner part of both arms | Lymph node enlargement, erythema and flu-like symptoms |
| [14] | USA, Dallas, 2014 | Asymptomatic HIV-1 infection; Baseline CD4+ T cell count >500 cells/mm3; Baseline PVL <50 copies/mL and within the previous 3 months while on ART; Nadir CD4+ T cells count ≥300 cells/mm3 | experienced | 670 (553–832) | 4 | Every 4 weeks | subcutaneous injection | upper and lower extremities | None reported |
| [6] | USA, Pittsburgh, 2015 | CD4+ T cell count ≥300 cells/mm3; 3000 < PVL < 100,000 copies/mL | naive | 486 (377–881) | 4 (3 doses while on ART, 1 dose after ATI) | Biweekly | subcutaneous injection | upper medial area of the arm (bilaterally) | Mild-to-moderate inflammation at the injection site; Two individuals experienced severe pruritus and pain at the injection site |
| [16] | USA, Philadelphia and Canada, Montreal, 2016 | PVL ≤200 copies/mL for at least 3 months prior to enrollment; PVL <50 copies/mL at screening; CD4+ T cell count ≥450 cells/mm3; Nadir CD4+ T cell count ≥200 cells/mm3; Pre-ART (within 3 months) plasma for virus isolation availability | experienced | 632 (513–765) | 4 | Every 4 weeks | intradermal injection | axillary lymph node | Mild local injection site reactions |
| Ref. | Country, City, Year | Vaccine Response Criterion | Enrolled | Placebo Arm | Comparator Arm | Vaccine Arm | Removed from Analysis n | Responders n | Non-Responders n | Study Follow-Up Length |
|---|---|---|---|---|---|---|---|---|---|---|
| [7] | USA, Stanford, 1998 | Any PVL decrease | 6 | 0 | 0 | 6 | 0 | 0 | 6 | 40 weeks |
| [8] | Brazil, Recife, 2004 | >90% PVL decrease by 1 year | 20 | 0 | 0 | 18 | 2 | 8 | 10 | 1 year |
| [9] | Spain, Barcelona, 2005 | PVL decrease of 0.5 log10 copies/mL 24 weeks after vaccination | 18 | 6 | 0 | 12 | 0 | 4 | 8 | 24 weeks |
| [5] | Japan, Tokyo, 2006 | PVL decrease of 0.5 log10 copies/mL | 4 | 0 | 0 | 4 | 0 | 0 | 4 | 12 weeks |
| [10] | USA, Boston and New York, 2009 | Average of the last two scheduled PVL evaluations during weeks 10–13 of ATI ≤5000 copies/mL | 29 | 0 | 15 | 14 | 1 | 4 | 9 | 12 weeks |
| [13] | Denmark, Copenhagen and Hvidovre, 2009 | A PVL decrease of 1.08 log10 copies/mL was the most pronounced change among responders | 12 | 0 | 0 | 12 | 0 | 5 | 7 | 40 weeks |
| [11] | Spain, Barcelona, 2011 | PVL decrease of 0.5 log10 copies/mL 24 weeks after vaccination | 24 | 12 | 0 | 12 | 4 | 4 | 4 | 48 weeks |
| [15] | Belgium, Brussel and Netherlands, Rotterdam, 2012 | Remaining off ART at 96 weeks following ATI | 17 | 0 | 0 | 17 | 0 | 6 | 11 | 110 weeks |
| [12] | Spain, Barcelona, 2013 | Post-vaccination PVL decrease ≥1 log | 36 | 12 | 0 | 24 | 2 | 12 | 10 | 48 weeks |
| [14] | USA, Dallas, 2014 | ATI maximum PVL <5 log10 copies/mL | 19 | 0 | 0 | 19 | 1 | 9 | 9 | 48 weeks |
| [6] | USA, Pittsburgh, 2015 | ATI PVL decrease >0.4 log10 copies/mL | 11 | 0 | 0 | 11 | 1 | 3 | 7 | 48 weeks |
| [16] | USA, Philadelphia and Canada, Montreal, 2016 | PVL in the vaccine arm is reduced by at least 1.1 log10 copies/mL | 54 | 17 | 0 | 37 | 2 | 0 | 35 | 2 years |
| Ref. | Loaded Molecules | Loaded Molecules (Summarized) | Dendritic Cell Number | Culture Medium | Days in Culture |
|---|---|---|---|---|---|
| [7] | Gag (residues 77 to 85, SLYNTVATL motif), Env (residues 120 to 128, KLTPLCVTL motif and residues 814 to 823, SLLNATDIAV motif) and Pol (residues 956 to 964, LLWKGEGAV motif; residues 464 to 472, ILKEPVHGV motif and residues 267 to 277, VLDVGDAYFSV motif) peptides from recombinant HIV-1 MN gp160 polypeptide | peptides | 2–8 × 106 (immature DCs) | RPMI-1640 | 2 |
| [8] | AT2 (chemically)-inactivated autologous virus | whole virus | 6 × 107 | CellGro® DC Medium | 7 |
| [9] | Heat-inactivated autologous virus | whole virus | 2 × 106 | MCM | 8 |
| [5] | Gag (residues 28 to 36, KYKLKHIVW and KYRLKHIVW motifs; residues 296 to 306, RDYVDRFYKTL motif), Nef (residues 138 to 147, RYPLTFGWCF and RFPLTFGWCF motifs) and Env (residues 584 to 594, RYLRDQQLLGI and RYLQDQQLLGI motifs) peptides | peptides | 0.7–1.8 ×106 | RPMI-1640 | 7 |
| [10] | Recombinant virus produced by a canarypox vector (ALVAC vCP1452) | whole virus | 1.5–6 × 106 | MCM | 6 |
| [13] | HLA A*0201-binding peptides (Gag, Pol, Env, Vpu and Vif) | peptides | 1 × 107 | X-VIVO™ 15 | 8 |
| [11] | Heat-inactivated autologous virus | whole virus | 8 × 106 | X-VIVO™ 15 | 7 |
| [15] | Mature DCs were electroporated with mRNA derived from pGEM-sig-Tat-DC-LAMP, pGEM-sigRev-DC-LAMP, pGEM-sig-Nef-DC-LAMP and pST1-sig-Gag-DCLAMP plasmids for peptides expression | mRNA (by electroporation) | 1 × 107 | X-VIVO™ 15 | 7 |
| [12] | Heat-inactivated autologous virus | whole virus | 2 × 106 | X-VIVO™ 15 | 7 |
| [14] | Viral epitopes from Gag (17 to 35, and 253 to 284 residues), Nef (66 to 97 and 116 to 145 residues) and Pol (residues 325 to 355) lipopeptides | peptides | 15 × 106 | CellGro® DC Medium | 3 |
| [6] | Autologous CD4+ T cells which had been superinfected with endogenous inactivated HIV-1 with psoralen and UVB irradiation | peptides (indirectly) | 1 × 107 | CellGro® DC Medium | 6 |
| [16] | Mature DCs were electroporated with autologous HIV-1 Gag, Nef, Rev, and Vpr mRNA for peptides expression | mRNA (by electroporation) | 1.2 × 107 | Not reported | 7 |
| Ref. | DC Maturation Procedure | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| GM-CSF Supplementation | IFN-α Supplementation | IFN-γ Supplementation | IL-1β Supplementation | IL-4 Supplementation | IL-6 Supplementation | PGE2 Supplementation | TNF-α Supplementation | Other Molecules Supplementation | |
| [7] | No | No | No | No | No | No | No | No | - |
| [8] | Yes | No | No | Yes | Yes | Yes | No | Yes | - |
| [9] | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | - |
| [5] | Yes | No | No | No | Yes | No | No | Yes | - |
| [10] | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | - |
| [13] | Yes | No | No | Yes | Yes | Yes | Yes | Yes | - |
| [11] | Yes | No | No | Yes | Yes | Yes | No | Yes | - |
| [15] | Yes | No | No | Yes | Yes | Yes | Yes | Yes | - |
| [12] | Yes | No | No | Yes | Yes | Yes | Yes | Yes | - |
| [14] | Yes | Yes | No | No | No | No | No | No | LPS |
| [6] | Yes | Yes | Yes | Yes | Yes | No | No | Yes | polyinosinic:polycytidylic acid |
| [16] | No | No | Yes | No | No | No | Yes | Yes | CD40L |
| Variable | Linear Univariate Meta-Regression Coefficient | Coefficient Standard Error | p-Value | Decision |
|---|---|---|---|---|
| Antigen: | ||||
| whole virus | Reference | - | - | |
| mRNA | −0.8419 | 0.5442 | 0.1219 | Pre-selected |
| peptides | −0.2937 | 0.3993 | 0.4621 | |
| Baseline (pre-vaccine) CD4+ T cell counts: | ||||
| more than 700 cells/mm3 | Reference | - | - | Pre-selected |
| between 600 and 700 cells/mm3 | −0.4542 | 0.4405 | 0.3025 | |
| between 500 and 600 cells/mm3 | −0.0367 | 0.5174 | 0.9434 | |
| less than 500 cells/mm3 | −0.9804 | 0.7178 | 0.1720 | |
| Days in culture (DCs maturation) | −0.0062 | 0.1118 | 0.9558 | Not pre-selected |
| DC maturation culture medium: | ||||
| RPMI-1640 | Reference | - | - | |
| CellGro® | 2.8743 | 0.8972 | 0.0014 | |
| MCM | 2.2890 | 0.9458 | 0.0155 | Pre-selected |
| X-VIVO™ 15 | 2.8780 | 0.8836 | 0.0011 | |
| GM-CSF supplementation (yes or no) | 3.1400 | 1.0378 | 0.0025 | Pre-selected |
| IFN-α supplementation (yes or no) | −0.1512 | 0.3660 | 0.6795 | Not pre-selected |
| IFN-γ supplementation (yes or no) | −1.2007 | 0.6481 | 0.0639 | Pre-selected; removed due to collinearity |
| IL-1β supplementation (yes or no) | 0.5968 | 0.5336 | 0.2633 | Pre-selected |
| IL-4 supplementation (yes or no) | 0.3529 | 0.5235 | 0.5002 | Not pre-selected |
| IL-6 supplementation (yes or no) | 0.5672 | 0.4479 | 0.2053 | Pre-selected; removed due to collinearity |
| Number of DCs per vaccine dose | −0.0032 | 0.0408 | 0.9369 | Not pre-selected |
| Number of vaccine doses | −0.5006 | 0.2386 | 0.0359 | Pre-selected; removed due to collinearity |
| Periodicity of vaccine doses (biweekly or every four weeks or more) | 0.4793 | 0.3577 | 0.1802 | Pre-selected; removed due to collinearity |
| PGE2 supplementation (yes or no) | −0.1765 | 0.3592 | 0.6231 | Not pre-selected |
| TNF-α supplementation (yes or no) | −0.1830 | 0.4887 | 0.7081 | Not pre-selected |
| Variable | Linear Multivariate Meta-Regression Coefficient | 95% Confidence Interval | p-Value |
|---|---|---|---|
| (Model Intercept) | −3.4826 | (−6.5475)–(−0.4176) | 0.0259 |
| Antigen: | |||
| whole virus | Reference | - | - |
| mRNA | −0.6289 | (−2.1234)–0.8657 | 0.4095 |
| peptides | 1.0689 | (−3.2088)–5.3466 | 0.6243 |
| Baseline (pre-vaccine) CD4+ T cell counts: | |||
| more than 700 cells/mm3 | Reference | - | - |
| between 600 and 700 cells/mm3 | −0.1513 | (−1.318)–1.0155 | 0.7994 |
| between 500 and 600 cells/mm3 | −1.5793 | (−6.0215)–2.8629 | 0.4859 |
| less than 500 cells/mm3 | −3.4955 | (−12.266)–5.2751 | 0.4347 |
| GM-CSF supplementation (yes or no) | 3.712 | (−3.8567)–11.2806 | 0.3364 |
| DC maturation culture medium: | |||
| RPMI-1640 | Reference | - | - |
| CellGro® | −1.147 | (−10.2552)–7.9612 | 0.805 |
| MCM | −3.4024 | (−16.7094)–9.9046 | 0.6163 |
| X-VIVO™ 15 | −2.5523 | (−15.9238)–10.8191 | 0.7083 |
| IL-1β supplementation (yes or no) | 2.4969 | (−6.0232)–11.01 | 0.5657 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coelho, A.V.C.; De Moura, R.R.; Kamada, A.J.; Da Silva, R.C.; Guimarães, R.L.; Brandão, L.A.C.; De Alencar, L.C.A.; Crovella, S. Dendritic Cell-Based Immunotherapies to Fight HIV: How Far from a Success Story? A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2016, 17, 1985. https://doi.org/10.3390/ijms17121985
Coelho AVC, De Moura RR, Kamada AJ, Da Silva RC, Guimarães RL, Brandão LAC, De Alencar LCA, Crovella S. Dendritic Cell-Based Immunotherapies to Fight HIV: How Far from a Success Story? A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences. 2016; 17(12):1985. https://doi.org/10.3390/ijms17121985
Chicago/Turabian StyleCoelho, Antonio Victor Campos, Ronald Rodrigues De Moura, Anselmo Jiro Kamada, Ronaldo Celerino Da Silva, Rafael Lima Guimarães, Lucas André Cavalcanti Brandão, Luiz Cláudio Arraes De Alencar, and Sergio Crovella. 2016. "Dendritic Cell-Based Immunotherapies to Fight HIV: How Far from a Success Story? A Systematic Review and Meta-Analysis" International Journal of Molecular Sciences 17, no. 12: 1985. https://doi.org/10.3390/ijms17121985