Systematic Analysis of Protein Interaction Network Associated with Azoospermia
Abstract
:1. Introduction
2. Results and Discussion
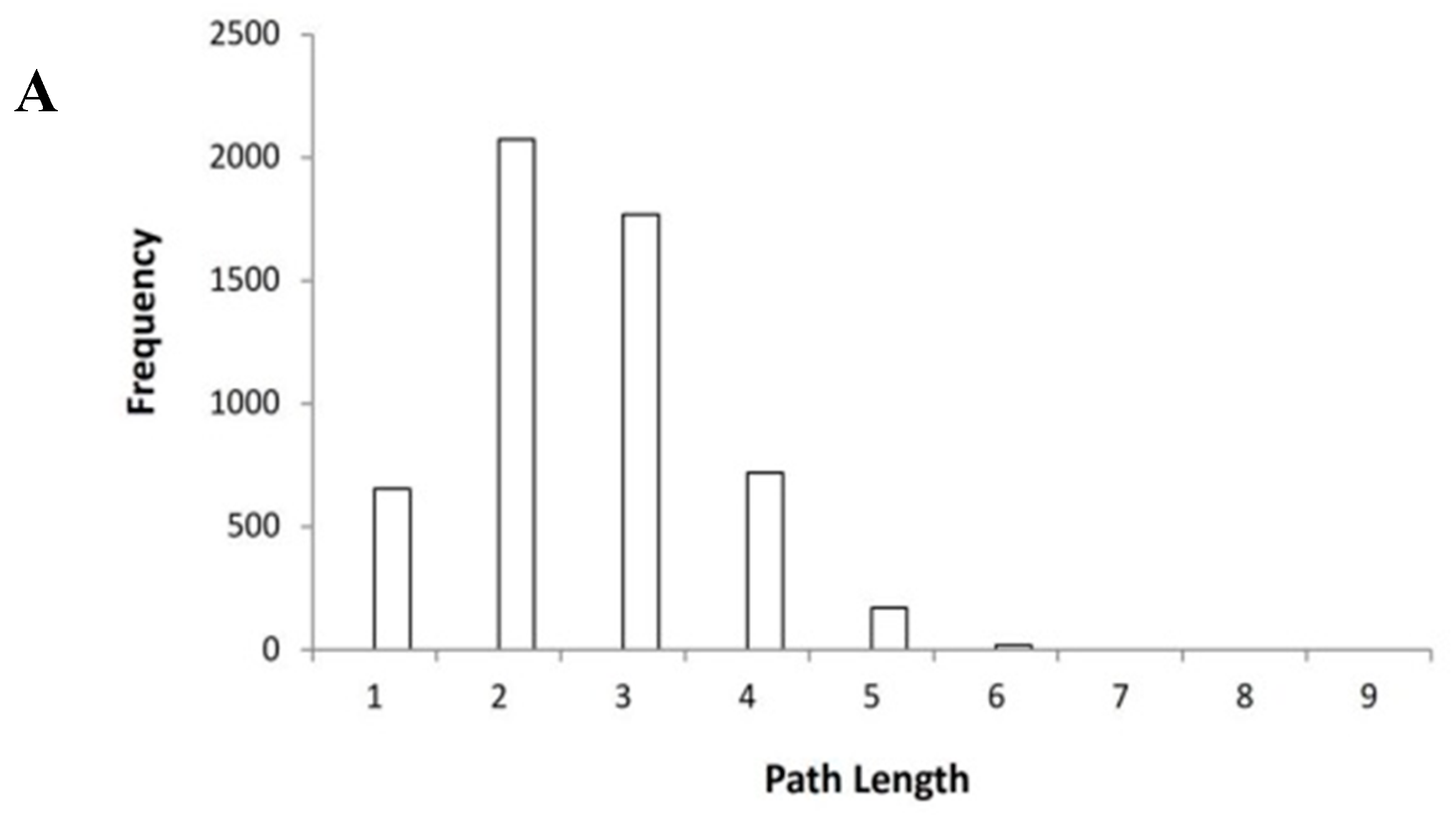
2.1. Construction and Parameters of Protein-Protein Interaction Protein-Protein Interaction (PPI Network)
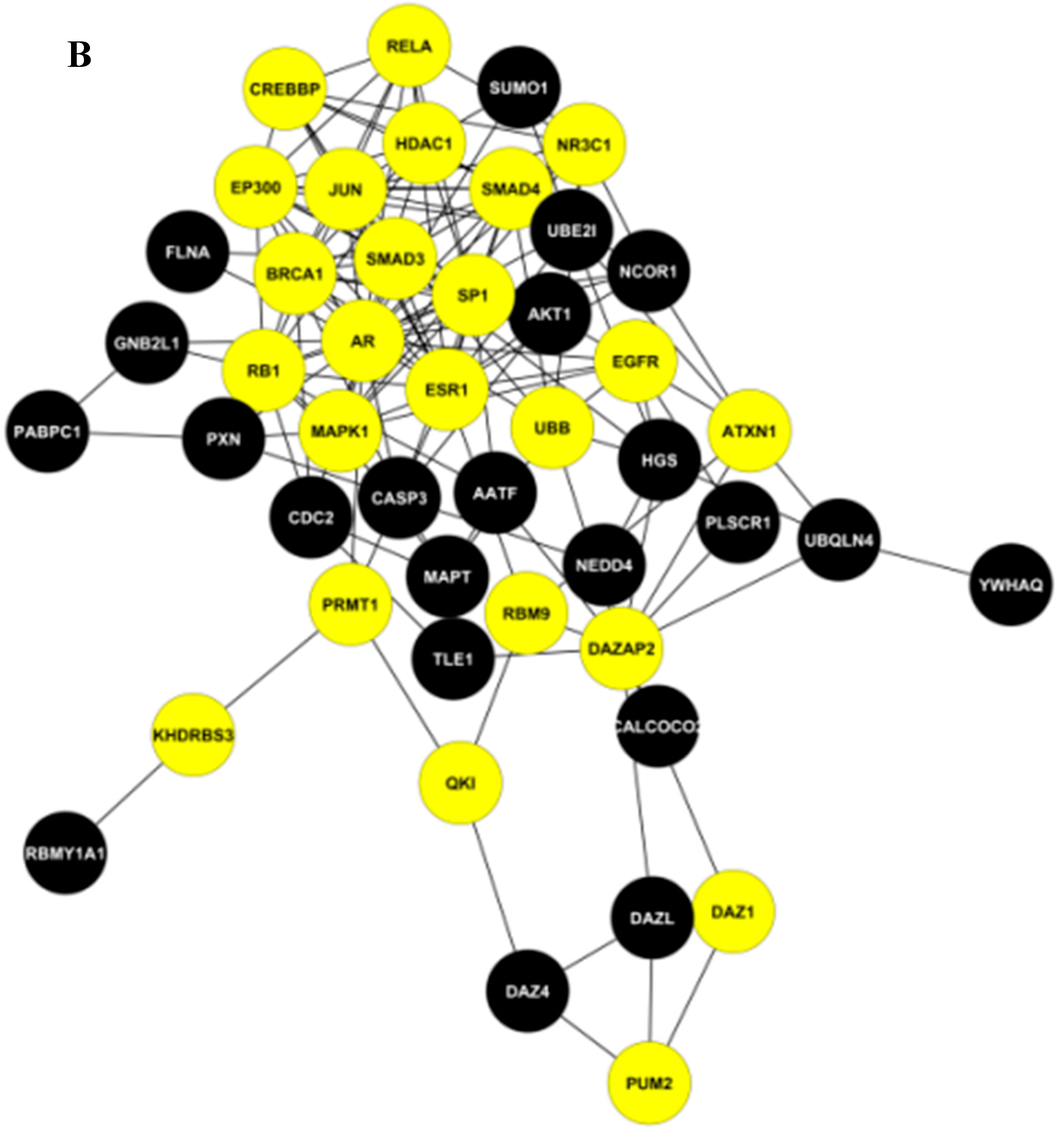
2.2. The Key Nodes of the PPI Network
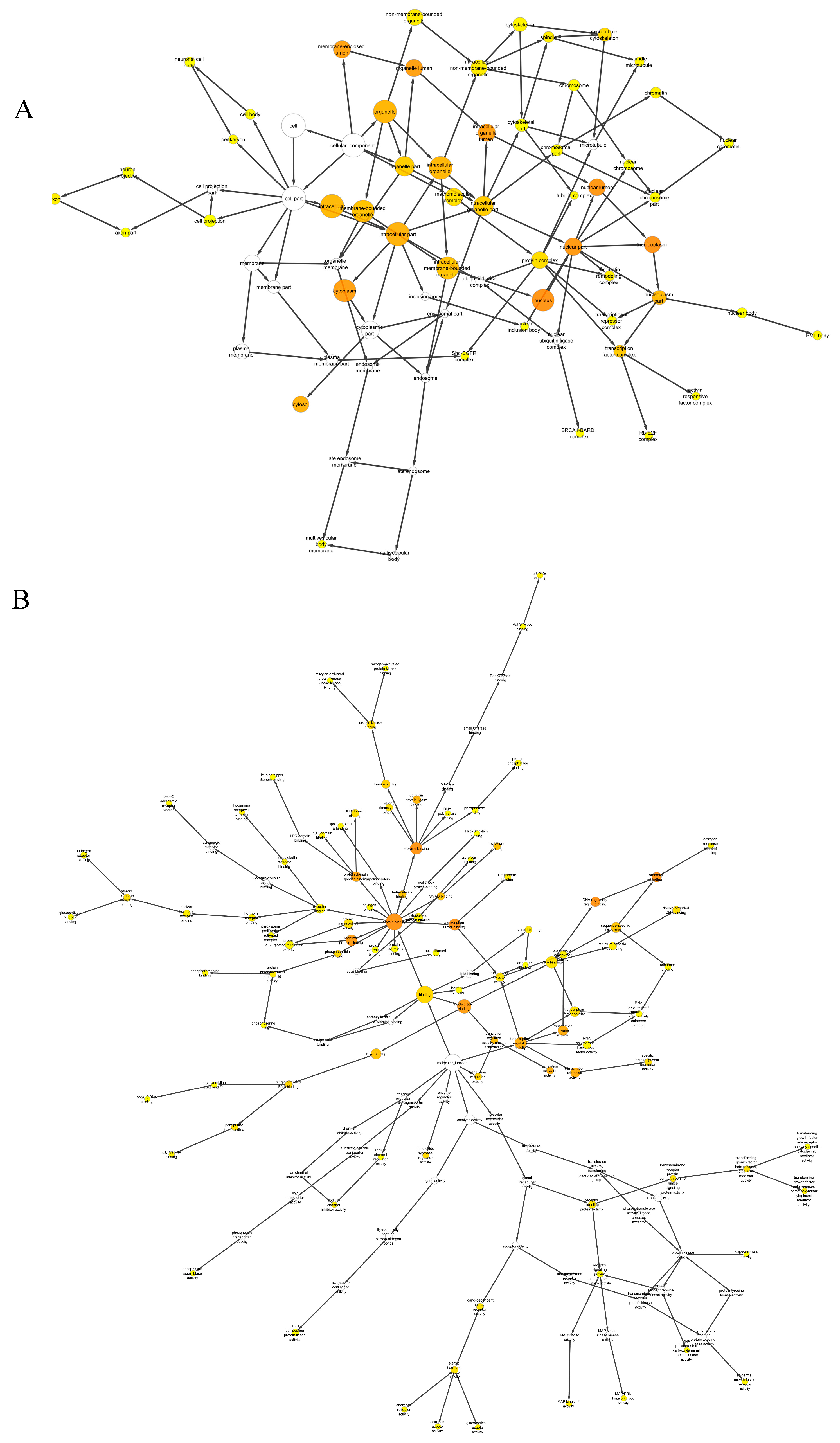
2.3. Gene Ontology (GO) Enrichment Analysis
2.4. DAVID (Database for Visualization, Annotation, and Integrated Discovery) Analysis
3. Materials and Methods
3.1. Selecting Associated Proteins with Azoospermia
3.2. Construction of the PPI Network
3.3. Topology and Parameter Analysis of PPI Network
3.4. Construction of a Small PPI Network with Important Nodes
3.5. GO Enrichment Analysis
3.6. DAVID v6.7 Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AR | Androgen Receptor |
| BC | Betweenness Centrality |
| CC | Closeness Centrality |
| DAZAP2 | Deleted in azoospermia associated protein 2 |
| ESR1 | Estrogen Receptor |
| GNRH | Gonadotropin-releasing hormone |
| HPRD | Human Protein Reference Database |
| ICSI | intracytoplasmic sperm injection |
| NOA | Non-obstructive azoospermia |
| OMIM | Online Mendelian Inheritance in Man |
| PPI | protein–protein interaction |
| SSC | spermatogonial stem cell |
References
- Sharlip, I.D.; Jarow, J.P.; Belker, A.M.; Lipshultz, L.I.; Sigman, M.; Thomas, A.J.; Schlegel, P.N.; Howards, S.S.; Nehra, A.; Damewood, M.D. Best practice policies for male infertility. Fertil. Steril. 2002, 77, 873–882. [Google Scholar] [CrossRef]
- Rengaraj, D.; Kwon, W.S.; Pang, M.G. Effects of motor vehicle exhaust on male reproductive function and associated proteins. J. Proteome Res. 2015, 14, 22–37. [Google Scholar] [CrossRef] [PubMed]
- Sabetian, S.; Shamsir, M.S. Identification of putative drug targets for human sperm-egg interaction defect using protein network approach. BMC Syst. Biol. 2015, 9. [Google Scholar] [CrossRef] [PubMed]
- Cocuzza, M.; Alvarenga, C.; Pagani, R. The epidemiology and etiology of azoospermia. Clinics 2013, 68, 15–26. [Google Scholar] [CrossRef]
- Ishikawa, T. Surgical recovery of sperm in non-obstructive azoospermia. Asian J. Androl. 2012, 14, 109–115. [Google Scholar] [CrossRef] [PubMed]
- De Croo, I.; van der Elst, J.; Everaert, K.; de Sutter, P.; Dhont, M. Fertilization, pregnancy and embryo implantation rates after icsi in cases of obstructive and non-obstructive azoospermia. Hum. Reprod. 2000, 15, 1383–1388. [Google Scholar] [CrossRef] [PubMed]
- Isidori, A.M.; Pozza, C.; Gianfrilli, D.; Isidori, A. Medical treatment to improve sperm quality. Reprod. Biomed. Online 2006, 12, 704–714. [Google Scholar] [CrossRef]
- Mengual, L.; Oriola, J.; Ascaso, C.; Ballescà, J.L.; Oliva, R. An increased cag repeat length in the androgen receptor gene in azoospermic icsi candidates. J. Androl. 2003, 24, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Küpker, W.; Schwinger, E.; Hiort, O.; Ludwig, M.; Nikolettos, N.; Schlegel, P.N.; Diedrich, K. Genetics of male subfertility: Consequences for the clinical work-up. Hum. Reprod. 1999, 14, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Massart, A.; Lissens, W.; Tournaye, H.; Stouffs, K. Genetic causes of spermatogenic failure. Asian J. Androl. 2012, 14, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Mancini, M.; Carmignani, L.; Gazzano, G.; Sagone, P.; Gadda, F.; Bosari, S.; Rocco, F.; Colpi, G. High prevalence of testicular cancer in azoospermic men without spermatogenesis. Hum. Reprod. 2007, 22, 1042–1046. [Google Scholar] [CrossRef] [PubMed]
- Rengaraj, D.; Kwon, W.S.; Pang, M.G. Bioinformatics annotation of human Y chromosome-encoded protein pathways and interactions. J. Proteome Res. 2015, 14, 3503–3518. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-Y.; Zou, X. Advances and challenges in protein-ligand docking. Int. J. Mol. Sci. 2010, 11, 3016–3034. [Google Scholar] [CrossRef] [PubMed]
- Barabási, A.-L.; Oltvai, Z.N. Network biology: Understanding the cell’s functional organization. Nat. Rev. Genet. 2004, 5, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Flórez, A.; Park, D.; Bhak, J.; Kim, B.-C.; Kuchinsky, A.; Morris, J.; Espinosa, J.; Muskus, C. Protein network prediction and topological analysis in leishmania major as a tool for drug target selection. BMC Bioinform. 2010, 11. [Google Scholar] [CrossRef]
- Albert, R. Scale-free networks in cell biology. J. Cell Sci. 2005, 118, 4947–4957. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.; Kinoshita, K.; Nakamura, H. Hub promiscuity in protein-protein interaction networks. Int. J. Mol. Sci. 2010, 11, 1930–1943. [Google Scholar] [CrossRef] [PubMed]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Johnson, A.; Alberts, B. Molecular Biology of The Cell; Garland Science: New York, NY, USA, 2002. [Google Scholar]
- Mitra, S.K.; Hanson, D.A.; Schlaepfer, D.D. Focal adhesion kinase: In command and control of cell motility. Nat. Rev. Mol. Cell Biol. 2005, 6, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, J.C.; Dempsey, P.J.; Reddy, S.; Bouton, A.H.; Coffey, R.J.; Hanks, S.K. Crk-associated substrate p130Cas interacts with nephrocystin and both proteins localize to cell–cell contacts of polarized epithelial cells. Exp. Cell Res. 2000, 256, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.-T.; Chiou, Y.-Y.; Wang, E.; Lin, H.-K.; Lee, S.-P.; Lu, H.-Y.; Wang, C.-K.L.; Tang, M.-J.; Li, H. Targeted disruption of Nphp1 causes male infertility due to defects in the later steps of sperm morphogenesis in mice. Hum. Mol. Genet. 2008, 17, 3368–3379. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.S.; Wolfe, A.; He, L.; Radovick, S.; Wondisford, F.E. CREB binding protein (CBP) activation is required for luteinizing hormone beta expression and normal fertility in mice. Mol. Cell. Biol. 2012, 32, 2349–2358. [Google Scholar] [CrossRef] [PubMed]
- Almog, T.; Naor, Z. The role of mitogen activated protein kinase (MAPK) in sperm functions. Mol. Cell. Endocrinol. 2010, 314, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.E.; Gwost, I.; Oatley, M.J.; Oatley, J.M. Retinoblastoma protein (RB1) controls fate determination in stem cells and progenitors of the mouse male germline. Biol. Reprod. 2013, 89. [Google Scholar] [CrossRef] [PubMed]
- Ferlin, A.; Arredi, B.; Foresta, C. Genetic causes of male infertility. Reprod. Toxicol. 2006, 22, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.I.; Yano, M.; Chiba, K.; Honda, M.; Kitahara, S. Cag repeat length in the androgen receptor gene is enhanced in patients with idiopathic azoospermia. Urology 1999, 54, 1078–1081. [Google Scholar] [CrossRef]
- Tsui, S.; Dai, T.; Roettger, S.; Schempp, W.; Salido, E.C.; Yen, P.H. Identification of two novel proteins that interact with germ-cell-specific RNA-binding proteins DAZ and DAZL1. Genomics 2000, 65, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Lukas, J.; Mazna, P.; Valenta, T.; Doubravska, L.; Pospichalova, V.; Vojtechova, M.; Fafilek, B.; Ivanek, R.; Plachy, J.; Novak, J. DAZAP2 modulates transcription driven by the Wnt effector TCF-4. Nucleic Acids Res. 2009, 37, 3007–3020. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.Q.; Hu, J.P.; Qu, Q.; Li, J.; Ren, W.; Zhang, J.M.; Zhong, Y.; Hu, W.X. The effects of promoter methylation on downregulation of DAZAP2 in multiple myeloma cell lines. PLoS ONE 2012, 7, e40475. [Google Scholar] [CrossRef] [PubMed]
- Moore, F.L.; Jaruzelska, J.; Dorfman, D.M.; Reijo-Pera, R.A. Identification of a novel gene, DZIP (DAZ-interacting protein), that encodes a protein that interacts with DAZ (deleted in azoospermia) and is expressed in embryonic stem cells and germ cells. Genomics 2004, 83, 834–843. [Google Scholar] [CrossRef] [PubMed]
- Lucas, T.F.; Pimenta, M.T.; Pisolato, R.; Lazari, M.F.M.; Porto, C.S. 17β-estradiol signaling and regulation of sertoli cell function. Spermatogenesis 2011, 1, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Sasagawa, I.; Itoh, K.; Ashida, J.; Muroya, K.; Ogata, T. Estrogen receptor α gene polymorphism is associated with idiopathic azoospermia. Fertil. Steril. 2002, 78, 1341–1343. [Google Scholar] [CrossRef]
- Sato, Y.; Jinam, T.; Iwamoto, T.; Yamauchi, A.; Imoto, I.; Inoue, I.; Tajima, A. Replication study and meta-analysis of human nonobstructive azoospermia in japanese populations 1. Biol. Reprod. 2013, 88. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Si, Y.; Slaymaker, S.; Li, J.; Zheng, H.; Young, D.L.; Aslanian, A.; Saunders, L.; Verdin, E.; Charo, I.F. Zmynd15 encodes a histone deacetylase-dependent transcriptional repressor essential for spermiogenesis and male fertility. J. Biol. Chem. 2010, 285, 31418–31426. [Google Scholar] [CrossRef] [PubMed]
- Batruch, I.; Smith, C.R.; Mullen, B.J.; Grober, E.; Lo, K.C.; Diamandis, E.P.; Jarvi, K.A. Analysis of seminal plasma from patients with non-obstructive azoospermia and identification of candidate biomarkers of male infertility. J. Proteome Res. 2012, 11, 1503–1511. [Google Scholar] [CrossRef] [PubMed]
- Mowat, N.; Edwards, C.; Fisher, R.; McNeilly, A.; Green, J.; Dawson, A. Hypothalamic-pituitary-gonadal function in men with cirrhosis of the liver. Gut 1976, 17, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Jequier, A.M. Male Infertility: A Clinical Guide; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Eisenberg, M.L.; Betts, P.; Herder, D.; Lamb, D.J.; Lipshultz, L.I. Increased risk of cancer among azoospermic men. Fertil. Steril. 2013, 100, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Hamosh, A.; Scott, A.F.; Amberger, J.S.; Bocchini, C.A.; McKusick, V.A. Online Mendelian inheritance in Man (OMIM), a knowledgebase of human genes and genetic disorders. Nucleic Acids Res. 2005, 33, D514–D517. [Google Scholar] [CrossRef] [PubMed]
- Peri, S.; Navarro, J.D.; Kristiansen, T.Z.; Amanchy, R.; Surendranath, V.; Muthusamy, B.; Gandhi, T.; Chandrika, K.; Deshpande, N.; Suresh, S. Human protein reference database as a discovery resource for proteomics. Nucleic Acids Res. 2004, 32, D497–D501. [Google Scholar] [CrossRef] [PubMed]
- Prasad, T.K.; Goel, R.; Kandasamy, K.; Keerthikumar, S.; Kumar, S.; Mathivanan, S.; Telikicherla, D.; Raju, R.; Shafreen, B.; Venugopal, A. Human protein reference database—2009 update. Nucleic Acids Res. 2009, 37, D767–D772. [Google Scholar] [CrossRef] [PubMed]
- Smoot, M.E.; Ono, K.; Ruscheinski, J.; Wang, P.-L.; Ideker, T. Cytoscape 2.8: New features for data integration and network visualization. Bioinformatics 2011, 27, 431–432. [Google Scholar] [CrossRef] [PubMed]
- Freeman, L.C. A set of measures of centrality based on betweenness. Sociometry 1977, 40, 35–41. [Google Scholar] [CrossRef]
- Brandes, U. A faster algorithm for betweenness centrality*. J. Math. Sociol. 2001, 25, 163–177. [Google Scholar] [CrossRef]
- Raman, K. Construction and analysis of protein-protein interaction networks. Autom. Exp. 2010, 2. [Google Scholar] [CrossRef] [PubMed]
- Maere, S.; Heymans, K.; Kuiper, M. Bingo: A cytoscape plugin to assess overrepresentation of gene ontology categories in biological networks. Bioinformatics 2005, 21, 3448–3449. [Google Scholar] [CrossRef] [PubMed]
- Mohamed-Hussein, Z.A.; Harun, S. Theoretical biology and medical modelling. Theor. Biol. Med. Model. 2009, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Bindea, G.; Mlecnik, B.; Hackl, H.; Charoentong, P.; Tosolini, M.; Kirilovsky, A.; Fridman, W.H.; Pages, F.; Trajanoski, Z.; Galon, J. Cluego: A cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 2009, 25, 1091–1093. [Google Scholar] [CrossRef] [PubMed]
- Dennis, G., Jr.; Sherman, B.T.; Hosack, D.A.; Yang, J.; Gao, W.; Lane, H.C.; Lempicki, R.A. David: Database for annotation, visualization, and integrated discovery. Genome Biol. 2003, 4. [Google Scholar] [CrossRef]
- Fard Jahromi, S.S.; Shamsir, M.S. Construction and analysis of the cell surface’s protein network for human sperm-egg interaction. ISRN Bioinform. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Gene Name |
|---|---|
| (Hub + Large BC) nodes | AR, DAZAP2, ESR1 |
| Hub nodes | PRMT1, ATXN1, UBB, KHDRBS3, DAZ1, RBM9, PUM2, QKI, EGFR |
| Large BC nodes | RB1, NR3C1, SMAD3, EP300, JUN, HDAC1, SMAD4, MAPK1, SP1, CREBBP, BCRA1, RELA |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabetian, S.; Shamsir, M.S. Systematic Analysis of Protein Interaction Network Associated with Azoospermia. Int. J. Mol. Sci. 2016, 17, 1857. https://doi.org/10.3390/ijms17111857
Sabetian S, Shamsir MS. Systematic Analysis of Protein Interaction Network Associated with Azoospermia. International Journal of Molecular Sciences. 2016; 17(11):1857. https://doi.org/10.3390/ijms17111857
Chicago/Turabian StyleSabetian, Soudabeh, and Mohd Shahir Shamsir. 2016. "Systematic Analysis of Protein Interaction Network Associated with Azoospermia" International Journal of Molecular Sciences 17, no. 11: 1857. https://doi.org/10.3390/ijms17111857





