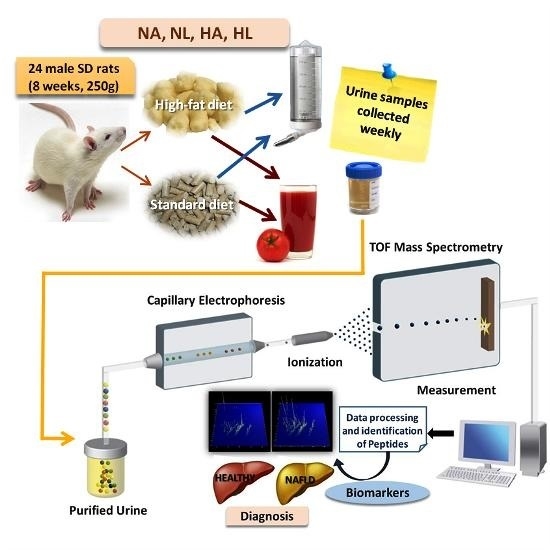
Tomato Juice Consumption Modifies the Urinary Peptide Profile in Sprague-Dawley Rats with Induced Hepatic Steatosis
Abstract
:1. Introduction
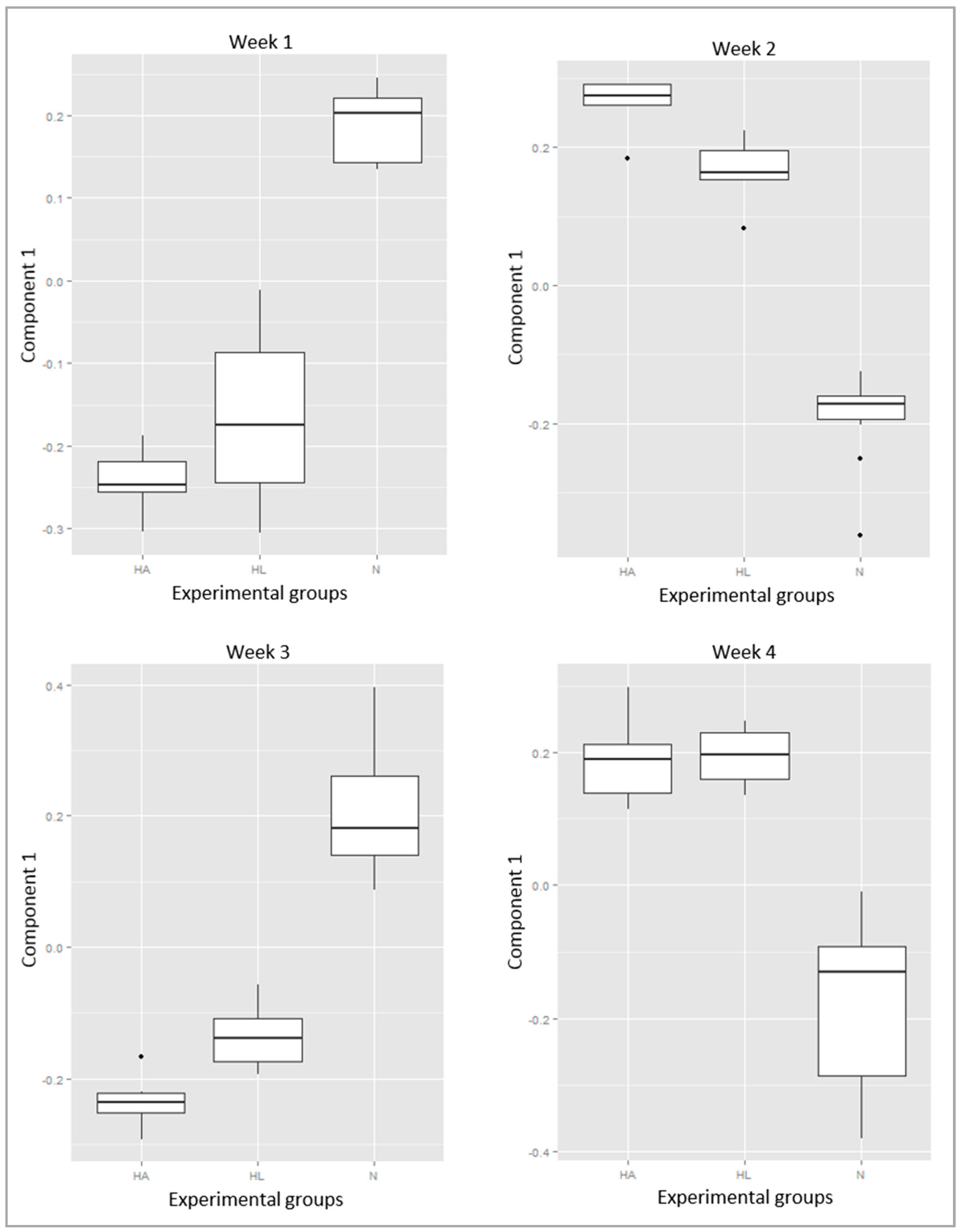
2. Results
3. Discussion
4. Materials and Methods
4.1. Tomato Juice
4.2. Animals and Experimental Design
4.3. NAFLD Confirmation
4.4. Sample Preparation
4.5. Protein Estimation
4.6. Capillary Electrophoresis Coupled to Mass Spectrometry (CE-MS) Analysis
4.7. CE-Data Processing
4.8. Statistical Analyses for Biochemical Parameters
4.9. Statistical Analysis for Biomarker Definition
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| Grouping | Weeks | Number of Components | Number of Peptides | Number of Combinations | Total |
|---|---|---|---|---|---|
| 16 groups (NAw1, NLw1, HAw1, HLw1, NAw2,…, HLw4) | na | 1–15 | 1–50 | 1 × 15 × 50 | 750 |
| 4 groups (NA, NL, HA, HL) | 4 | 1–3 | 1–50 | 4 × 3 × 50 | 600 |
| 3 groups (N, HA HL) | 4 | 1–2 | 1–50 | 4 × 2 × 50 | 400 |
| 1750 |
References
- Tessari, P.; Coracina, A.; Cosma, A.; Tiengo, A. Hepatic lipid metabolism and non-alcoholic fatty liver disease. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Koek, G.H.; Liedorp, P.R.; Bast, A. The role of oxidative stress in non-alcoholic steatohepatitis. Clin. Chim. Acta 2011, 412, 1297–1305. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Rector, R.S.; Thyfault, J.P.; Ibdah, J.A. Nonalcoholic fatty liver disease and mitochondrial dysfunction. World J. Gastroenterol. 2008, 14, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Musso, G.; Gambino, R.; Cassader, M. Recent insights into hepatic lipid metabolism in non-alcoholic fatty liver disease (NAFLD). Prog. Lipid Res. 2009, 48, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Day, C.P.; James, O.F. Steatohepatitis: A tale of two hits? Gastroenterology 1998, 114, 842–845. [Google Scholar] [CrossRef]
- Hernaez, R. Genetics of non-alcoholic fatty liver disease and associated metabolic disorders. Av. Diabetol. 2011, 27, 186–197. [Google Scholar] [CrossRef]
- Yilmaz, Y. Review article: Is non-alcoholic fatty liver disease a spectrum, or are steatosis and non-alcoholic steatohepatitis distinct conditions? Aliment. Pharmacol. Ther. 2012, 36, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Targher, G.; Arcaro, G. Non-alcoholic fatty liver disease and increased risk of cardiovascular disease. Atherosclerosis 2007, 191, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Fon Tacer, K.; Rozman, D. Nonalcoholic fatty liver disease: Focus on lipoprotein and lipid deregulation. J. Lipids 2011, 2011, 783976. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.P.M.; de Jesús, R.P.; Freire, T.O.; Oliveira, C.P.; Castro Lyra, A.; Lyra, L.G.C. Possible molecular mechanisms soy-mediated in preventing and treating nonalcoholic fatty liver disease. Nutr. Hosp. 2012, 27, 991–998. [Google Scholar] [PubMed]
- Sharma, A.K.; Bharti, S.; Bhatia, J.; Nepal, S.; Malik, S.; Ray, R.; Kumari, S.; Arya, D.S. Sesamol alleviates diet-induced cardiometabolic syndrome in rats via up-regulating PPARγ, PPARα and e-NOS. J. Nutr. Biochem. 2012, 23, 1482–1489. [Google Scholar] [CrossRef] [PubMed]
- Kontogianni, M.D.; Tileli, N.; Margariti, A.; Georgoulis, M.; Deutsch, M.; Tiniakos, D.; Fragopoulou, E.; Zafiropoulou, R.; Manios, Y.; Papatheodoridis, G. Adherence to the Mediterranean diet is associated with the severity of non-alcoholic fatty liver disease. Clin. Nutr. 2014, 33, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.C.; Itsiopoulos, C.; Thodis, T.; Ward, G.; Trost, N.; Hofferberth, S.; O’Dea, K.; Desmond, P.V.; Johnson, N.A.; Wilson, A.M. The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J. Hepatol. 2013, 59, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Jesús Periago, M.; García-Alonso, J.; Jacob, K.; Belén Olivares, A.; José Bernal, M.; Dolores Iniesta, M.; Martínez, C.; Ros, G. Bioactive compounds, folates and antioxidant properties of tomatoes (Lycopersicum esculentum) during vine ripening. Int. J. Food Sci. Nutr. 2009, 60, 694–708. [Google Scholar] [CrossRef] [PubMed]
- García-Valverde, V.; Navarro-González, I.; García-Alonso, J.; Periago, M. Antioxidant bioactive compounds in selected industrial processing and fresh consumption tomato cultivars. Food Bioprocess Technol. 2013, 6, 391–102. [Google Scholar] [CrossRef]
- Visioli, F.; Riso, P.; Grande, S.; Galli, C.; Porrini, M. Protective activity of tomato products on in vivo markers of lipid oxidation. Eur. J. Nutr. 2003, 42, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Jacob, K.; Periago, M.J.; Böhm, V.; Berruezo, G.R. Influence of lycopene and vitamin C from tomato juice on biomarkers of oxidative stress and inflammation. Br. J. Nutr. 2008, 99, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.M.; Lai, C.H.; Chang, C.Y.; Fan, C.T.; Chen, C.T.; Wu, C.H. Characterizing the lipid-lowering effects and antioxidant mechanisms of tomato paste. Biosci. Biotechnol. Biochem. 2008, 72, 677–685. [Google Scholar] [CrossRef] [PubMed]
- García-Alonso, F.J.; Jorge-Vidal, V.; Ros, G.; Periago, M.J. Effect of consumption of tomato juice enriched with n-3 polyunsaturated fatty acids on the lipid profile, antioxidant biomarker status, and cardiovascular disease risk in healthy women. Eur. J. Nutr. 2012, 51, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Vitaglione, P.; Morisco, F.; Caporaso, N.; Fogliano, V. Dietary antioxidant compounds and liver health. Crit. Rev. Food Sci. Nutr. 2004, 44, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Bernal, C.; Martín-Pozuelo, G.; Lozano, A.B.; Sevilla, Á.; García-Alonso, J.; Canovas, M.; Periago, M.J. Lipid biomarkers and metabolic effects of lycopene from tomato juice on liver of rats with induced hepatic steatosis. J. Nutr. Biochem. 2013, 24, 1870–1881. [Google Scholar] [CrossRef] [PubMed]
- Melendez-Martinez, A.J.; Nascimento, A.F.; Wang, Y.; Liu, C.; Mao, Y.; Wang, X.D. Effect of tomato extract supplementation against high-fat diet-induced hepatic lesions. Hepatobiliary Surg. Nutr. 2013, 2, 198–208. [Google Scholar] [PubMed]
- Ip, B.C.; Liu, C.; Lichtenstein, A.H.; von Lintig, J.; Wang, X.D. Lycopene and apo-10’-lycopenoic acid have differential mechanisms of protection against hepatic steatosis in β-carotene-9’,10’-oxygenase knockout male mice. J. Nutr. 2015, 145, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Martín-Pozuelo, G.; Navarro-González, I.; González-Barrio, R.; Santaella, M.; García-Alonso, J.; Hidalgo, N.; Gómez-Gallego, C.; Ros, G.; Periago, M.J. The effect of tomato juice supplementation on biomarkers and gene expression related to lipid metabolism in rats with induced hepatic steatosis. Eur. J. Nutr. 2014, 54, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ausman, L.M.; Greenberg, A.S.; Russell, R.M.; Wang, X.D. Dietary lycopene and tomato extract supplementations inhibit nonalcoholic steatohepatitis-promoted hepatocarcinogenesis in rats. Int. J. Cancer 2010, 126, 1788–1796. [Google Scholar] [CrossRef] [PubMed]
- Bahcecioglu, I.H.; Kuzu, N.; Metin, K.; Ozercan, I.H.; Ustündag, B.; Sahin, K.; Kucuk, O. Lycopene prevents development of steatohepatitis in experimental non-alcoholic steatohepatitis model induced by high-fat diet. Vet. Med. Int. 2010, 2010. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.V.; Lu, X.; Standley, M.; Pattee, P.; Neelima, G.; Girisesh, G.; Dakshinamurthy, K.V.; Roberts, C.T.; Nagalla, S.R. Proteomic identification of urinary biomarkers of diabetic nephropathy. Diabetes Care 2007, 30, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Albalat, A.; Bitsika, V.; Zurbig, P.; Siwy, J.; Mullen, W. High resolution proteome/peptidome analysis of body fluids by capillary electrophoresis coupled with MS. Methods Mol. Biol. 2013, 984, 153–165. [Google Scholar] [PubMed]
- Kolch, W.; Neusüß, C.; Pelzing, M.; Mischak, H. Capillary electrophoresis-mass spectrometry as a powerful tool in clinical diagnosis and biomarker discovery. Mass Spectrom. Rev. 2005, 24, 959–977. [Google Scholar] [CrossRef] [PubMed]
- Dakna, M.; He, Z.; Yu, W.C.; Mischak, H.; Kolch, W. Technical, bioinformatical and statistical aspects of liquid chromatography-mass spectrometry (LC-MS) and capillary electrophoresis-mass spectrometry (CE-MS) based clinical proteomics: A critical assessment. J. Chromatogr. B 2009, 877, 1250–1258. [Google Scholar] [CrossRef] [PubMed]
- Albalat, A.; Mischak, H.; Mullen, W. Urine proteomics in clinical applications: Technologies, principal considerations and clinical implementation. Prilozi 2011, 32, 13–44. [Google Scholar] [PubMed]
- Bedossa, P. Current histological classification of NAFLD: Strength and limitations. Hepatol. Int. 2013, 7, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Baranova, A.; Ziegler, K.; Del Giacco, L.; Schlauch, K.; Born, T.L.; Elariny, H.; Gorreta, F.; VanMeter, A.; Younoszai, A.; et al. A genomic and proteomic study of the spectrum of non-alcoholic fatty liver disease. Hepatology 2005, 42, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Bell, L.N.; Theodorakis, J.L.; Vuppalanchi, R.; Saxena, R.; Bemis, K.G.; Wang, M.; Chalasani, N. Serum proteomics and biomarker discovery across the spectrum of non-alcoholic fatty liver disease. Hepatology 2010, 51, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Brunt, E.M.; Janney, C.G.; Di Bisceglie, A.M.; Neuschwander-Tetri, B.A.; Bacon, B.R. Nonalcoholic steatohepatitis: A proposal for grading and staging the histological lesions. Am. J. Gastroenterol. 1999, 94, 2467–2474. [Google Scholar] [CrossRef] [PubMed]
- Mullen, W.; Gonzalez, J.; Siwy, J.; Franke, J.; Sattar, N.; Mullan, A.; Roberts, S.; Delles, C.; Mischak, H.; Albalat, A. A pilot study on the effect of short-term consumption of a polyphenol rich drink on biomarkers of coronary artery disease defined by urinary proteomics. J. Agric. Food Chem. 2011, 59, 12850–12857. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, E.; Dhawan, A. Noninvasive biomarkers in non-alcoholic fatty liver disease: Current status and a glimpse of the future. World J. Gastroenterol. 2014, 20, 10851–10863. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Kohan, A.B.; Lo, C.M.; Liu, M.; Howles, P.; Tso, P. Apolipoprotein A-IV: A protein intimately involved in metabolism. J. Lipid Res. 2015, 56, 1403–1418. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.W.; Dillon, J.; Miller, M. Proteomic and genomic studies of non-alcoholic fatty liver disease-clues in the pathogenesis. World J. Gastroenterol. 2014, 20, 8325–8340. [Google Scholar] [CrossRef] [PubMed]
- Ried, K.; Fakler, P. Protective effect of lycopene on serum cholesterol and blood pressure: Meta-analyses of intervention trials. Maturitas 2011, 68, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Mordente, A.L.; Guantario, B.; Meucci, E.; Silvestrini, A.; Lombardi, E.; Martorana, G.E; Giardina, B.; Bohm, V. Lycopene and cardiovascular diseases: An update. Curr. Med. Chem. 2011, 18, 1146–1163. [Google Scholar] [CrossRef] [PubMed]
- Navarro-González, I.; Pérez-Sánchez, H.; Martín-Pozuelo, G.; García-Alonso, J.; Periago, M.J. The inhibitory effects on bioactive compounds of tomato juice binding to hepatic HMGCR: In vivo study and molecular modelling. PLoS ONE 2014, 9, e83968. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, V.N.; Leite-Mór, M.M.B.; Kondo, M.; Martins, J.R.; Nader, H.; Lanzoni, V.P.; Parise, E.R. Serum laminin, type IV collagen and hyaluronan as fibrosis markers in non-alcoholic fatty liver disease. Braz. J. Med. Biol. Res. 2005, 38, 747–753. [Google Scholar] [CrossRef] [Green Version]
- Yoneda, M.; Mawatari, H.; Fujita, K.; Yonemitsu, K.; Kato, S.; Takahashi, H.; Kirikoshi, H.; Inamori, M.; Nozaki, Y.; Abe, Y.; et al. Type IV collagen 7s domain is an independent clinical marker of the severity of fibrosis in patients with non-alcoholic steatohepatitis before the cirrhotic stage. J. Gastroenterol. 2007, 42, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, M.; Shima, T.; Oya, H.; Mitsumoto, Y.; Mizuno, C.; Isoda, S.; Kuramoto, M.; Taniguchi, M.; Noda, M.; Sakai, K.; et al. Classification of patients with non-alcoholic fatty liver disease using rapid immunoassay of serum type IV collagen compared with liver histology and other fibrosis markers. Hepatol. Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Matafora, V.; Zagato, L.; Ferrandi, M.; Molinari, I.; Zerbini, G.; Casamassima, N.; Lanzani, C.; Carpini, S.D.; Trepiccione, F.; Manunta, P.; et al. Quantitative proteomics reveals novel therapeutic and diagnostic markers in hypertension. BBA Clin. 2014, 2, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Verwaerde, P.; Sénard, J.M.; Galinier, M.; Rouge, P.; Massabuau, P.; Galitzky, J.; Berlan, M.; Lafontan, M.; Montastruc, J.L. Changes in short-term variability of blood pressure and heart rate during the development of obesity-associated hypertension in high-fat fed dogs. J. Hypertens. 1999, 17, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Gajendragadkar, P.R.; Hubsch, A.; Mäki-Petäjä, K.M.; Serg, M.; Wilkinson, I.B.; Cheriyan, J. Effects of oral lycopene supplementation on vascular function in patients with cardiovascular disease and healthy volunteers: A randomized controlled trial. PLoS ONE 2014, 9, e99070. [Google Scholar] [CrossRef] [PubMed]
- Hammes, H.P.; Du, X.; Edelstein, D.; Taguchi, T.; Matsumura, T.; Ju, Q.; Lin, J.; Bierhaus, A.; Nawroth, P.; Hannak, D.; et al. Benfotiamine blocks three major pathways of hyperglycemic damage and prevents experimental diabetic retinopathy. Nat. Med. 2003, 9, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Boren, J.; Ramos-Montoya, A.; Bosch, K.S.; Vreeling, H.; Jonker, A.; Centelles, J.J.; Cascante, M.; Frederiks, W.M. In situ localization of transketolase activity in epithelial cells of different rat tissues and subcellularly in liver parenchymal cells. J. Histochem. Cytochem. 2006, 54, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Vitali, A. Proline-rich peptides: Multifunctional bioactive molecules as new potential therapeutic drugs. Curr. Protein Pept. Sci. 2015, 16, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Fukai, T.; Ushio-Fukai, M. Superoxide dismutases: Role in redox signaling, vascular function and diseases. Antioxid. Redox Signal. 2011, 15, 1583–1606. [Google Scholar] [CrossRef] [PubMed]
- Siwy, J.; Zoja, C.; Klein, J.; Benigni, A.; Mullen, W.; Mayer, B.; Mischak, H.; Jankowski, J.; Stevens, R.; Vlahou, A.; et al. Evaluation of the Zucker diabetic fatty (ZDF) rat as a model for human disease based on urinary peptidomic profiles. PLoS ONE 2012, 7, e51334. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Fu, G.; Lei, T. Two urinary peptides associated closely with type 2 diabetes mellitus. PLoS ONE 2015, 10, e0122950. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A.; Dubouchaud, H.; Brown, M.; Sicurello, J.P.; Butz, E. Role of mitochondrial lactate dehydrogenase and lactate oxidation in the intracellular lactate shuttle. Proc, Natl. Acad. Sci. USA 1999, 96, 1129–1134. [Google Scholar] [CrossRef]
- Theodorescu, D.; Fliser, D.; Wittke, S.; Mischak, H.; Krebs, R.; Walden, M.; Ross, M.; Eltze, E.; Bettendorf, O.; Wulfing, C.; et al. Pilot study of capillary electrophoresis coupled to mass spectrometry as a tool to define potential prostate cancer biomarkers in urine. Electrophoresis 2005, 26, 2797–2808. [Google Scholar] [CrossRef] [PubMed]
- Good, D.M.; Thongboonkerd, V.; Novak, J.; Bascands, J.L.; Schanstra, J.P.; Coon, J.J.; Dominiczak, A.; Mischak, H. Body fluid proteomics for biomarkers discovery: Lessons from the past hold the key to success in the future. J. Proteome Res. 2007, 6, 4549–4555. [Google Scholar] [CrossRef] [PubMed]
- Coon, J.J.; Zürbig, P.; Dakna, M.; Dominiczak, A.F.; Decramer, S.; Fliser, D.; Frommberger, M.; Golovko, I.; Good, D.M.; Herget-Rosenthal, S.; et al. CE-MS analysis of the human urinary proteome for biomarker discovery and disease diagnostics. Proteom. Clin. Appl. 2008, 2, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Mischak, H.; Espandiari, P.; Sadrieh, N.; Hanig, J. Profiling of rat urinary proteomic patterns associated with drug-induced nephrotoxicity using CE coupled with MS as a potential model for detection of drug-induced adverse effects. Proteom. Clin. Appl. 2009, 3, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Skotland, T.; Berge, V.; Sandvig, K.; Llorente, A. Exosomal proteins as prostate cancer biomarkers in urine: From mass spectrometry discovery to immunoassay-based validation. Eur. J. Pharm. Sci. 2016. [Google Scholar] [CrossRef] [PubMed]
- Charan, J.; Kantharia, N.D. How to calculate sample size in animal studies? J. Pharmacol. Pharmacother. 2013, 4, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Neuhoff, N.V.; Kaiser, T.; Wittke, S.; Krebs, R.; Pitt, A.; Burchard, A.; Sundmacher, A.; Schlegelberger, B.; Kolch, W.; Mischak, H. Mass spectrometry for the detection of differentially expressed proteins: A comparison of surface-enhanced laser desorption/ionization and capillary electrophoresis/mass spectrometry. Rapid Commun. Mass Spectrom. 2004, 18, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Lê Cao, K.A.; Boitard, S.; Besse, P. Sparse PLS discriminant analysis: Biologically relevant feature selection and graphical displays for multiclass problems. BMC Bioinform. 2011, 12, 253. [Google Scholar] [CrossRef] [PubMed]
- MixOmics: Omics Data Integration Project R Package Version 6.00. 2016. Available online: https://CRAN.R-project.org/package=mixOmics (accessed on 1 May 2016).
- R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria. Available online: http://www.R-project.org/ (accessed on 1 May 2016).



| Parameters | NA | NL | HA | HL |
|---|---|---|---|---|
| Total Cholesterol (mg/dL) | 99 ± 4.1 b | 81 ± 6.5 b | 167 ± 14 a | 162 ± 14 a |
| LDL-cholesterol (mg/dL) | 26 ± 1.4 b | 17 ± 2.4 b | 94 ± 11 a | 96 ± 8.2 a |
| HDL-cholesterol (mg/dL) | 54 ± 2.3 a | 44 ± 3.5 a,b | 40 ± 2.1 b | 49 ± 2.7 a,b |
| Triglycerides (mg/dL) | 80 ± 4.6 b | 78 ± 8.3 b | 121 ± 7.1 a | 98 ± 14 a,b |
| ALT (U/L) | 32 ± 1.9 b | 41 ± 2.9 b | 79 ± 11 a | 79 ± 12 a |
| AST (U/L) | 68 ± 7.1 b | 67 ± 4.9 b | 150 ± 7.6 a | 143 ± 20 a |
| Week | Peptide ID | Peptide Mass (Da) | CE Time (min) | Sequence Peptide | Protein Identity | Mean Rel ab N | Freq N | Mean Rel ab HA | Freq HA | Mean Rel ab HL | Freq HL | Fold Change HA | Fold Change HL |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 7035 | 1197.6 | 37.2 | DSYVGDEAQSK | Actin. α skeletal muscle | 482.2 | 91.7 | 195.8 | 16.7 | 0.0 | 0.0 | −2.46 | na |
| 1 | 10615 | 1433.7 | 39.2 | GEVGPpGPpGPAGEKG | Collagen α-1(I) chain precursor | 506.7 | 100.0 | 164.9 | 50.0 | 483.4 | 40.0 | −3.07 | −1.05 |
| 1 | 13476 | 1652.8 | 40.9 | GEpGEpGQTGPAGSRGPA | Collagen α-2(I) chain | 452.8 | 91.7 | 0.0 | 0.0 | 67.6 | 20.0 | na | −6.70 |
| 1 | 13640 | 1668.9 | 35.1 | WGKVNPDDVGGEALGR | Hemoglobin subunit beta-1 | 0.0 | 0.0 | 5343.6 | 100.0 | 3360.0 | 100.0 | na | na |
| 1 | 17766 | 2088.1 | 36.3 | AGRpGEVGPpGPpGPAGEKGSpG | Collagen α-1(I) chain precursor | 102.5 | 100.0 | 0.0 | 0.0 | 93.6 | 20.0 | na | −1.09 |
| 1 | 7744 | 1240.6 | 31.4 | AGYQRELNYK | α-1-inhibitor 3 precursor | 0.0 | 0.0 | 4339.0 | 100.0 | 2920.1 | 100.0 | na | na |
| 1 | 14576 | 1759.9 | 34.0 | SADTLWDIQKDLKDL | l-lactate dehydrogenase B chain | 615.4 | 91.7 | 67.1 | 66.7 | 66.0 | 60.0 | −9.17 | −9.32 |
| 1 | 13862 | 1691.9 | 35.2 | DGKTGPpGPAGQDGRPGp | Collagen α-1(I) chain | 336.8 | 100.0 | 89.1 | 16.7 | 250.8 | 60.0 | −3.78 | −1.34 |
| 1 | 11124 | 1470.8 | 32.6 | FSHIDVSPGSAQVK | Hemoglobin subunit α-1/2 | 39.0 | 8.3 | 1755.1 | 100.0 | 1770.7 | 100.0 | 45.04 | 45.44 |
| 1 | 10647 | 1435.7 | 31.1 | SLKGFSQQTQQKG | Seminal vesicle secretory protein 2 precursor | 838.1 | 75.0 | 2676.8 | 100.0 | 2623.0 | 80.0 | 3.19 | 3.13 |
| 1 | 13451 | 1649.8 | 41.3 | TDVTQQLNTLFQDK | Apolipoprotein A-IV | 1863.1 | 100.0 | 847.2 | 100.0 | 1080.5 | 100.0 | −2.20 | −1.72 |
| 1 | 11115 | 1469.7 | 39.3 | GSpGAPGApGHpGPpGP | Collagen α-1(III) chain | 271.6 | 91.7 | 25.4 | 16.7 | 0.0 | 0.0 | −10.70 | na |
| 2 | 10013 | 1388.7 | 39.2 | RpGEVGPpGPpGPAG | Collagen α-1(I) chain | 3710.4 | 100.0 | 892.4 | 80.0 | 1133.4 | 100.0 | −4.16 | −3.27 |
| 2 | 13862 | 1691.9 | 35.2 | DGKTGPpGPAGQDGRPGp | Collagen α-1(I) chain | 466.0 | 100.0 | 0.0 | 0.0 | 306.4 | 16.7 | na | −1.52 |
| 2 | 14941 | 1795.9 | 41.6 | GETGNKGEpGSAGAQGPpGP | Collagen α-2(I) chain | 146.7 | 100.0 | 0.0 | 0.0 | 14.9 | 16.7 | na | −9.88 |
| 2 | 8057 | 1260.7 | 38.4 | RpGEVGPpGPpGP | Collagen α-1(I) chain | 675.8 | 100.0 | 0.0 | 0.0 | 271.4 | 16.7 | na | −2.49 |
| 2 | 24835 | 3124.6 | 32.9 | DELVRDKPYGPKVSGGSFGEEASEEISSR | Seminal vesicle protein 2 precursor | 0.0 | 0.0 | 1421.0 | 100.0 | 947.8 | 100.0 | na | na |
| 2 | 17689 | 2081.0 | 26.9 | DGPpGRDGQpGHKGERGYpG | Collagen α-2(I) chain | 1593.7 | 100.0 | 615.3 | 80.0 | 829.7 | 100.0 | −2.59 | −1.92 |
| 2 | 11667 | 1511.7 | 39.3 | AGPpGPpGpPGSIGHpG | Procollagen. type IX. α 3 (predicted). isoform CRA a | 125.4 | 91.7 | 0.0 | 0.0 | 0.0 | 0.0 | na | na |
| 2 | 9863 | 1378.7 | 39.2 | ApGEDGRpGPpGPQ | Collagen α-1(II) chain | 825.0 | 100.0 | 705.3 | 20.0 | 281.0 | 83.3 | −1.17 | −2.94 |
| 2 | 10377 | 1414.7 | 38.7 | LYQAEAFIADFK | Contrapsin-like protease inhibitor 3 precursor | 324.1 | 100.0 | 75.4 | 40.0 | 0.0 | 0.0 | −4.30 | na |
| 2 | 13429 | 1647.8 | 40.5 | GApGPAGPAGERGEQGPAG | Collagen α-1(I) chain | 101.1 | 16.7 | 0.0 | 0.0 | 762.1 | 66.7 | na | 7.54 |
| 2 | 13543 | 1659.8 | 39.7 | GAPGAKGNVGppGEPGPpG | α 4 type V collagen | 156.0 | 100.0 | 307.6 | 20.0 | 260.1 | 100.0 | 1.97 | 1.67 |
| 2 | 15097 | 1811.9 | 42.1 | GGAGPpGPEGGKGPAGPpGPpG | Collagen α-1(III) chain | 188.6 | 66.7 | 535.0 | 100.0 | 0.0 | 0.0 | 2.84 | na |
| 2 | 7243 | 1210.6 | 38.0 | QLQEGPPEWK | Fibrinogen α chain precursor | 99.2 | 66.7 | 499.0 | 80.0 | 101.7 | 16.7 | 5.03 | 1.03 |
| 2 | 8984 | 1320.7 | 38.2 | GPpGENGKPGEpGP | Collagen α-1(III) chain | 861.3 | 100.0 | 798.5 | 80.0 | 1560.3 | 100.0 | −1.08 | 1.81 |
| 2 | 2712 | 933.5 | 47.2 | PGpAGPpGPVG | Collagen α-1(II) chain | 13.3 | 33.3 | 0.0 | 0.0 | 27.4 | 66.7 | na | 2.06 |
| 2 | 4970 | 1066.5 | 36.7 | GEDGRpGPpGP | Collagen α-1(II) chain | 408.7 | 75.0 | 984.6 | 100.0 | 377.9 | 33.3 | 2.41 | −1.08 |
| 2 | 9955 | 1384.7 | 32.7 | VDLADRLDLVEK | Extracellular superoxide dismutase (Cu-Zn) precursor | 415.5 | 66.7 | 2275.0 | 100.0 | 266.7 | 33.3 | 5.47 | −1.56 |
| 2 | 10240 | 1405.7 | 39.1 | GLpGPAGPpGEAGKpG | Collagen α-1(I) chain | 1005.1 | 91.7 | 1430.2 | 60.0 | 1830.2 | 100.0 | 1.42 | 1.82 |
| 2 | 11153 | 1472.7 | 49.2 | GPpGPSGNAGppGPpGP | Collagen α-1(I) chain | 0.0 | 0.0 | 1478.7 | 80.0 | 170.1 | 33.3 | na | na |
| 2 | 10124 | 1396.7 | 31.8 | SRLAPLAEGVQEK | Apolipoprotein A-IV | 132.4 | 66.7 | 378.2 | 80.0 | 254.3 | 16.7 | 2.86 | 1.92 |
| 2 | 17855 | 2096.1 | 35.8 | FQDKLGNINTYADDLQNK | Apolipoprotein A-IV precursor | 0.0 | 0.0 | 1731.4 | 100.0 | 642.7 | 100.0 | na | na |
| 2 | 9540 | 1354.6 | 48.8 | EpGTTGPpGTAGPQG | Collagen α-2(I) chain | 1212.4 | 100.0 | 382.3 | 40.0 | 697.7 | 50.0 | −3.17 | −1.74 |
| 2 | 12640 | 1584.8 | 34.0 | DGQPGAKGEpGDTGVKG | Collagen α-1(I) chain | 323.8 | 100.0 | 101.6 | 100.0 | 120.8 | 66.7 | −3.19 | −2.68 |
| 2 | 13476 | 1652.8 | 40.9 | GEpGEpGQTGPAGSRGPA | Collagen α-2(I) chain | 541.2 | 91.7 | 304.1 | 20.0 | 0.0 | 0.0 | −1.78 | na |
| 2 | 14976 | 1799.9 | 41.5 | PPTGPFVEPPDLFFLK | Proline-rich protein | 441.3 | 83.3 | 51010.5 | 100.0 | 14119.9 | 100.0 | 115.59 | 32.00 |
| 2 | 4768 | 1051.5 | 36.5 | TGVKGDAGPPGP | Collagen α-1(I) chain | 134.6 | 100.0 | 106.8 | 60.0 | 220.0 | 100.0 | −1.26 | 1.63 |
| 2 | 20632 | 2428.3 | 26.6 | DGILGRDTLPHEDQGKGRQLHS | Contrapsin-like protease inhibitor 1 precursor | 84.2 | 16.7 | 254.8 | 80.0 | 983.1 | 100.0 | 3.03 | 11.68 |
| 2 | 15844 | 1887.9 | 40.9 | AKALYQAEAFTADFQQS | Contrapsin-like protease inhibitor 6 precursor | 62.1 | 33.3 | 450.6 | 80.0 | 135.7 | 16.7 | 7.25 | 2.18 |
| 2 | 4524 | 1035.5 | 35.6 | GPpGPEGGKGPA | Collagen α-1(III) chain | 461.9 | 58.3 | 471.4 | 60.0 | 896.5 | 100.0 | 1.02 | 1.94 |
| 3 | 13476 | 1652.8 | 40.9 | GEpGEpGQTGPAGSRGPA | Collagen α-2(I) chain | 624.1 | 100.0 | 171.8 | 33.3 | 192.1 | 50.0 | −3.63 | −3.25 |
| 3 | 14544 | 1756.9 | 41.9 | GNFIDQTRVLNLGPIT | Uromodulin | 0.0 | 0.0 | 2190.6 | 100.0 | 976.5 | 100.0 | na | na |
| 3 | 10648 | 1435.7 | 39.6 | SpGSPGPDGKTGPpGP | Collagen α-1(I) chain precursor | 1141.3 | 100.0 | 147.2 | 16.7 | 0.0 | 0.0 | −7.75 | na |
| 3 | 8116 | 1263.6 | 32.3 | ISSDLDGHPVPK | Transketolase | 392.6 | 100.0 | 66.1 | 50.0 | 104.0 | 100.0 | −5.94 | −3.77 |
| 3 | 13451 | 1649.8 | 41.3 | TDVTQQLNTLFQDK | Apolipoprotein A-IV | 1740.8 | 100.0 | 509.9 | 100.0 | 922.7 | 100.0 | −3.41 | −1.89 |
| 3 | 17002 | 2008.0 | 31.2 | DGESGRpGRpGERGLpGPpG | Collagen α-1(III) chain | 507.3 | 100.0 | 68.9 | 33.3 | 136.2 | 100.0 | −7.36 | −3.72 |
| 3 | 14576 | 1759.9 | 34.0 | SADTLWDIQKDLKDL | l-lactate dehydrogenase B chain | 683.8 | 100.0 | 31.0 | 16.7 | 150.3 | 83.3 | −22.03 | −4.55 |
| 3 | 16532 | 1960.0 | 35.5 | RpGEVGPpGPpGPAGEKGSpG | Collagen α-1(I) chain | 170.7 | 100.0 | 0.0 | 0.0 | 97.1 | 50.0 | na | −1.76 |
| 3 | 17766 | 2088.1 | 36.3 | AGRpGEVGPpGPpGPAGEKGSpG | Collagen α-1(I) chain precursor | 164.6 | 100.0 | 20.0 | 16.7 | 79.9 | 50.0 | −8.22 | −2.06 |
| 3 | 13970 | 1702.0 | 22.8 | ELEKETKKETKKDP | α-1-acid glycoprotein precursor | 0.0 | 0.0 | 75.3 | 50.0 | 303.6 | 100.0 | na | na |
| 3 | 19332 | 2260.0 | 26.3 | MADEAASEAHQEGDTRTTKRG | Fibrinogen α chain precursor | 28.4 | 9.1 | 10.6 | 16.7 | 47.4 | 83.3 | −2.69 | 1.67 |
| 3 | 4486 | 1032.5 | 36.9 | pGPpGPRGPpG | Collagen α-1(XVII) chain | 12.4 | 36.4 | 8.1 | 16.7 | 21.3 | 83.3 | −1.52 | 1.72 |
| 3 | 8197 | 1269.6 | 39.2 | GFpGAAGRTGPpGP | Collagen α-2(I) chain | 30.4 | 45.5 | 130.6 | 83.3 | 13.9 | 50.0 | 4.30 | −2.19 |
| 3 | 5870 | 1124.6 | 30.8 | DLEDVAGHGGR | Cd99 protein | 10.5 | 9.1 | 0.0 | 0.0 | 21.0 | 50.0 | na | 1.99 |
| 3 | 21145 | 2497.4 | 38.7 | DLPGQQPVSEQAQQKLPPLALLK | Serine (Or cysteine) peptidase inhibitor. clade F. member 2 | 34.6 | 9.1 | 71.2 | 33.3 | 161.9 | 83.3 | 2.06 | 4.69 |
| 3 | 18962 | 2216.1 | 26.6 | DHGKELSSSLGPALVDKAPAPK | Similar to CG7896-PA | 0.0 | 0.0 | 22.4 | 33.3 | 36.1 | 83.3 | na | na |
| 3 | 17050 | 2013.0 | 36.7 | DEQYPDATDEDLTSRMK | Osteopontin precursor | 26.9 | 54.5 | 58.8 | 33.3 | 57.7 | 100.0 | 2.19 | 2.15 |
| 3 | 5198 | 1079.5 | 47.6 | GQpGPpGPpGTA | Collagen α-1(III) chain | 9.2 | 63.6 | 0.0 | 0.0 | 15.0 | 66.7 | na | 1.62 |
| 4 | 6975 | 1194.6 | 37.7 | SpGPDGKTGPpGP | Collagen α-1(I) chain | 12172.0 | 100.0 | 6619.6 | 100.0 | 6543.0 | 100.0 | −1.84 | −1.86 |
| 4 | 11644 | 1509.8 | 27.3 | GLpGpKGDRGDAGPKG | Collagen α-1(I) chain precursor | 52.9 | 83.3 | 50.7 | 16.7 | 187.6 | 100.0 | −1.04 | 3.55 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-Pozuelo, G.; González-Barrio, R.; Barberá, G.G.; Albalat, A.; García-Alonso, J.; Mullen, W.; Mischak, H.; Periago, M.J. Tomato Juice Consumption Modifies the Urinary Peptide Profile in Sprague-Dawley Rats with Induced Hepatic Steatosis. Int. J. Mol. Sci. 2016, 17, 1789. https://doi.org/10.3390/ijms17111789
Martín-Pozuelo G, González-Barrio R, Barberá GG, Albalat A, García-Alonso J, Mullen W, Mischak H, Periago MJ. Tomato Juice Consumption Modifies the Urinary Peptide Profile in Sprague-Dawley Rats with Induced Hepatic Steatosis. International Journal of Molecular Sciences. 2016; 17(11):1789. https://doi.org/10.3390/ijms17111789
Chicago/Turabian StyleMartín-Pozuelo, Gala, Rocío González-Barrio, Gonzalo G. Barberá, Amaya Albalat, Javier García-Alonso, William Mullen, Harald Mischak, and María Jesús Periago. 2016. "Tomato Juice Consumption Modifies the Urinary Peptide Profile in Sprague-Dawley Rats with Induced Hepatic Steatosis" International Journal of Molecular Sciences 17, no. 11: 1789. https://doi.org/10.3390/ijms17111789