An Overview of Hardware for Protein Crystallization in a Magnetic Field
Abstract
:1. Introduction
2. Magnet Systems That Provide a Strong and Stable Magnetic Field
2.1. Electromagnets
2.2. Permanent Magnets
2.3. Resistive Magnets
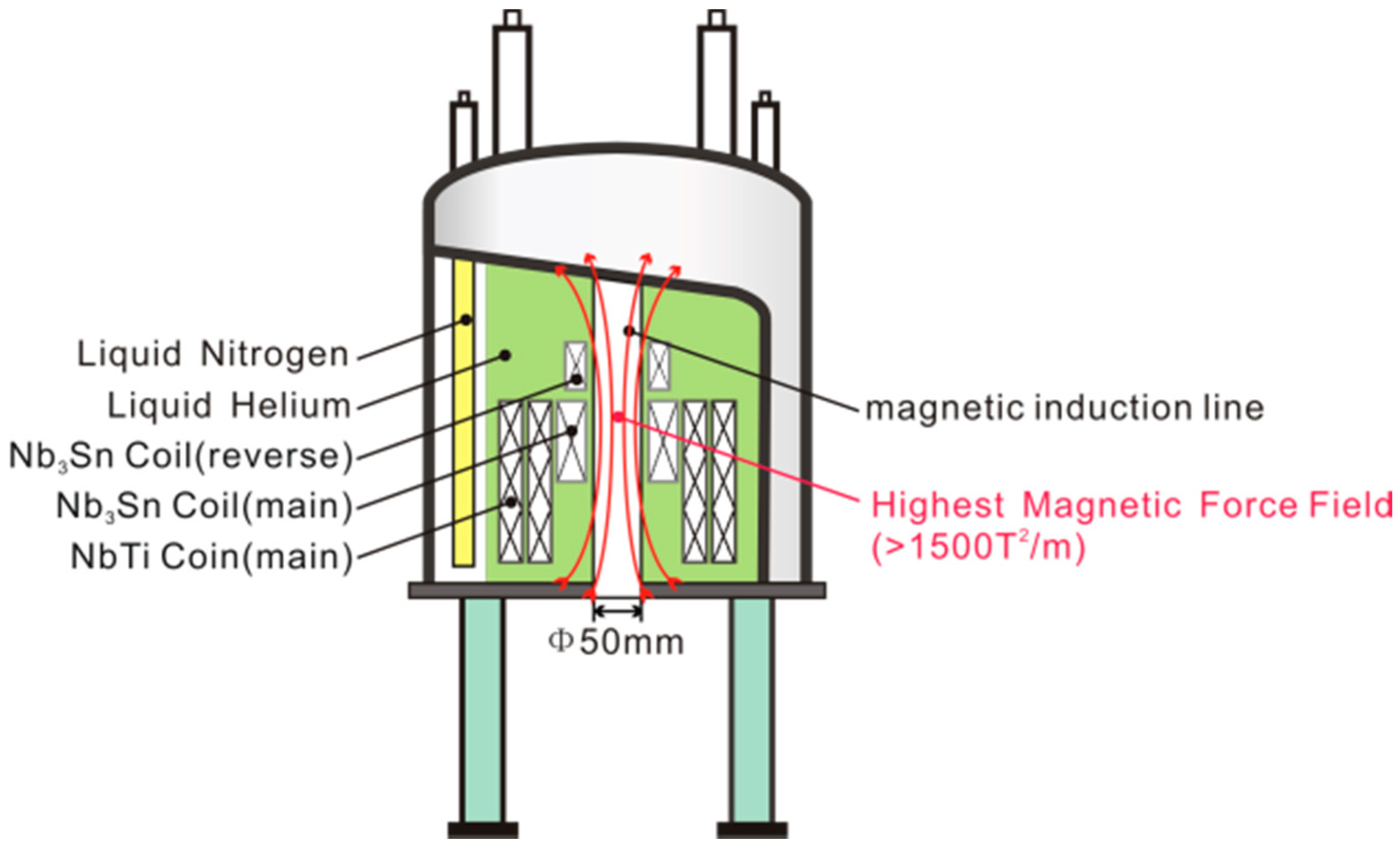
2.4. Superconducting Magnets
2.4.1. Common Superconducting Magnets
2.4.2. Nuclear Magnetic Resonance (NMR) Magnets
3. Apparatuses for Protein Crystal Growth inside a Magnetic Field
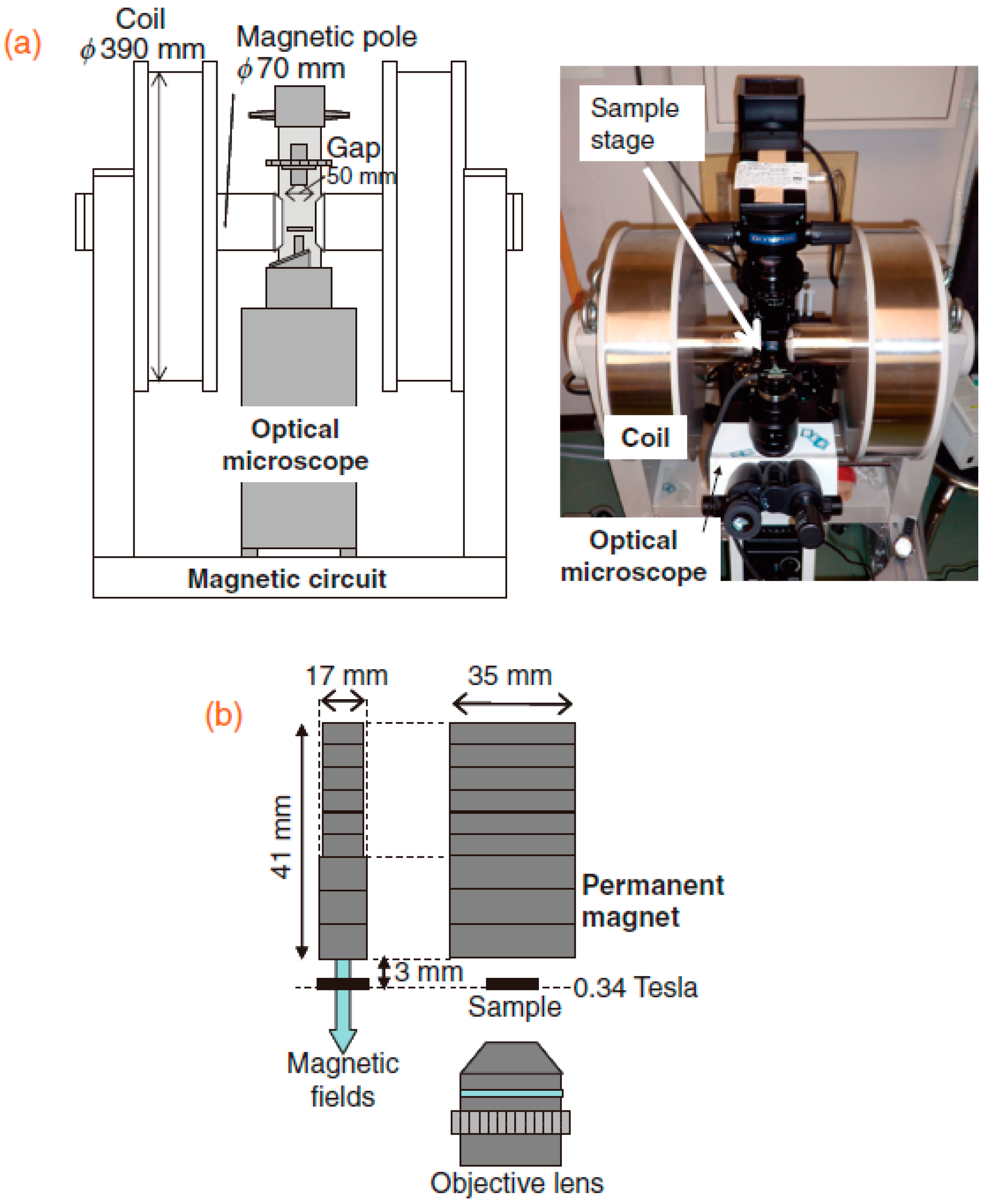
3.1. Requirement for Setup Using Protein Crystallization inside the Magnet
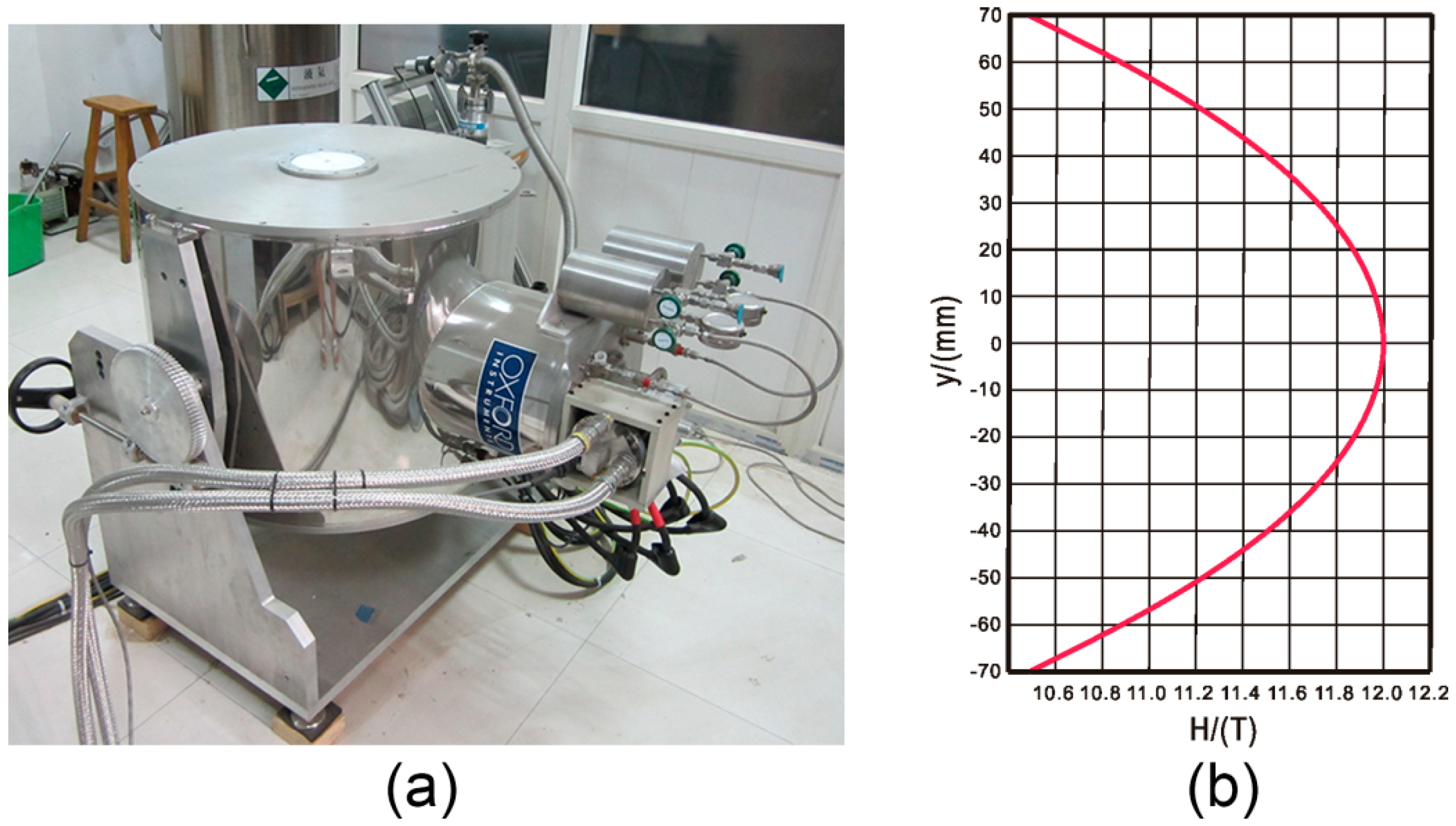
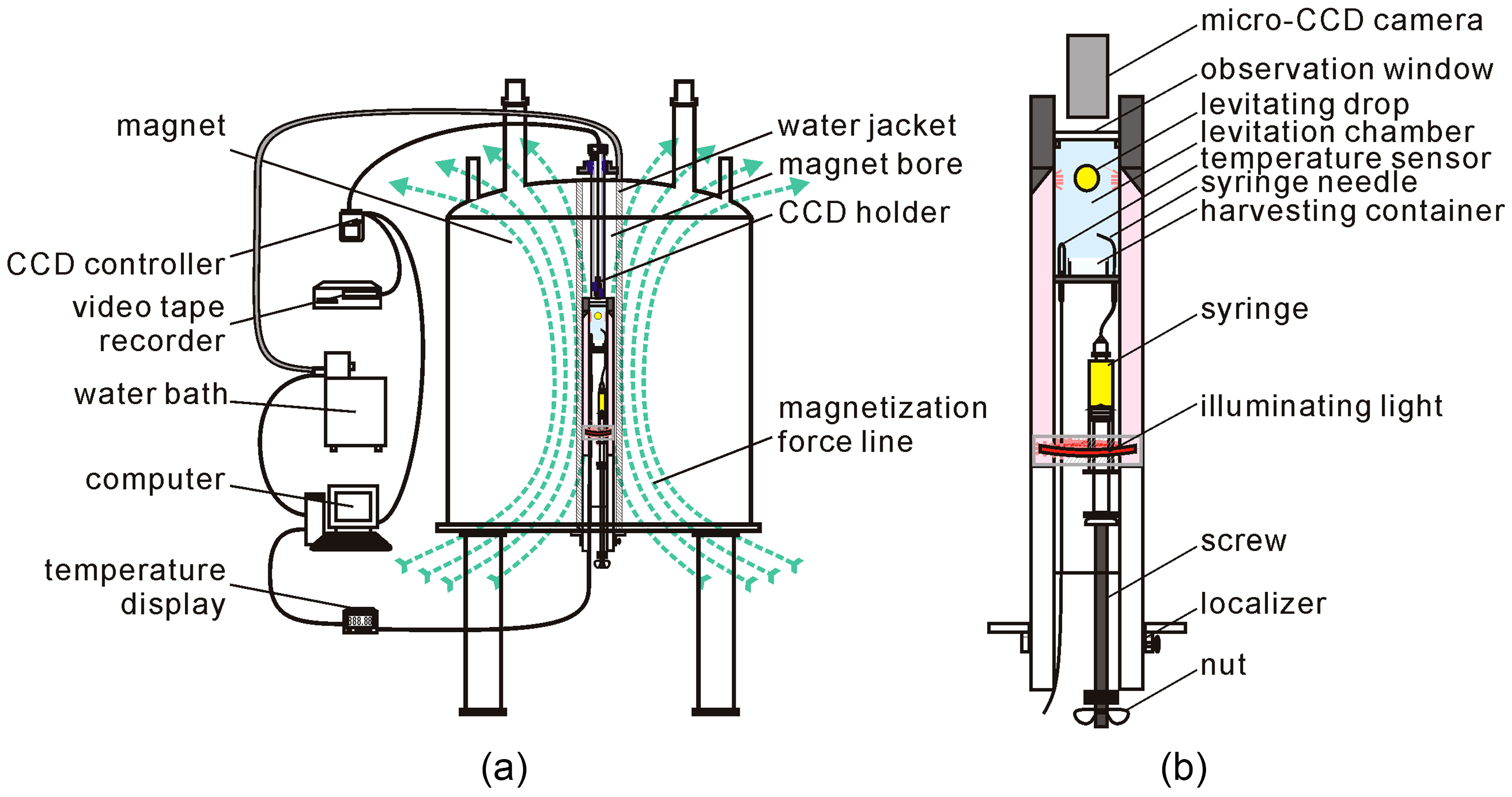
3.2. Protein Crystallization Apparatus inside the Magnetic Field
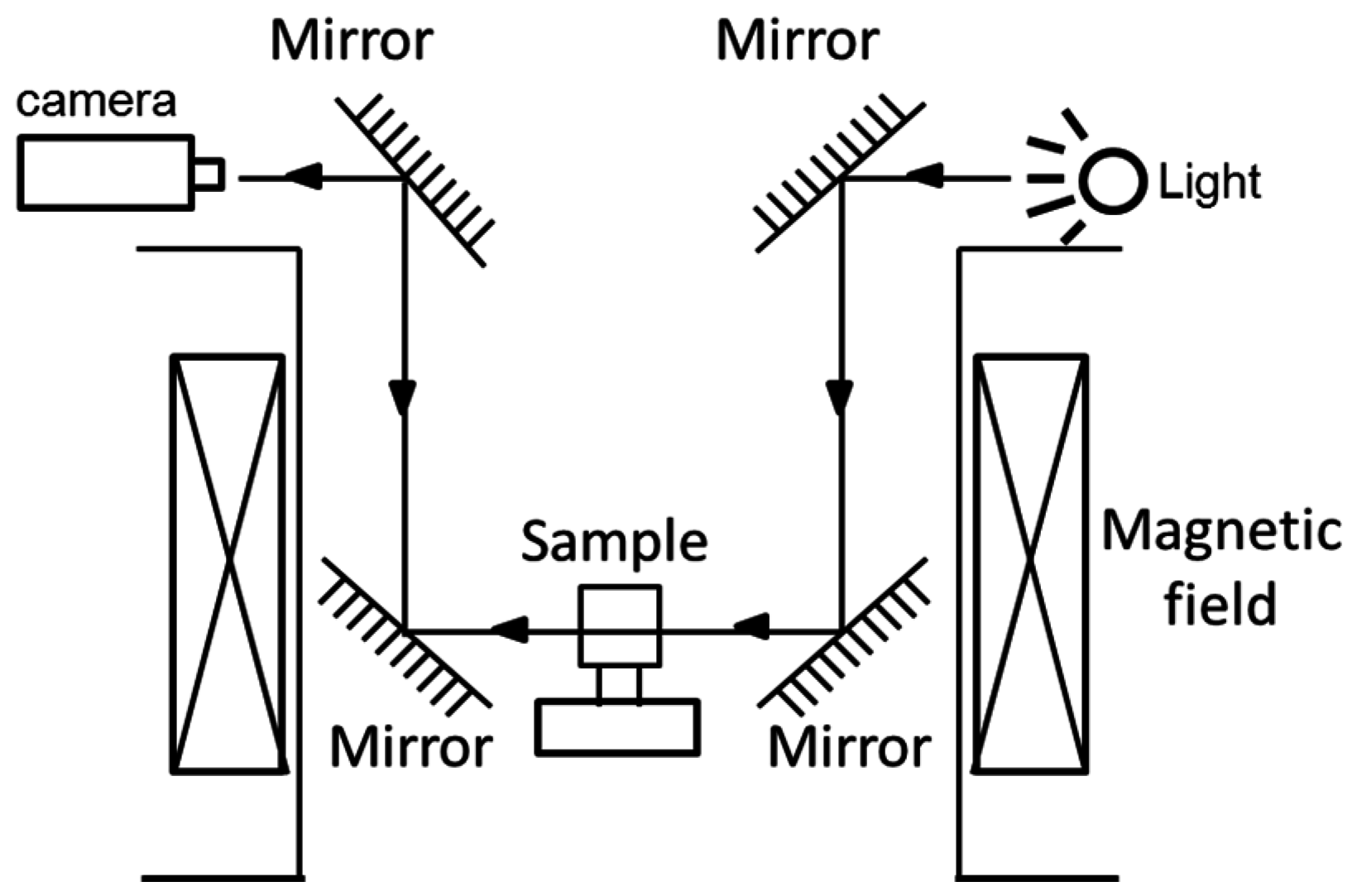
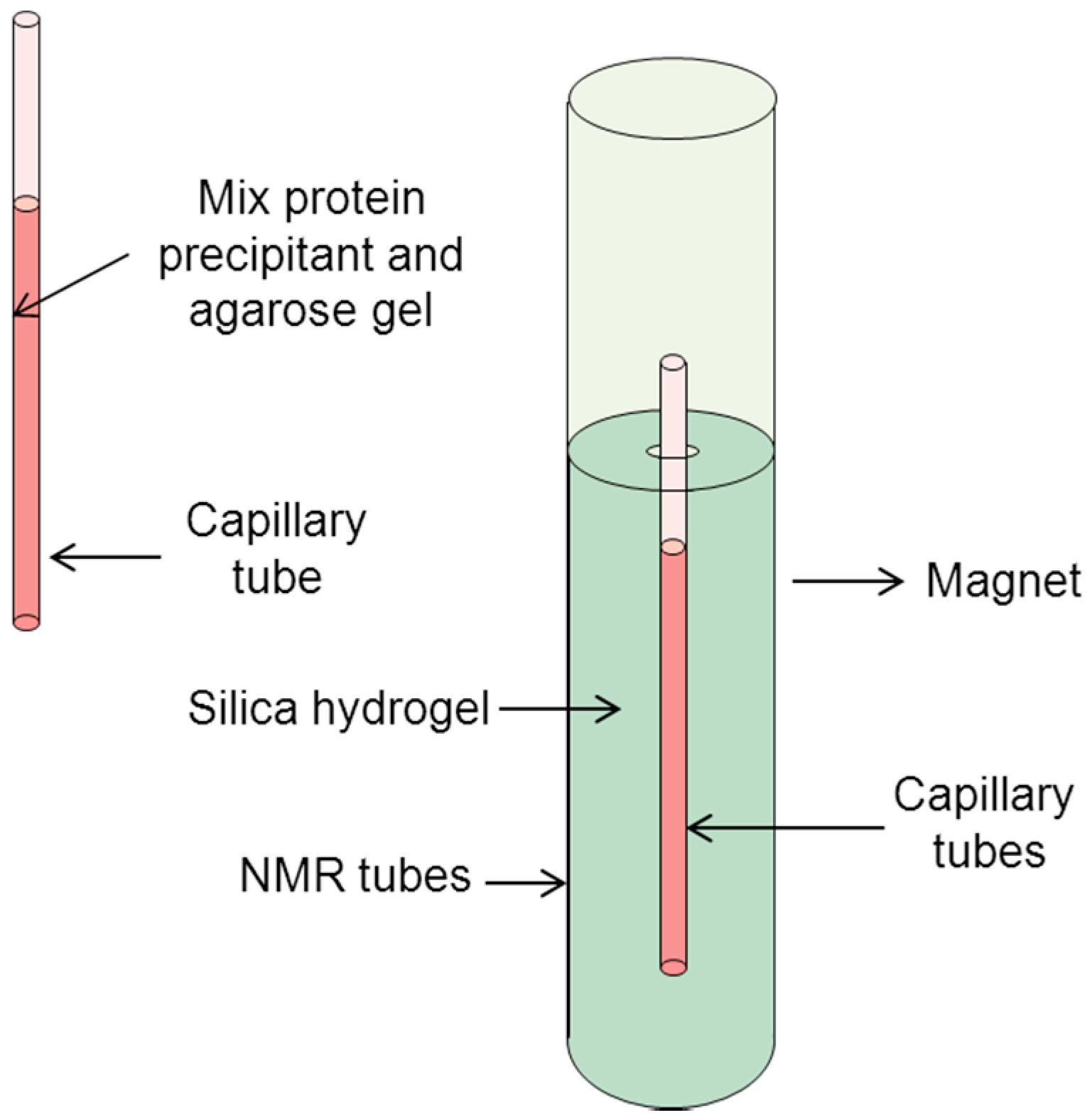
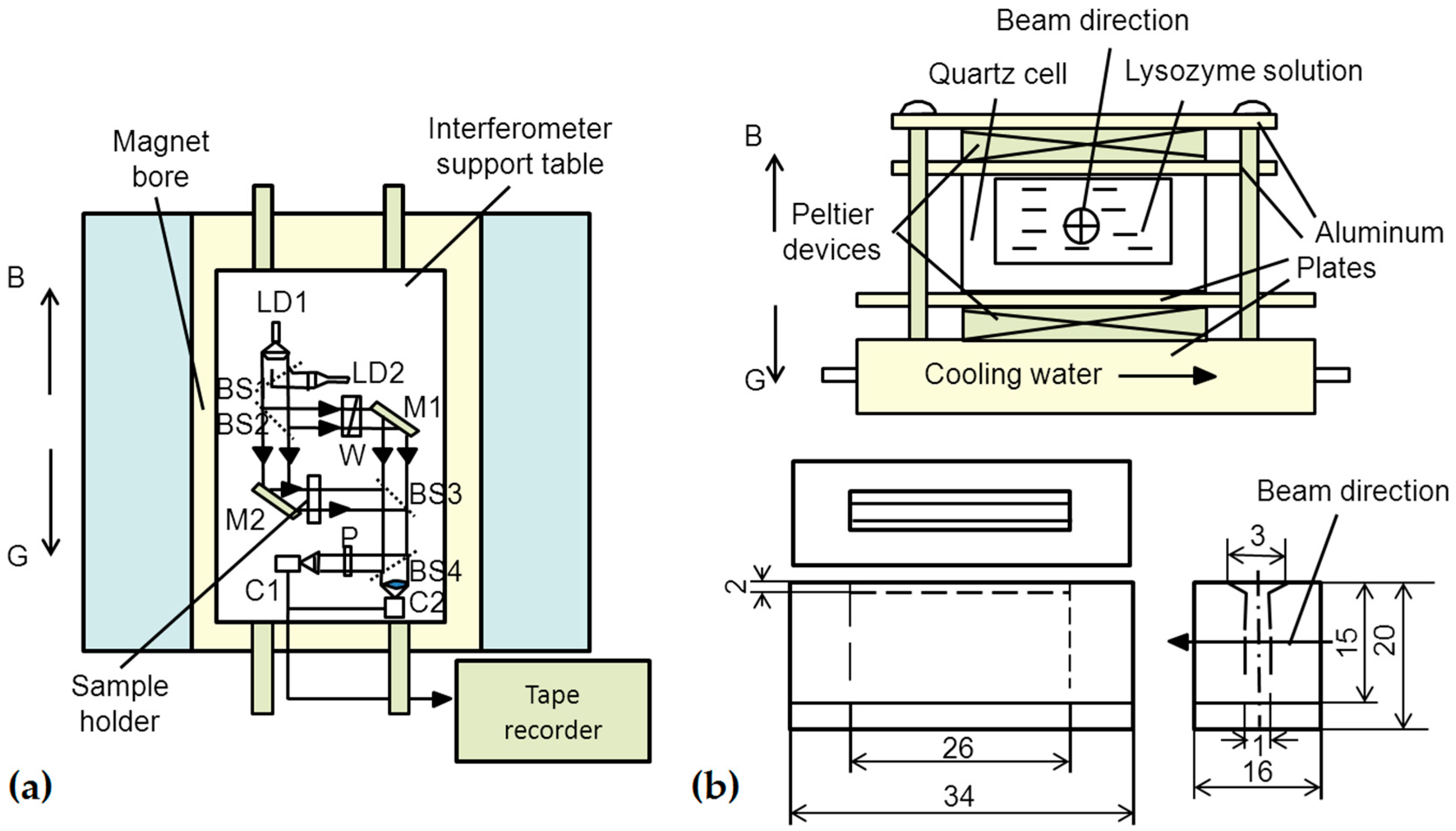
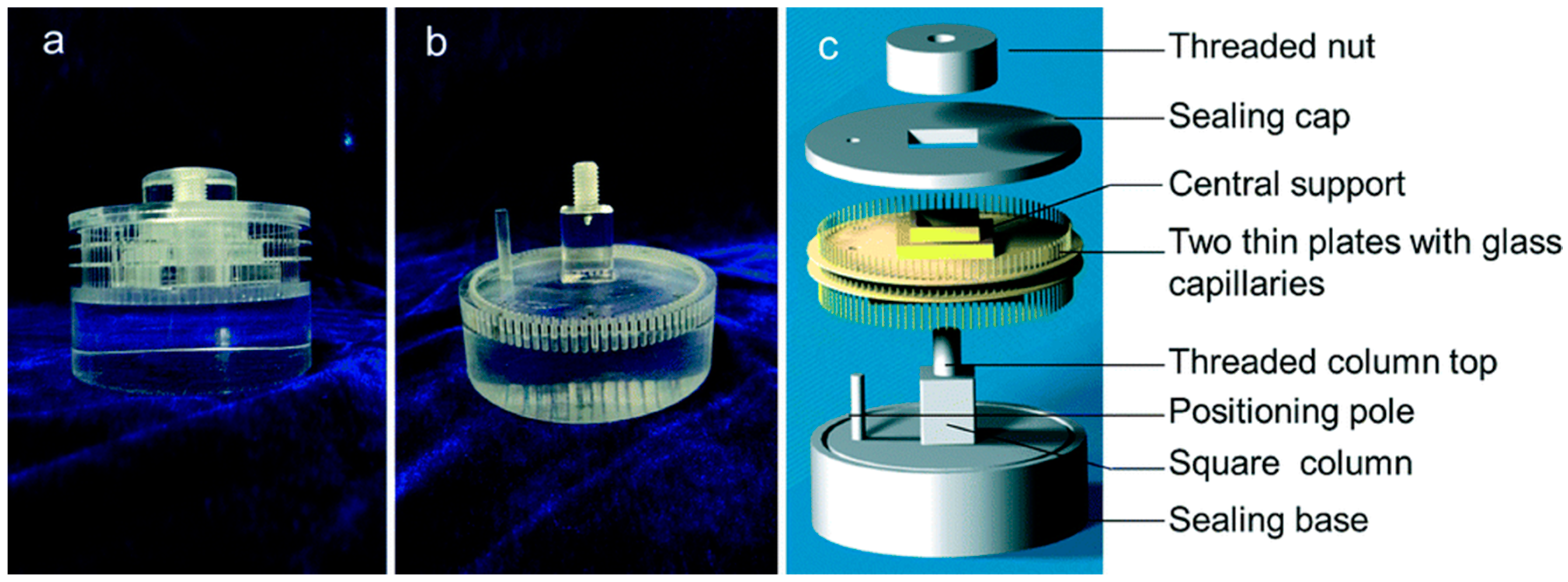
3.3. Other Equipment Used for Protein Crystals under a Magnetic Field
4. Future Prospects and Concluding Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ataka, M.; Wakayama, N.I. Effects of a magnetic field and magnetization force on protein crystal growth. Why does a magnet improve the quality of some crystals? Acta Cryst. D 2002, 58, 1708–1710. [Google Scholar] [CrossRef]
- Wakayama, N.I. Effects of a strong magnetic field on protein crystal growth. Cryst. Growth Des. 2003, 3, 17–24. [Google Scholar] [CrossRef]
- Vergara, A.; Lorber, B.; Zagari, A.; Giege, R. Physical aspects of protein crystal growth investigated with the Advanced Protein Crystallization Facility in reduced-gravity environments. Acta Cryst. D 2003, 59, 2–15. [Google Scholar] [CrossRef]
- Sazaki, G. Crystal quality enhancement by magnetic fields. Biophys. Mol. Biol. 2009, 101, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.C. Protein crystallization in a magnetic field. Cryst. Growth Charact. Mater. 2015, 61, 1–26. [Google Scholar] [CrossRef]
- Lubbert, D.; Meents, A.; Weckert, E. Accurate rocking-curve measurements on protein crystals grown in a homogeneous magnetic field of 2.4 T. Acta Cryst. D 2004, 60, 987–998. [Google Scholar] [CrossRef] [PubMed]
- Astier, J.P.; Veesler, S.; Boistelle, R. Protein crystals orientation in a magnetic field. Acta Cryst. D 1998, 54, 703–706. [Google Scholar] [CrossRef]
- Perenboom, J.A.A.J.; Wiegers, S.A.J.; Christianen, P.C.M.; Zeitler, U.; Maan, J.C. Research in high magnetic fields: The installation at the University of Nijmegen. J. Low Temp. Phys. 2003, 133, 181–201. [Google Scholar] [CrossRef]
- Wiegers, S.; Christianen, P.; Engelkamp, H.; den Ouden, A.; Perenboom, J.; Zeitler, U.; Maan, J. The High Field Magnet Laboratory at Radboud University Nijmegen. J. Low Temp. Phys. 2010, 159, 389–393. [Google Scholar] [CrossRef]
- Zhao, B. Effect of magnetic gradient on the growth rate of l-Alanine crystal. IEEE Trans. Appl. Supercond. 2004, 14, 1596–1599. [Google Scholar] [CrossRef]
- Yin, D.C.; Lu, H.M.; Geng, L.Q.; Shi, Z.H.; Luo, H.M.; Li, H.S.; Ye, Y.J.; Guo, W.H.; Shang, P.; Wakayama, N.I. Growing and dissolving protein crystals in a levitated and containerless droplet. J. Cryst. Growth 2008, 310, 1206–1212. [Google Scholar] [CrossRef]
- Wakayama, N. Quantitative study of crystallization kinetics of hen egg-white lysozyme using magnetic orientation. J. Cryst. Growth 1998, 191, 199–205. [Google Scholar] [CrossRef]
- Sakurazawa, S.; Kubota, T.; Ataka, M. Orientation of protein crystals grown in a magnetic field. J. Cryst. Growth 1999, 196, 325–331. [Google Scholar] [CrossRef]
- Tanimoto, Y.; Yamaguchi, R.; Kanazawa, Y.; Fujiwara, M. Magnetic orientation of lysozyme crystals. Bull. Chem. Soc. Jpn. 2002, 75, 1133–1134. [Google Scholar] [CrossRef]
- Iwasaka, M.; Miyashita, Y.; Mizukawa, Y.; Suzuki, K.; Toyota, T.; Sugawara, T. Biaxial alignment control of guanine crystals by diamagnetic orientation. Appl. Phys. Express 2013, 6, 037002. [Google Scholar] [CrossRef]
- Wakayama, N.I.; Ataka, M.; Abe, H. Effect of a magnetic field gradient on the crystallization of hen lysozyme. J. Cryst. Growth 1997, 178, 653–656. [Google Scholar] [CrossRef]
- Heijna, M.C.R.; Poodt, P.W.G.; Tsukamoto, K.; de Grip, W.J.; Christianen, P.C.M.; Maan, J.C.; Hendrix, J.L.A.; van Enckevort, W.J.P.; Vlieg, E. Magnetically controlled gravity for protein crystal growth. Appl. Phys. Lett. 2007, 90, 264105. [Google Scholar] [CrossRef] [Green Version]
- Leslie, F.W.; Ramachandran, N. Stability of magnetically suppressed solutal convection in crystal growth from solutions. J. Cryst. Growth 2007, 303, 597–606. [Google Scholar] [CrossRef]
- Okada, H.; Hirota, N.; Matsumoto, S.; Wada, H. A simulation study of solution flow under magnetic force field. IEEE Trans. Appl. Supercond. 2012, 22, 4904204. [Google Scholar] [CrossRef]
- Okada, H.; Hirota, N.; Matsumoto, S.; Wada, H. A flow simulation study of protein solution under magnetic forces. J. Appl. Phys. 2013, 113, 073913. [Google Scholar] [CrossRef]
- Wakayama, N.I. Damping of solute convection during crystal growth by applying magnetic field gradients and floating crystals at the air-solution interface. Jpn. J. Appl. Phys. 2006, 45, 253–255. [Google Scholar] [CrossRef]
- Watanabe, K.; Awaji, S.; Sakuraba, J.; Watazawa, K.; Hasebe, T.; Jikihara, K.; Yamada, Y.; Ishihara, M. 11 T liquid helium-free superconducting magnet. Cryogenics 1996, 36, 1019–1025. [Google Scholar] [CrossRef]
- Sazaki, G.; Yoshida, E.; Komatsu, H.; Nakada, T.; Miyashita, S.; Watanabe, K. Effects of a magnetic field on the nucleation and growth of protein crystals. J. Cryst. Growth 1997, 173, 231–234. [Google Scholar] [CrossRef]
- Yanagiya, S.-I.; Sazaki, G.; Durbin, S.D.; Miyashita, S.; Nakada, T.; Komatsu, H.; Watanabe, K.; Motokawa, M. Effect of a magnetic field on the orientation of hen egg-white lysozyme crystals. J. Cryst. Growth 1999, 196, 319–324. [Google Scholar] [CrossRef]
- Kimura, F.; Mizutani, K.; Mikami, B.; Kimurat, T. Single-crystal X-ray diffraction study of a magnetically oriented microcrystal array of lysozyme. Cryst. Growth Des. 2011, 11, 12–15. [Google Scholar] [CrossRef]
- Huang, L.J.; Cao, H.L.; Ye, Y.J.; Liu, Y.M.; Zhang, C.Y.; Lu, Q.Q.; Hou, H.; Shang, P.; Yin, D.C. A new method to realize high-throughput protein crystallization in a superconducting magnet. CrystEngComm 2015, 17, 1237–1241. [Google Scholar] [CrossRef]
- Sato, T.; Yamada, Y.; Saijo, S.; Hori, T.; Hirose, R.; Tanaka, N.; Sazaki, G.; Nakajima, K.; Igarashi, N.; Tanaka, M.; et al. Enhancement in the perfection of orthorhombic lysozyme crystals grown in a high magnetic field (10 T). Acta Cryst. D 2000, 56, 1079–1083. [Google Scholar] [CrossRef]
- Sato, T.; Yamada, Y.; Saijo, S.; Hori, T.; Hirose, R.; Tanaka, N.; Sazaki, G.; Nakajima, K.; Igarashi, N.; Tanaka, M.; et al. Improvement in diffraction maxima in orthorhombic HEWL crystal grown under high magnetic field. J. Cryst. Growth 2001, 232, 229–236. [Google Scholar] [CrossRef]
- Lin, S.X.; Zhou, M.; Azzi, A.; Xu, G.J.; Wakayama, N.I.; Ataka, M. Magnet used for protein crystallization: Novel attempts to improve the crystal quality. Biochem. Biophys. Res. Commun. 2000, 275, 274–278. [Google Scholar] [CrossRef] [PubMed]
- DeLucas, L.J.; Moore, K.M.; Long, M.M.; Rouleau, R.; Bray, T.; Crysel, W.; Weise, L. Protein crystal growth in space, past and future. J. Cryst. Growth 2002, 237, 1646–1650. [Google Scholar] [CrossRef]
- Kinoshita, T.; Ataka, M.; Warizaya, M.; Neya, M.; Fujii, T. Improving quality and harvest period of protein crystals for structure-based drug design: Effects of a gel and a magnetic field on bovine adenosine deaminase crystals. Acta Cryst. D 2003, 59, 1333–1335. [Google Scholar] [CrossRef]
- Yin, D.C.; Oda, Y.; Wakayama, N.I.; Ataka, M. New morphology, symmetry, orientation and perfection of lysozyme crystals grown in a magnetic field when paramagnetic salts (NiCl2, CoCl2 and MnCl2) are used as crystallizing agents. J. Cryst. Growth 2003, 252, 618–625. [Google Scholar] [CrossRef]
- Yin, D.C.; Wakayama, N.I.; Wada, H.; Huang, W.D. Significant effects of magnetic and gravitational fields on the morphology of protein crystals (orthorhombic lysozyme crystals grown using NiCl2 as crystallization agent). J. Phys. Chem. B 2003, 107, 14140–14144. [Google Scholar] [CrossRef]
- Sazaki, G.; Moreno, A.; Nakajima, K. Novel coupling effects of the magnetic and electric fields on protein crystallization. J. Cryst. Growth 2004, 262, 499–502. [Google Scholar] [CrossRef]
- Saijo, S.; Yamada, Y.; Sato, T.; Tanaka, N.; Matsui, T.; Sazaki, G.; Nakajima, K.; Matsuura, Y. Structural consequences of hen egg-white lysozyme orthorhombic crystal growth in a high magnetic field: Validation of X-ray diffraction intensity, conformational energy searching and quantitative analysis of B factors and mosaicity. Acta Cryst. D 2005, 61, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.L.; Sun, L.H.; Li, J.; Tang, L.; Lu, H.M.; Guo, Y.Z.; He, J.; Liu, Y.M.; Xie, X.-Z.; Shen, H.-F. A quality comparison of protein crystals grown under containerless conditions generated by diamagnetic levitation, silicone oil and agarose gel. Acta Cryst. D 2013, 69, 1901–1910. [Google Scholar] [CrossRef] [PubMed]
- Numoto, N.; Shimizu, K.; Matsumoto, K.; Miki, K.; Kita, A. Observation of the orientation of membrane protein crystals grown in high magnetic force fields. J. Cryst. Growth 2013, 367, 53–56. [Google Scholar] [CrossRef] [Green Version]
- Wada, H.; Hirota, N.; Matsumoto, S.; Okada, H.; Kiyohara, M.; Ode, T.; Tanokura, M.; Nakamura, A.; Ohtsuka, J.; Kita, A.; et al. Application of high-field superconducting magnet to protein crystallization. Phys. Procedia 2012, 36, 953–957. [Google Scholar] [CrossRef]
- Yin, D.C.; Geng, L.Q.; Lu, Q.Q.; Lu, H.M.; Shang, P.; Wakayama, N.I. Multiple orientation responses of lysozyme crystals to magnetic field when paramagnetic salts are used as the crystallization agents. Cryst. Growth Des. 2009, 9, 5083–5091. [Google Scholar] [CrossRef]
- Nakamura, A.; Ohtsuka, J.; Miyazono, K.-I.; Yamamura, A.; Kubota, K.; Hirose, R.; Hirota, N.; Ataka, M.; Sawano, Y.; Tanokura, M. Improvement in quality of protein crystals grown in a high magnetic field gradient. Cryst. Growth Des. 2012, 12, 1141–1150. [Google Scholar] [CrossRef]
- Hirose, R.; Saito, K.; Watanabe, Y.; Tanimoto, Y. Development of a superconducting magnet for high magnetic force application. IEEE Trans. Appl. Supercond. 2004, 14, 1693–1695. [Google Scholar] [CrossRef]
- Yin, D.C.; Wakayama, N.I.; Harata, K.; Fujiwara, M.; Kiyoshi, T.; Wada, H.; Niimura, N.; Arai, S.; Huang, W.D.; Tanimoto, Y. Formation of protein crystals (orthorhombic lysozyme) in quasi-microgravity environment obtained by superconducting magnet. J. Cryst. Growth 2004, 270, 184–191. [Google Scholar] [CrossRef]
- Wakayama, N.I.; Yin, D.C.; Tanimoto, Y.; Fujiwara, M.; Harata, K.; Wada, H. Protein crystal growth in low gravity provided by a new type of superconducting magnet. Mater. Proc. Magn. Fields 2005, 285–291. [Google Scholar]
- Lu, H.M.; Yin, D.C.; Li, H.S.; Geng, L.Q.; Zhang, C.Y.; Lu, Q.Q.; Guo, Y.Z.; Guo, W.H.; Shang, P.; Wakayama, N.I. A containerless levitation setup for liquid processing in a superconducting magnet. Rev. Sci. Instrum. 2008, 79, 093903. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.; Quiroz-Garcia, B.; Yokaichiya, F.; Stojanoff, V.; Rudolph, P. Protein crystal growth in gels and stationary magnetic fields. Cryst. Res. Technol. 2007, 42, 231–236. [Google Scholar] [CrossRef]
- Moreno, A.; Yokaichiya, F.; Dimasi, E.; Stojanoff, V. Growth and characterization of high-quality protein crystals for X-ray Crystallography. Ann. N. Y. Acad. Sci. 2009, 1161, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Gavira, J.A.; Garcia-Ruiz, J.M. Effects of a magnetic field on lysozyme crystal nucleation and growth in a diffusive environment. Cryst. Growth Des. 2009, 9, 2610–2615. [Google Scholar] [CrossRef]
- Surade, S.; Ochi, T.; Nietlispach, D.; Chirgadze, D.; Moreno, A. Investigations into protein crystallization in the presence of a strong magnetic field. Cryst. Growth Des. 2010, 10, 691–699. [Google Scholar] [CrossRef]
- Sazaki, G.; Durbin, S.D.; Miyashita, S.; Ujihara, T.; Nakajima, K.; Motokawa, M. Magnetic damping of the temperature-driven convection in NaCl aqueous solution using a static and homogeneous field of 10 T. Jpn. J. Appl. Phys. 1999, 38, 842–844. [Google Scholar] [CrossRef]
- Yanagiya, S.-I.; Sazaki, G.; Durbin, S.D.; Miyashita, S.; Nakajima, K.; Komatsu, H.; Watanabe, K.; Motokawa, M. Effects of a magnetic field on the growth rate of tetragonal lysozyme crystals. J. Cryst. Growth 2000, 208, 645–650. [Google Scholar] [CrossRef]
- Maki, S.; Oda, Y.; Ataka, M. High-quality crystallization of lysozyme by magneto-Archimedes levitation in a superconducting magnet. J. Cryst. Growth 2004, 261, 557–565. [Google Scholar] [CrossRef]
- Poodt, P.W.G.; Heijna, M.C.R.; Christianen, P.C.M.; van Enckevort, W.J.P.; de Grip, W.J.; Tsukamoto, K.; Maan, J.C.; Vlieg, E. Using gradient magnetic fields to suppress convection during crystal growth. Cryst. Growth Des. 2006, 6, 2275–2280. [Google Scholar] [CrossRef]
- Okada, H.; Hirota, N.; Matsumoto, S.; Wada, H.; Kiyohara, M.; Ode, T.; Tanokura, M.; Nakamura, A.; Ohtsuka, J. Development of a protein crystal formation system with a superconducting magnet. IEEE Trans. Appl. Supercond. 2013, 23, 3700104. [Google Scholar] [CrossRef]
- Wang, Y.; Yin, D.C.; Liu, Y.M.; Shi, J.Z.; Lu, H.M.; Shi, Z.H.; Qian, A.R.; Shang, P. Design of shared instruments to utilize simulated gravities generated by a large-gradient, high-field superconducting magnet. Rev. Sci. Instrum. 2011, 82, 034705. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.Y.; Xie, C.; Wang, J.K. Effects of low magnetic field on batch crystallisation of glycine. Mater. Res. Innov. 2009, 13, 112–115. [Google Scholar] [CrossRef]
- Yin, D.C.; Inatomi, Y.; Kuribayashi, K. Study of lysozyme crystal growth under a strong magnetic field using a Mach–Zehnder interferometer. J. Cryst. Growth 2001, 226, 534–542. [Google Scholar] [CrossRef]
- Yin, D.C.; Inatomi, Y.; Wakayama, N.; Huang, W.; Kuribayashi, K. An investigation of magnetic field effects on the dissolution of lysozyme crystal and related phenomena. Acta Cryst. D 2002, 58, 2024–2030. [Google Scholar] [CrossRef]
- Zhong, C.; Wakayama, N.I. Effect of a high magnetic field on the viscosity of an aqueous solution of protein. J. Cryst. Growth 2001, 226, 327–332. [Google Scholar] [CrossRef]
- Zhong, C.; Wang, L.; Wakayama, N.I. Effect of a high magnetic field on protein crystal growth-magnetic field induced order in aqueous protein solutions. J. Cryst. Growth 2001, 233, 561–566. [Google Scholar] [CrossRef]
- Ramachandran, N.; Leslie, F.W. Using magnetic fields to control convection during protein crystallization-analysis and validation studies. J. Cryst. Growth 2005, 274, 297–306. [Google Scholar] [CrossRef]
- Nakamura, A.; Ohtsuka, J.; Kashiwagi, T.; Numoto, N.; Hirota, N.; Ode, T.; Okada, H.; Nagata, K.; Kiyohara, M.; Suzuki, E.-I. In-situ and real-time growth observation of high-quality protein crystals under quasi-microgravity on earth. Sci. Rep. 2016, 6, 22127. [Google Scholar] [CrossRef] [PubMed]





| Magnets | Flux Density (T) (Accessible Range) | Features | References |
|---|---|---|---|
| Electromagnets | 0–2.4 (0–2.0) | Low cost; continuous function in the range of hours | [6] |
| Permanent magnets | 0.1–1.25 (0.1–4) | Negligible cost; prolonged durability | [7] |
| Hybrid magnets (resistive magnets) | 33–45 (11–45) | Large power consumption; continuous function in the range of hours | [8,9] |
| Superconducting magnets | 4–16.1 (4–27) | Low running cost; sustainable working state for years | [10,11] |
| Magnets | Intensity (T) | Manufacturer | Types of Protein | References |
|---|---|---|---|---|
| Liquid-helium-free superconducting magnet | 11 | Institute for Materials Research, Tohoku University | Lysozyme, horse spleen ferritin | [22,23,24] |
| Cryogen-free superconducting magnet | 8 | Sumitomo Heavy Industry | Lysozyme microcrystals | [25] |
| Cryogen-free superconducting magnet | 12 | OXFORD Instruments, UK (Abingdon, UK) | Lysozyme | [26] |
| Low-temperature superconducting magnet | 5 | Japan Magnet Technology, Inc. (Kobe, Japan) | l-alanine | [10] |
| Liquid-helium-free superconducting magnet | 10 | Japan Magnet Technology, Inc. | Human estrogenic 17 β-hydroxysteroid dehydrogenase, lysozyme snake muscle fructose-1,6-bisphosphatase, bovine adenosine deaminase | [27,28,29,30,31,32,33,34,35] |
| Low-temperature superconducting magnet | 16.12 | Japan Superconductor Technology, Inc. (Hyogo, Japan) | Lysozyme, light-harvesting complex 2, protein K, concanavalin, HSP90N thaumatin, catalase, trichosanthin | [11,36,37,38,39] |
| Laboratory-size superconducting magnet | 15 | Japan Superconductor Technology, Inc. | Lysozyme, acylphosphatase, nucleoside diphosphate kinase, ST0811, monomeric sarcosine oxidase, flap endonuclease 1 | [40,41,42,43] |
| Protein | Resolution (Å) (In the Control) | Resolution (Å) (In a Magnetic Field) | Best Resolution (Å) (In the PDB Database) | Magnetic Field Intensity (T) | Types of Diffractometers | References |
|---|---|---|---|---|---|---|
| Lysozyme | 1.3 | 1.13 | 0.65 | 10 | Rigaku AFC5 four-circle diffractometer | [27] |
| Bovine adenosine deaminase crystals | 2.5 | 2.0 | 1.52 | 10 | Rigaku rotating-anode generator | [31] |
| Lysozyme | 1.33 | 1.13 | 0.65 | 10 | BL18B at the Photon Factory, Tsukuba, Japan | [35] |
| Fru-1,6-Pase crystals | 3.15 | 2.9 | 6 (upper) | Rigaku rotating-anode generator | [29] | |
| 2.9 | 2.1 | 10 (middle) | ||||
| 6 | 3.9 (lower) | |||||
| Lysozyme | 1.20 | 0.95 | 0.65 | 12 | Macromolecular Crystallography Beamline (BL17U1) at the SSRF | [36] |
| Protease K | 1.14 | 0.95 | 0.98 | |||
| Trichosanthin | 1.07 | 1.12 | 1.6 | |||
| Concanavalin A | 1.78 | 1.23 | 0.94 | |||
| Thaumatin | 2.70 | 1.35 | 0.94 | |||
| Catalase | 4.64 | 2.28 | 0.88 | |||
| Heat shock protein90N | 2.89 | 1.61 | 1.2 | |||
| ST0811 | 1.59 | 1.10 | 2.0 | 8–11 | Synchrotron beamlines (SPring-8 or Photon Hyogo, Japan) | [40] |
| Nucleoside diphosphate kinase | 2.61 | 2.16 | 1.25 | |||
| Flap endonuclease 1 | 1.90 | 1.85 | 1.88 | |||
| Pyrococcus horikoshii OT3 | 1.70 | 1.50 | 1.2 | |||
| Monomeric sarcosine oxidase | 2.70 | 1.95 | 1.6 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, E.-K.; Zhang, C.-Y.; He, J.; Yin, D.-C. An Overview of Hardware for Protein Crystallization in a Magnetic Field. Int. J. Mol. Sci. 2016, 17, 1906. https://doi.org/10.3390/ijms17111906
Yan E-K, Zhang C-Y, He J, Yin D-C. An Overview of Hardware for Protein Crystallization in a Magnetic Field. International Journal of Molecular Sciences. 2016; 17(11):1906. https://doi.org/10.3390/ijms17111906
Chicago/Turabian StyleYan, Er-Kai, Chen-Yan Zhang, Jin He, and Da-Chuan Yin. 2016. "An Overview of Hardware for Protein Crystallization in a Magnetic Field" International Journal of Molecular Sciences 17, no. 11: 1906. https://doi.org/10.3390/ijms17111906






