Peculiarities of the Super-Folder GFP Folding in a Crowded Milieu
Abstract
:1. Introduction
2. Results
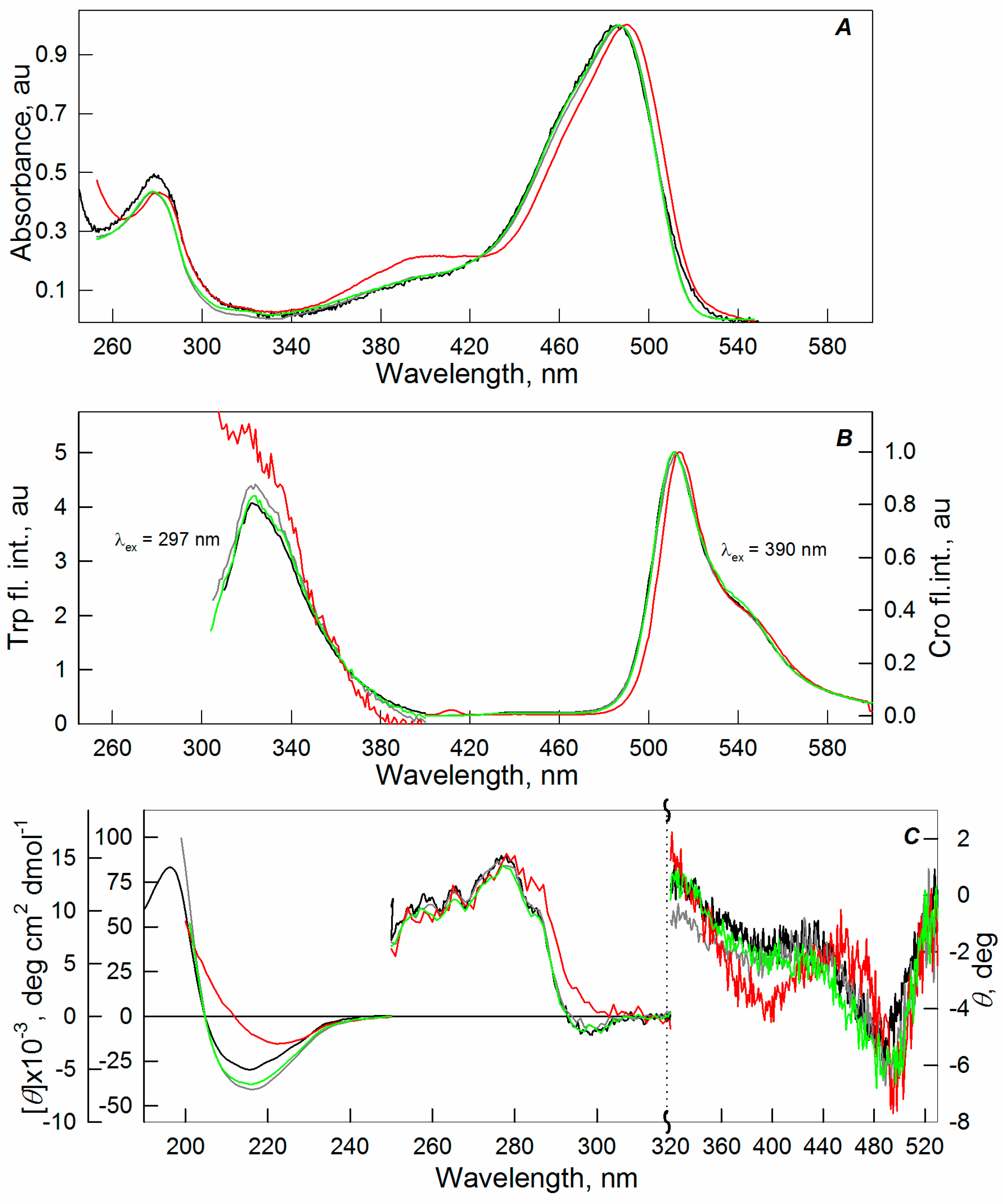
2.1. Spectral Properties of sfGFP in the Presence of Crowding Agents
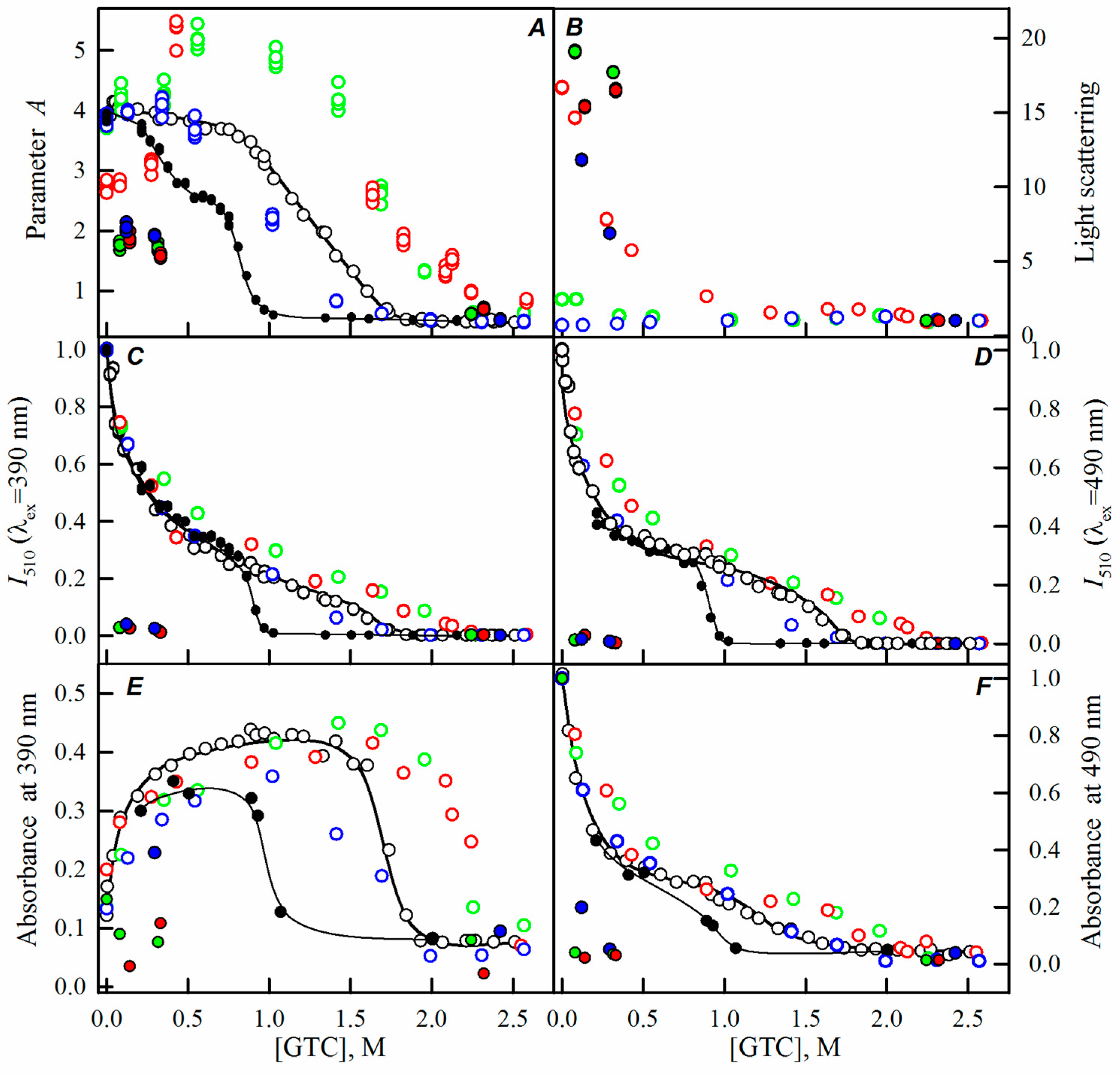
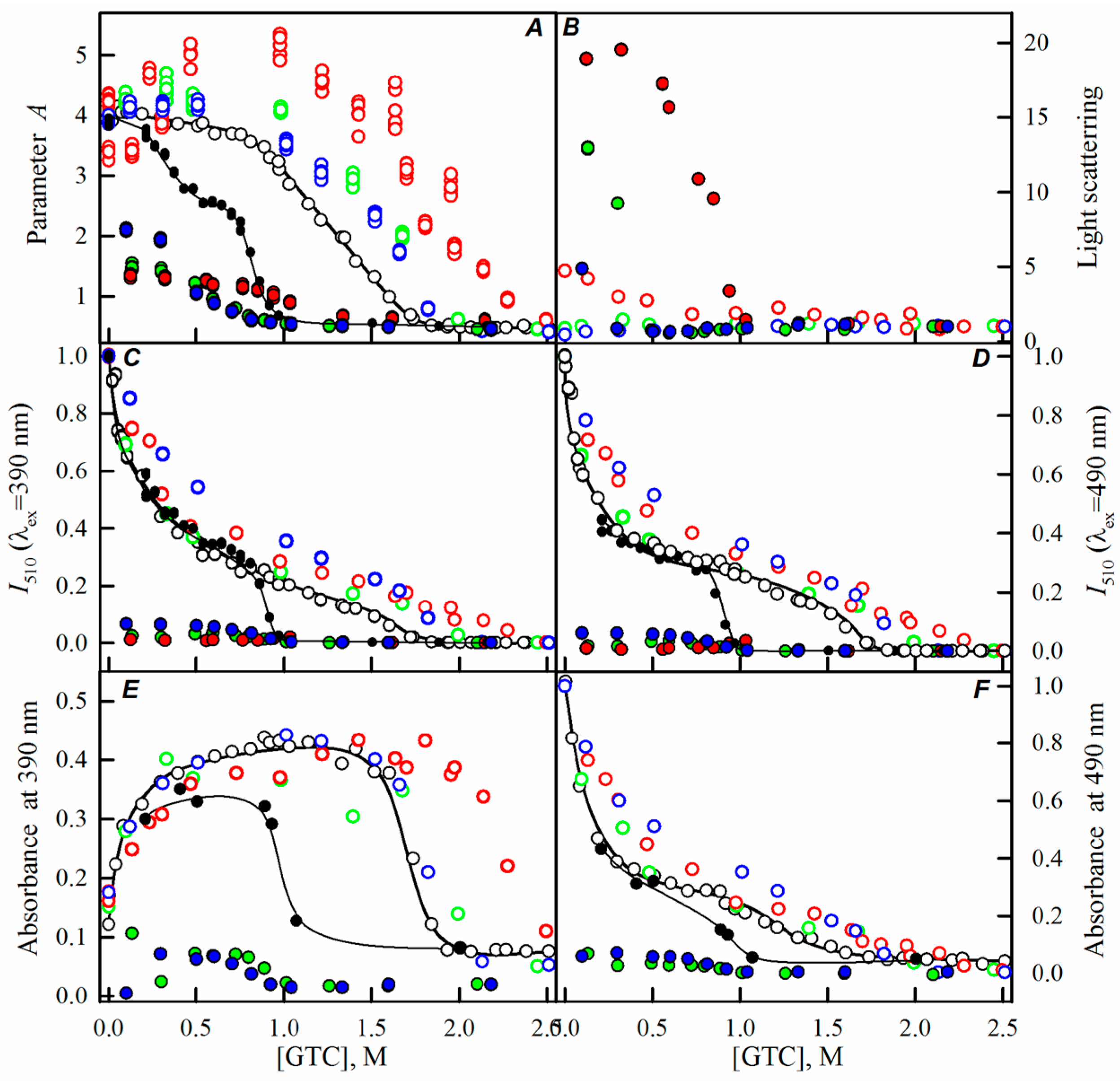
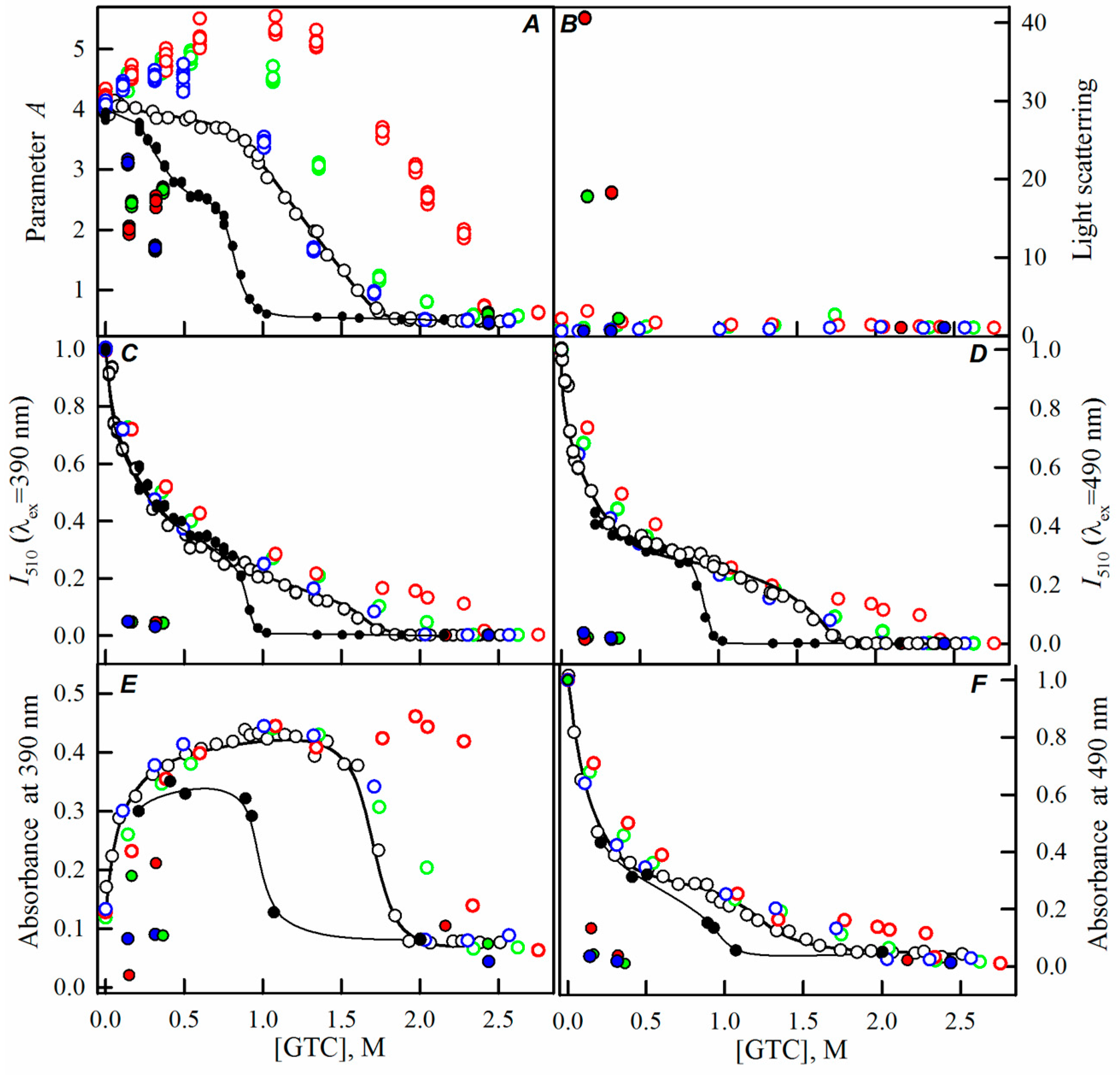
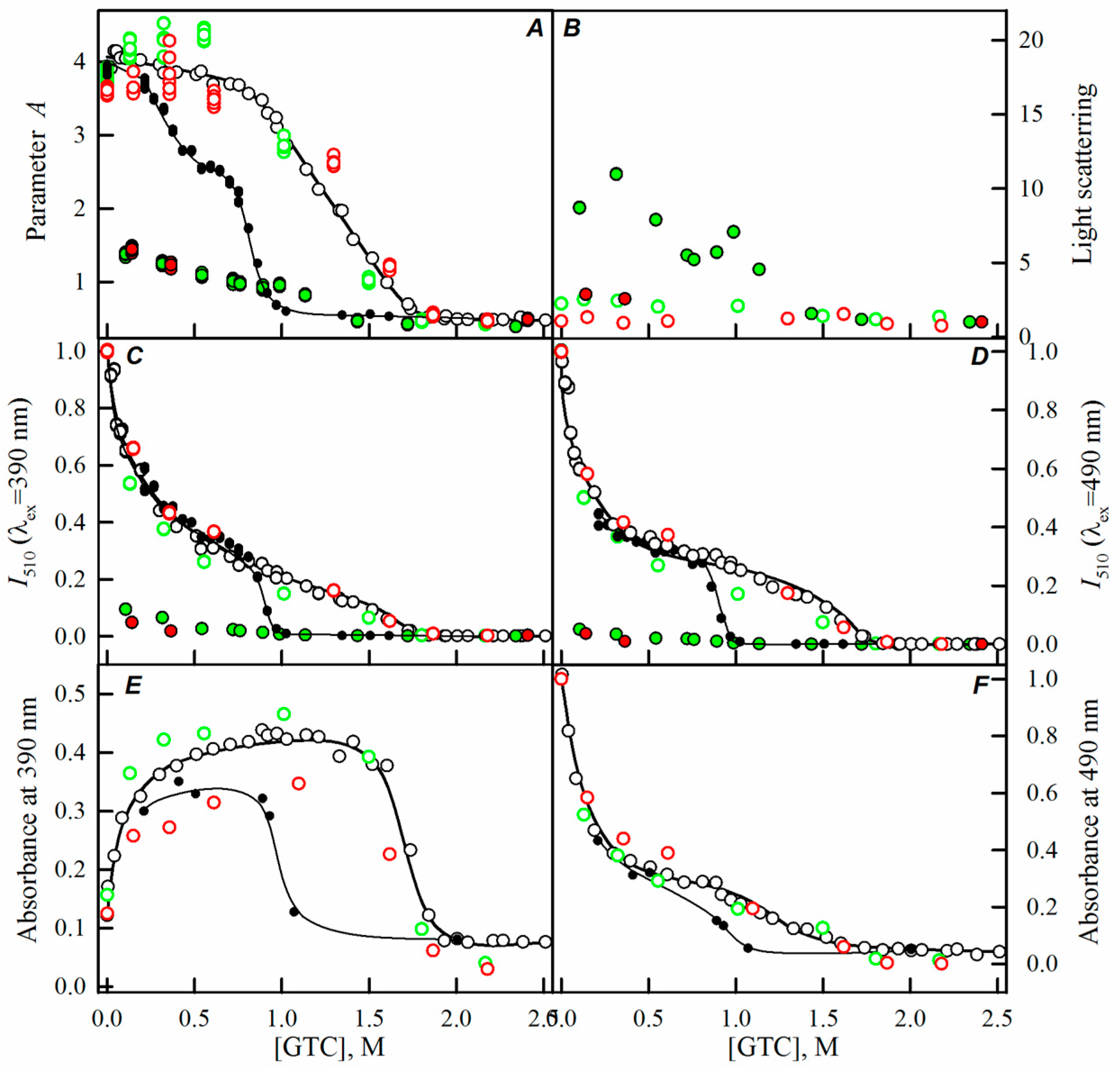
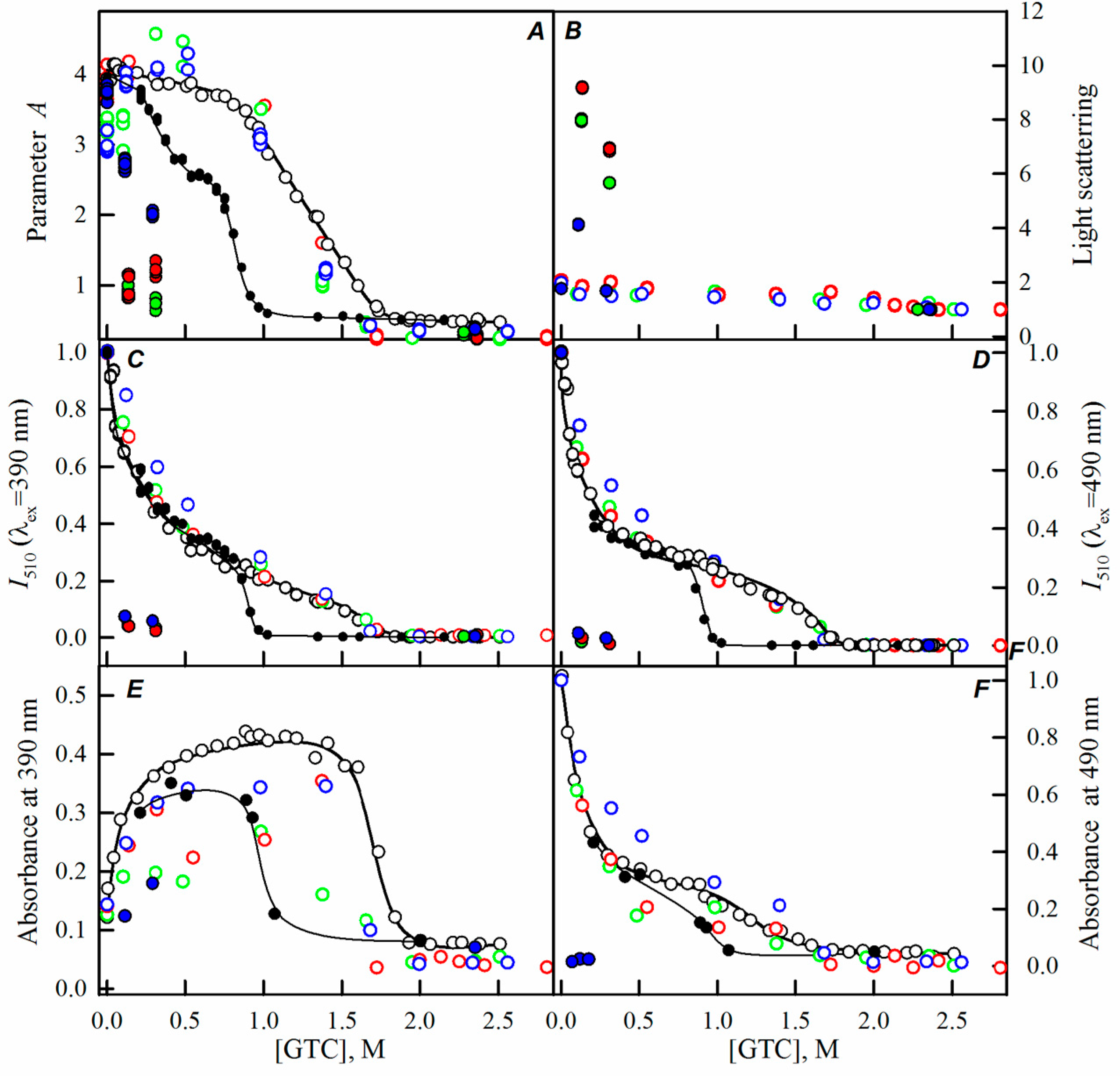
2.2. Unfolding-Refolding of sfGFP in the Crowded Milieu
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Gene Expression and Protein Purification
4.3. Spectrophotometric Experiments
4.4. Fluorescence Spectroscopy
4.5. Circular Dichroism Measurements
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chudakov, D.M.; Matz, M.V.; Lukyanov, S.; Lukyanov, K.A. Fluorescent proteins and their applications in imaging living cells and tissues. Physiol. Rev. 2010, 90, 1103–1163. [Google Scholar] [CrossRef] [PubMed]
- Frommer, W.B.; Davidson, M.W.; Campbell, R.E. Genetically encoded biosensors based on engineered fluorescent proteins. Chem. Soc. Rev. 2009, 38, 2833–2841. [Google Scholar] [CrossRef] [PubMed]
- Pakhomov, A.A.; Martynov, V.I. GFP family: Structural insights into spectral tuning. Chem. Biol. 2008, 15, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Shcherbakova, D.M.; Subach, O.M.; Verkhusha, V.V. Red fluorescent proteins: Advanced imaging applications and future design. Angew. Chem. Int. Ed. Engl. 2012, 51, 10724–10738. [Google Scholar] [CrossRef] [PubMed]
- Stepanenko, O.V.; Stepanenko, O.V.; Shcherbakova, D.M.; Kuznetsova, I.M.; Turoverov, K.K.; Verkhusha, V.V. Modern fluorescent proteins: From chromophore formation to novel intracellular applications. Biotechniques 2011, 51, 313–314. [Google Scholar] [CrossRef] [PubMed]
- Subach, F.V.; Piatkevich, K.D.; Verkhusha, V.V. Directed molecular evolution to design advanced red fluorescent proteins. Nat. Methods 2011, 8, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Piatkevich, K.D.; Lionnet, T.; Singer, R.H.; Verkhusha, V.V. Modern fluorescent proteins and imaging technologies to study gene expression, nuclear localization, and dynamics. Curr. Opin. Cell Biol. 2011, 23, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Tsien, R.Y. Nobel lecture: Constructing and exploiting the fluorescent protein paintbox. Integr. Biol. 2010, 2, 77–93. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, H.; Arai, M.; Kuwajima, K. Folding of green fluorescent protein and the cycle3 mutant. Biochemistry 2000, 39, 12025–12032. [Google Scholar] [CrossRef] [PubMed]
- Pedelacq, J.D.; Cabantous, S.; Tran, T.; Terwilliger, T.C.; Waldo, G.S. Engineering and characterization of a superfolder green fluorescent protein. Nat. Biotechnol. 2006, 24, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Stepanenko, O.V.; Verkhusha, V.V.; Shavlovsky, M.M.; Kuznetsova, I.M.; Uversky, V.N.; Turoverov, K.K. Understanding the role of Arg96 in structure and stability of green fluorescent protein. Proteins 2008, 73, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.T.; Blaser, G.; Jackson, S.E. The folding, stability and conformational dynamics of β-barrel fluorescent proteins. Chem. Soc. Rev. 2009, 38, 2951–2965. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wu, D.; Lu, Z.; Chen, W.; Hu, X.; Ding, Y. A novel method for high-level production of tev protease by superfolder GFP tag. J. Biomed. Biotechnol. 2009, 2009. [Google Scholar] [CrossRef] [PubMed]
- Al-Homsi, L.; Al-Okla, S.; Abbady, A.Q. Preparation of specific polyclonal antibody against the recombinant mutacin produced by sfGFP fusion protein technology. Open Microbiol. J. 2015, 9, 70–80. [Google Scholar] [PubMed]
- Stepanenko, O.V.; Stepanenko, O.V.; Kuznetsova, I.M.; Shcherbakova, D.M.; Verkhusha, V.V.; Turoverov, K.K. Distinct effects of guanidine thiocyanate on the structure of superfolder GFP. PLoS ONE 2012, 7, e48809. [Google Scholar] [CrossRef] [PubMed]
- Stepanenko, O.V.; Stepanenko, O.V.; Kuznetsova, I.M.; Verkhusha, V.V.; Turoverov, K.K. β-barrel scaffold of fluorescent proteins: Folding, stability and role in chromophore formation. Int. Rev. Cell Mol. Biol. 2013, 302, 221–278. [Google Scholar] [PubMed]
- Andrews, B.T.; Gosavi, S.; Finke, J.M.; Onuchic, J.N.; Jennings, P.A. The dual-basin landscape in GFP folding. Proc. Natl. Acad. Sci. USA 2008, 105, 12283–12288. [Google Scholar] [CrossRef] [PubMed]
- Andrews, B.T.; Roy, M.; Jennings, P.A. Chromophore packing leads to hysteresis in GFP. J. Mol. Biol. 2009, 392, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Andrews, B.T.; Schoenfish, A.R.; Roy, M.; Waldo, G.; Jennings, P.A. The rough energy landscape of superfolder GFP is linked to the chromophore. J. Mol. Biol. 2007, 373, 476–490. [Google Scholar] [CrossRef] [PubMed]
- Follenius-Wund, A.; Bourotte, M.; Schmitt, M.; Iyice, F.; Lami, H.; Bourguignon, J.J.; Haiech, J.; Pigault, C. Fluorescent derivatives of the GFP chromophore give a new insight into the GFP fluorescence process. Biophys. J. 2003, 85, 1839–1850. [Google Scholar] [CrossRef]
- Niwa, H.; Inouye, S.; Hirano, T.; Matsuno, T.; Kojima, S.; Kubota, M.; Ohashi, M.; Tsuji, F.I. Chemical nature of the light emitter of the aequorea green fluorescent protein. Proc. Natl. Acad. Sci. USA 1996, 93, 13617–13622. [Google Scholar] [CrossRef] [PubMed]
- Remington, S.J. Fluorescent proteins: Maturation, photochemistry and photophysics. Curr. Opin. Struct. Biol. 2006, 16, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Stepanenko, O.V.; Stepanenko, O.V.; Kuznetsova, I.M.; Verkhusha, V.V.; Turoverov, K.K. Sensitivity of superfolder GFP to ionic agents. PLoS ONE 2014, 9, e110750. [Google Scholar]
- Zimmerman, S.B.; Minton, A.P. Macromolecular crowding: Biochemical, biophysical, and physiological consequences. Annu. Rev. Biophys. Biomol. Struct. 1993, 22, 27–65. [Google Scholar] [CrossRef] [PubMed]
- Minton, A.P. The influence of macromolecular crowding and macromolecular confinement on biochemical reactions in physiological media. J. Biol. Chem. 2001, 276, 10577–10580. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, S.B.; Trach, S.O. Estimation of macromolecule concentrations and excluded volume effects for the cytoplasm of Escherichia coli. J. Mol. Biol. 1991, 222, 599–620. [Google Scholar] [CrossRef]
- Minton, A.P. Models for excluded volume interaction between an unfolded protein and rigid macromolecular cosolutes: Macromolecular crowding and protein stability revisited. Biophys. J. 2005, 88, 971–985. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, I.M.; Turoverov, K.K.; Uversky, V.N. What macromolecular crowding can do to a protein. Int. J. Mol. Sci. 2014, 15, 23090–23140. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, I.M.; Zaslavsky, B.Y.; Breydo, L.; Turoverov, K.K.; Uversky, V.N. Beyond the excluded volume effects: Mechanistic complexity of the crowded milieu. Molecules 2015, 20, 1377–1409. [Google Scholar] [CrossRef] [PubMed]
- Chebotareva, N.A.; Filippov, D.O.; Kurganov, B.I. Effect of crowding on several stages of protein aggregation in test systems in the presence of α-crystallin. Int. J. Biol. Macromol. 2015, 80, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Hatters, D.M.; Minton, A.P.; Howlett, G.J. Macromolecular crowding accelerates amyloid formation by human apolipoprotein C-II. J. Biol. Chem. 2002, 277, 7824–7830. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N.; Cooper, E.M.; Bower, K.S.; Li, J.; Fink, A.L. Accelerated α-synuclein fibrillation in crowded milieu. FEBS Lett. 2002, 515, 99–103. [Google Scholar] [CrossRef]
- Ferreira, L.A.; Povarova, O.I.; Stepanenko, O.V.; Sulatskaya, A.I.; Madeira, P.P.; Kuznetsova, I.M.; Turoverov, K.K.; Uversky, V.N.; Zaslavsky, B.Y. Effects of low urea concentrations on protein-water interactions. J. Biomol. Struct. Dyn. 2016. [Google Scholar] [CrossRef] [PubMed]
- Stepanenko, O.V.; Povarova, O.I.; Sulatskaya, A.I.; Ferreira, L.A.; Zaslavsky, B.Y.; Kuznetsova, I.M.; Turoverov, K.K.; Uversky, V.N. Protein unfolding in crowded milieu: What crowding can do to a protein undergoing unfolding? J. Biomol. Struct. Dyn. 2016. [Google Scholar] [CrossRef] [PubMed]
- Stepanenko, O.V.; Roginskii, D.O.; Stepanenko, O.V.; Kuznetsova, I.M.; Uversky, V.N.; Turoverov, K.K. Structure and stability of recombinant bovine odorant-binding protein: III. Peculiarities of the wild type bOBP unfolding in crowded milieu. PeerJ 2016, 4. [Google Scholar] [CrossRef] [PubMed]
- Costantini, L.M.; Fossati, M.; Francolini, M.; Snapp, E.L. Assessing the tendency of fluorescent proteins to oligomerize under physiologic conditions. Traffic 2012, 13, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Ormo, M.; Cubitt, A.B.; Kallio, K.; Gross, L.A.; Tsien, R.Y.; Remington, S.J. Crystal structure of the aequorea victoria green fluorescent protein. Science 1996, 273, 1392–1395. [Google Scholar] [CrossRef] [PubMed]
- Tsien, R.Y. The green fluorescent protein. Annu. Rev. Biochem. 1998, 67, 509–544. [Google Scholar] [CrossRef] [PubMed]
- Cheung, M.S.; Klimov, D.; Thirumalai, D. Molecular crowding enhances native state stability and refolding rates of globular proteins. Proc. Natl. Acad. Sci. USA 2005, 102, 4753–4758. [Google Scholar] [CrossRef] [PubMed]
- Minton, A.P. Macromolecular crowding and molecular recognition. J. Mol. Recognit. 1993, 6, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Tokuriki, N.; Kinjo, M.; Negi, S.; Hoshino, M.; Goto, Y.; Urabe, I.; Yomo, T. Protein folding by the effects of macromolecular crowding. Protein Sci. 2004, 13, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.L.; Wu, L.J.; Chen, J.; Liang, Y. Effects of macromolecular crowding on the structural stability of human α-lactalbumin. Acta Biochim. Biophys. Sin. 2012, 44, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Phillip, Y.; Schreiber, G. Formation of protein complexes in crowded environments—From in vitro to in vivo. FEBS Lett. 2013, 587, 1046–1052. [Google Scholar] [CrossRef] [PubMed]
- Venturoli, D.; Rippe, B. Ficoll and dextran vs. Globular proteins as probes for testing glomerular permselectivity: Effects of molecular size, shape, charge, and deformability. Am. J. Physiol. Ren. Physiol. 2005, 288, F605–F613. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N. What does it mean to be natively unfolded? Eur. J. Biochem. 2002, 269, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Di Donato, M.; van Wilderen, L.J.; van Stokkum, I.H.; Stuart, T.C.; Kennis, J.T.; Hellingwerf, K.J.; van Grondelle, R.; Groot, M.L. Proton transfer events in GFP. Phys. Chem. Chem. Phys. 2011, 13, 16295–16305. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Frontiera, R.R.; Tran, R.; Mathies, R.A. Mapping GFP structure evolution during proton transfer with femtosecond raman spectroscopy. Nature 2009, 462, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Kitano, H.; Ichikawa, K.; Ide, M.; Fukuda, M.; Mizuno, W. Fourier transform infrared study on the state of water sorbed to poly(ethylene glycol) films. Langmuir 2001, 17, 1889–1895. [Google Scholar] [CrossRef]
- Mishin, A.S.; Subach, F.V.; Yampolsky, I.V.; King, W.; Lukyanov, K.A.; Verkhusha, V.V. The first mutant of the aequorea victoria green fluorescent protein that forms a red chromophore. Biochemistry 2008, 47, 4666–4673. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Fonin, A.V.; Sulatskaya, A.I.; Kuznetsova, I.M.; Turoverov, K.K. Fluorescence of dyes in solutions with high absorbance. Inner filter effect correction. PLoS ONE 2014, 9, e103878. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, I.M.; Sulatskaya, A.I.; Povarova, O.I.; Turoverov, K.K. Reevaluation of ANS binding to human and bovine serum albumins: Key role of equilibrium microdialysis in ligand–receptor binding characterization. PLoS ONE 2012, 7, e40845. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, I.M.; Stepanenko, O.V.; Stepanenko, O.V.; Povarova, O.I.; Biktashev, A.G.; Verkhusha, V.V.; Shavlovsky, M.M.; Turoverov, K.K. The place of inactivated actin and its kinetic predecessor in actin folding-unfolding. Biochemistry 2002, 41, 13127–13132. [Google Scholar] [CrossRef] [PubMed]
- Turoverov, K.K.; Verkhusha, V.V.; Shavlovsky, M.M.; Biktashev, A.G.; Povarova, O.I.; Kuznetsova, I.M. Kinetics of actin unfolding induced by guanidine hydrochloride. Biochemistry 2002, 41, 1014–1019. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.A.; Madeira, P.P.; Breydo, L.; Reichardt, C.; Uversky, V.N.; Zaslavsky, B.Y. Role of solvent properties of aqueous media in macromolecular crowding effects. J. Biomol. Struct. Dyn. 2016, 34, 92–103. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stepanenko, O.V.; Stepanenko, O.V.; Kuznetsova, I.M.; Uversky, V.N.; Turoverov, K.K. Peculiarities of the Super-Folder GFP Folding in a Crowded Milieu. Int. J. Mol. Sci. 2016, 17, 1805. https://doi.org/10.3390/ijms17111805
Stepanenko OV, Stepanenko OV, Kuznetsova IM, Uversky VN, Turoverov KK. Peculiarities of the Super-Folder GFP Folding in a Crowded Milieu. International Journal of Molecular Sciences. 2016; 17(11):1805. https://doi.org/10.3390/ijms17111805
Chicago/Turabian StyleStepanenko, Olesya V., Olga V. Stepanenko, Irina M. Kuznetsova, Vladimir N. Uversky, and Konstantin K. Turoverov. 2016. "Peculiarities of the Super-Folder GFP Folding in a Crowded Milieu" International Journal of Molecular Sciences 17, no. 11: 1805. https://doi.org/10.3390/ijms17111805