Cloning and Expression of Ecdysone Receptor and Retinoid X Receptor from Procambarus clarkii: Induction by Eyestalk Ablation
Abstract
:1. Introduction
2. Results
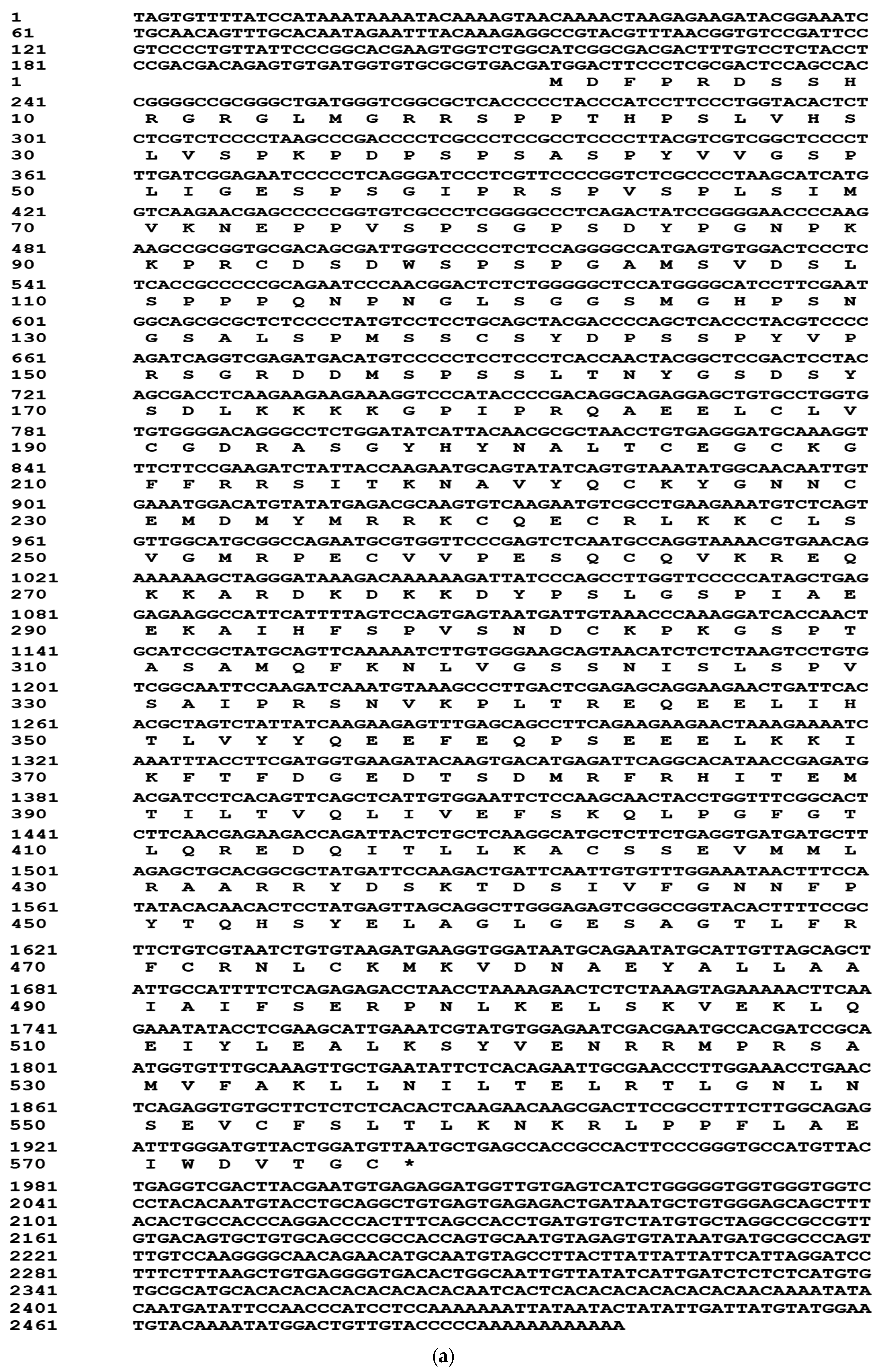
2.1. Cloning of Full Length cDNAs of PcEcR and PcRXR
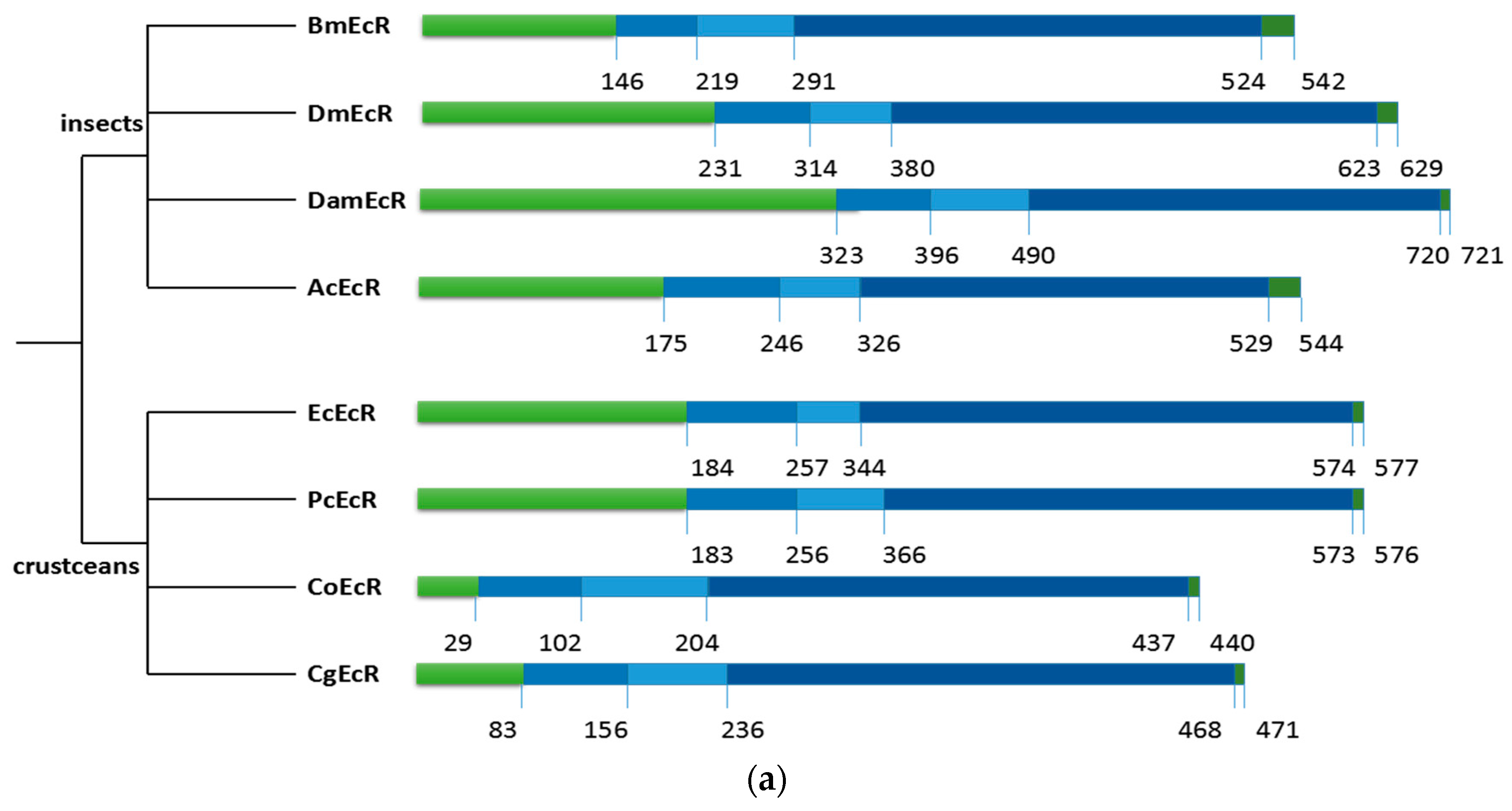
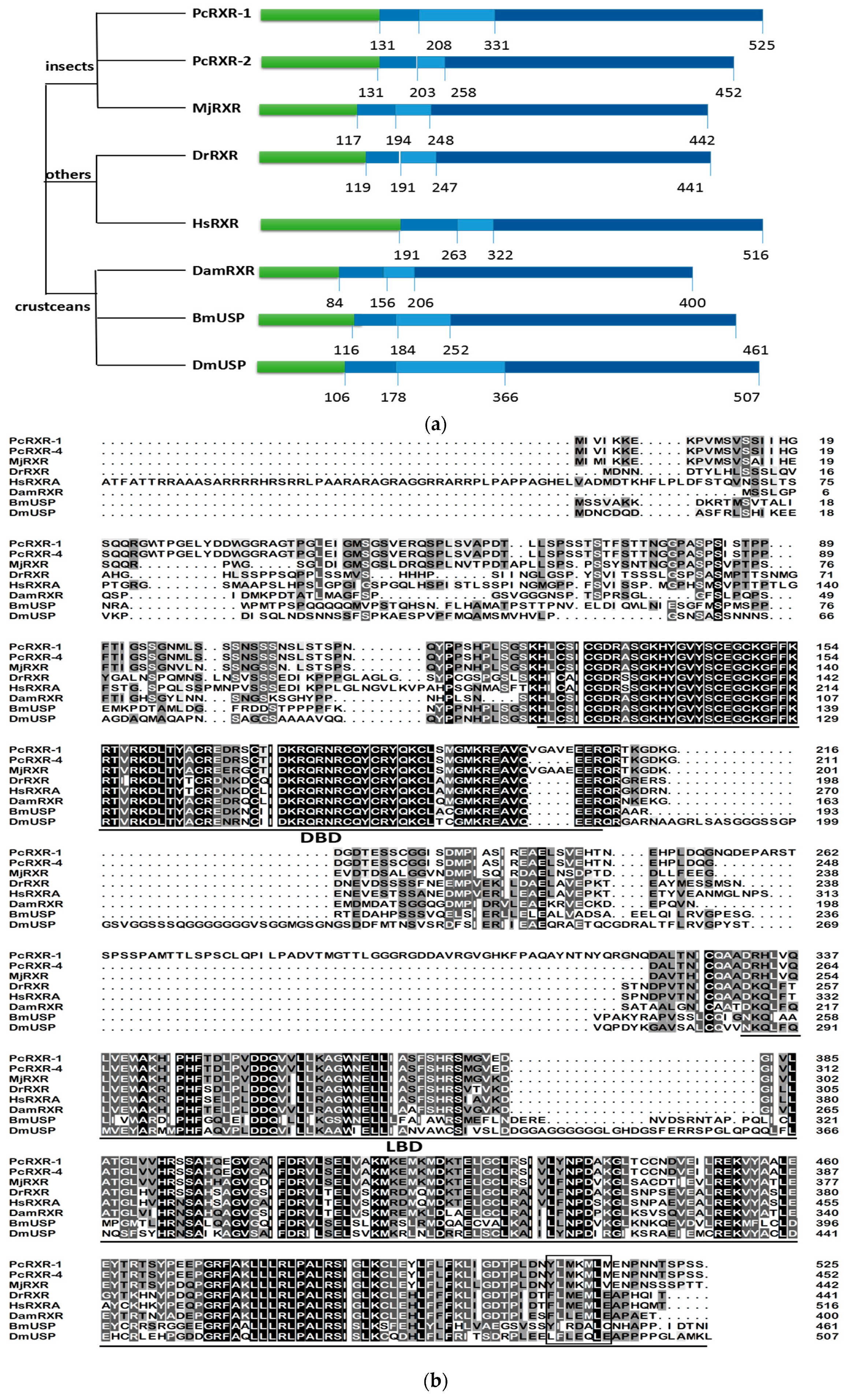
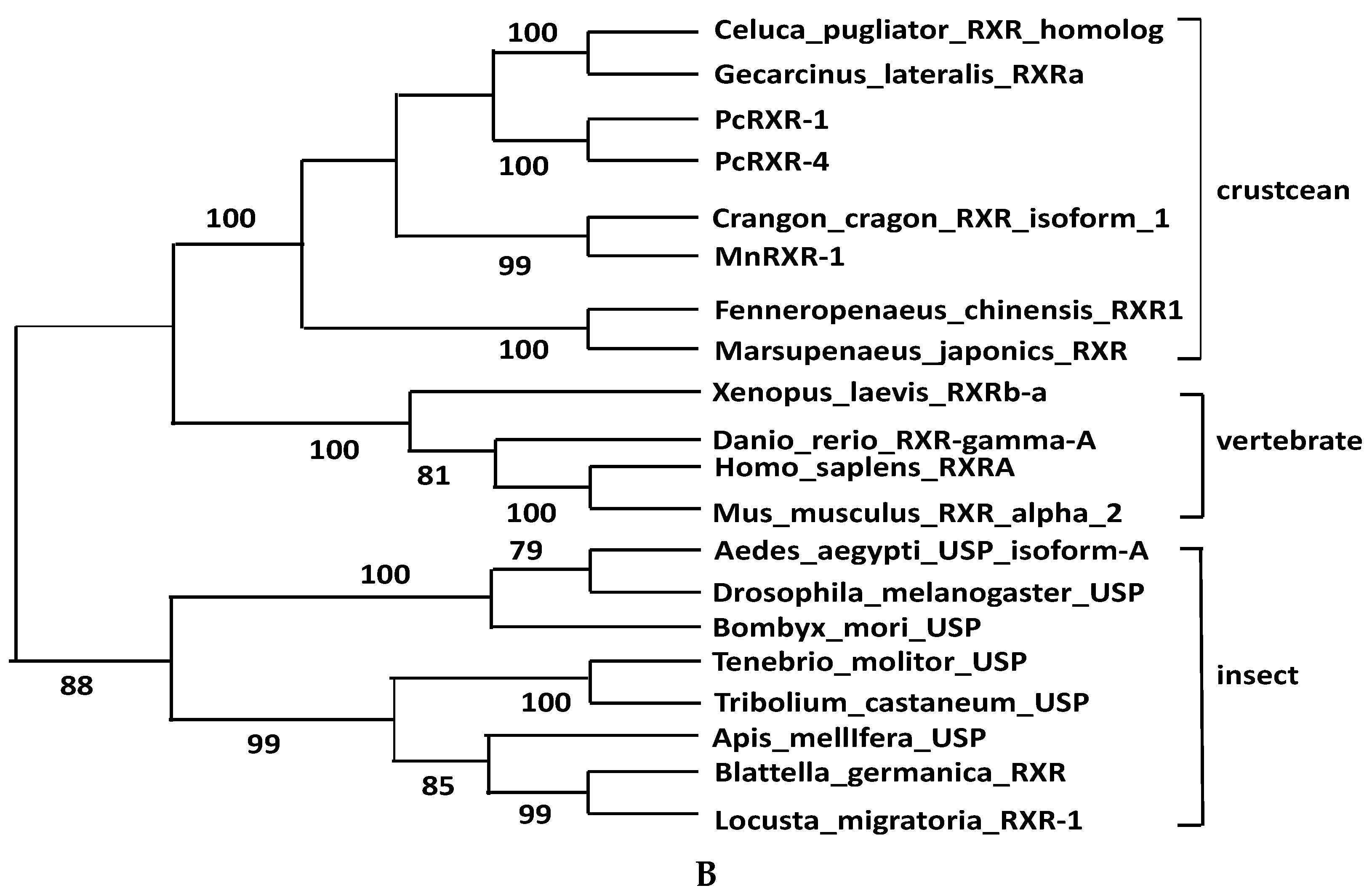
2.2. Sequence Alignments and Phylogenic Trees of PcEcR and PcRXR
2.3. Expression of PcEcR and PcRXR in Different Tissues
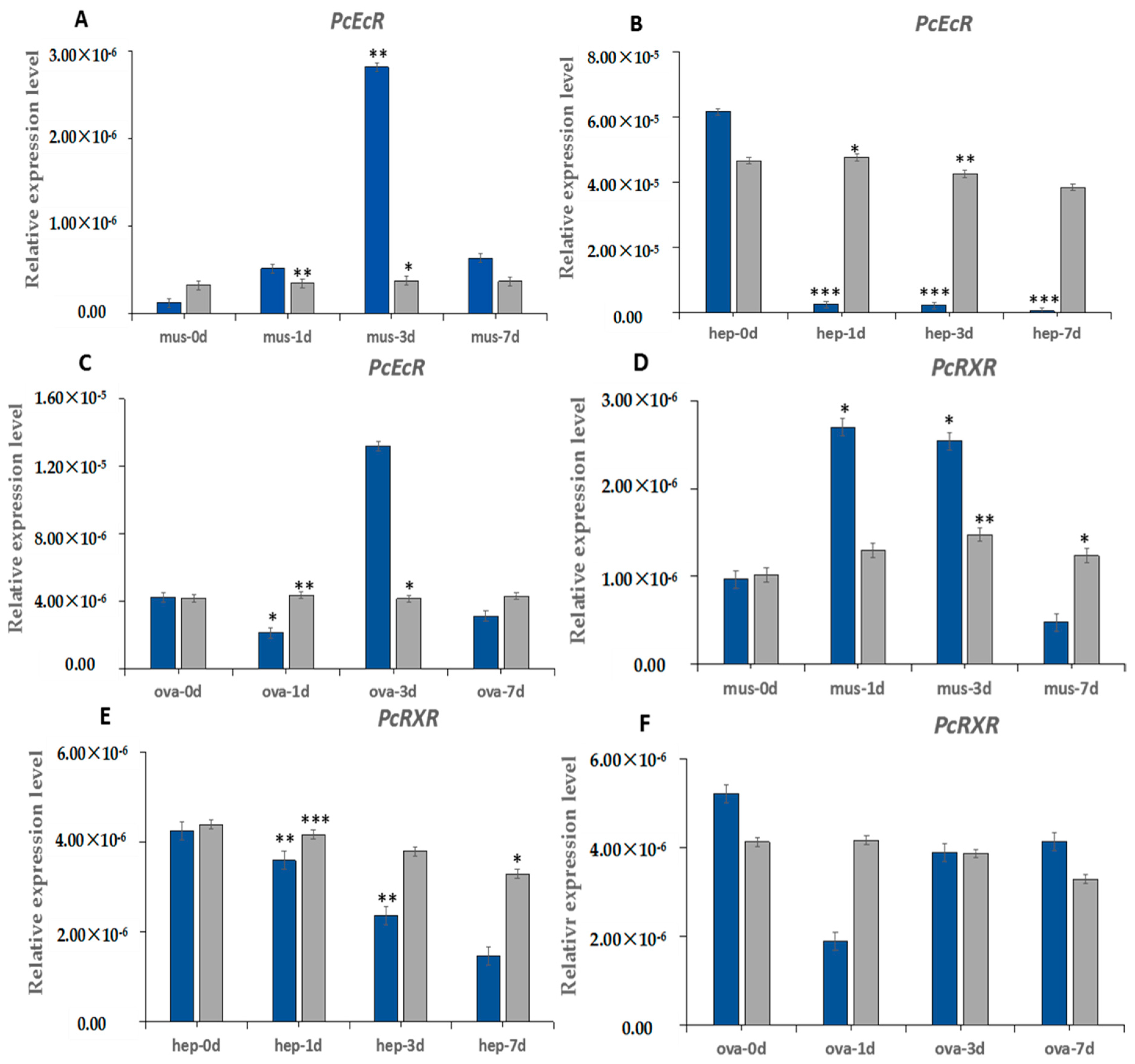
2.4. The Induction Expression of PcEcR and PcRXR after Eyestalk Ablation
3. Discussion
3.1. Analysis of PcEcR and PcRXR
3.2. Expression in Different Tissues and Synergistic Expression of PcEcR and PcRXR in Different Tissues
3.3. Induction of PcEcR and PcRXR after ESA in Different Tissues
4. Material and Methods
4.1. Animal Collection, Preparation of Total RNA, and cDNA Synthesis
4.2. Cloning and Sequencing of Full-Length PcEcR and PcRXR cDNA
4.3. Sequence Alignments and Phylogenetic Analysis
4.4. Quantitation of PcEcR and PcRXR Transcripts by Real-Time PCR
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yue, G.H.; Wang, G.L.; Zhu, B.Q.; Wang, C.M.; Zhu, Z.Y.; Lo, L.C. Discovery of four natural clones in a crayfish species Procambarus clarkii. Int. J. Biol. Sci. 2008, 4, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Gu, W.; Ding, Z.; Ren, Y.; Chen, J.; Hou, Y. A novel Spiroplasma pathogen causing systemic infection in the crayfish Procambarus clarkii (Crustacea: Decapod), in China. FEMS Microbiol. Lett. 2005, 249, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Henrich, V.C. Arthropod nuclear receptors and their role in molting. FEBS J. 2009, 276, 6128–6157. [Google Scholar] [CrossRef] [PubMed]
- Yao, T.P.; Forman, B.M.; Jiang, Z.; Cherbas, L.; Chen, J.D.; McKeown, M.; Cherbas, P.; Evans, R.M. Functional ecdysone receptor is the product of EcR and ultraspiracle genes. Nature 1993, 366, 476–479. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Lee, S.G.; Mykles, D.L. Ecdysteroid-responsive genes, RXR and E75, in the tropical land crab, Gecarcinus lateralis: Differential tissue expression of multiple RXR isoforms generated at three alternative splicing sites in the hinge and ligand-binding domains. Mol. Cell. Endocrinol. 2005, 242, 80–95. [Google Scholar] [CrossRef] [PubMed]
- Shechter, A.; Tom, M.; Yudkovski, Y.; Weil, S.; Chang, S.A.; Chang, E.S.; Chalifa-Caspi, V.; Berman, A.; Sagi, A. Search for hepatopancreatic ecdysteroid-responsive genes during the crayfish molt cycle: From a single gene to multigenicity. J. Exp. Biol. 2007, 210, 3525–3537. [Google Scholar] [CrossRef] [PubMed]
- King-Jones, K.; Charles, J.P.; Lam, G.; Thummel, C.S. The ecdysone-induced DHR4 orphan nuclear receptor coordinates growth and maturation in Drosophila. Cell 2005, 121, 773–784. [Google Scholar] [CrossRef] [PubMed]
- Nikolenko, J.V.; Krasnov, A.N. Nuclear receptors: structure and mechanisms of action. Genetika 2007, 43, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Renaud, J.P.; Moras, D. Structural studies on nuclear receptors. Cell. Mol. Life Sci. 2000, 57, 1748–1769. [Google Scholar] [CrossRef] [PubMed]
- Thomson, S.A.; Baldwin, W.S.; Wang, Y.H.; Kwon, G.; Leblanc, G.A. Annotation, phylogenetics, and expression of the nuclear receptors in Daphnia pulex. BMC Genom. 2009, 10, 500. [Google Scholar] [CrossRef] [PubMed]
- Chung, A.C.; Durica, D.S.; Clifton, S.W.; Roe, B.A.; Hopkins, P.M. Cloning of crustacean ecdysteroid receptor and retinoid-X receptor gene homologs and elevation of retinoid-X receptor mrna by retinoic acid. Mol. Cell. Endocrinol. 1998, 139, 209–227. [Google Scholar] [CrossRef]
- Kim, H.W.; Chang, E.S.; Mykles, D.L. Three calpains and ecdysone receptor in the land crab gecarcinus lateralis: Sequences, expression and effects of elevated ecdysteroid induced by eyestalk ablation. J. Exp. Biol. 2005, 208, 3177–3197. [Google Scholar] [CrossRef] [PubMed]
- Asazuma, H.; Nagata, S.; Kono, M.; Nagasawa, H. Molecular cloning and expression analysis of ecdysone receptor and retinoid X receptor from the kuruma prawn, marsupenaeus japonicus. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2007, 148, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Kobayashi, K.; Oda, S.; Tatarazako, N.; Watanabe, H.; Iguchi, T. Cloning and characterization of the ecdysone receptor and ultraspiracle protein from the water flea daphnia magna. J. Endocrinol. 2007, 193, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.S.; Lee, J.S.; Lee, K.W.; Rhee, J.S.; Han, J.; Lee, J.; Park, G.S.; Lee, Y.M. Cloning and expression of ecdysone receptor (EcR) from the intertidal copepod, Tigriopus japonicus. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2010, 151, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Verhaegen, Y.; Parmentier, K.; Swevers, L.; Renders, E.; Rouge, P.; de Coen, W.; Cooreman, K.; Smagghe, G. The heterodimeric ecdysteroid receptor complex in the brown shrimp Crangon crangon: EcR and RXR isoform characteristics and sensitivity towards the marine pollutant tributyltin. Gen. Comp. Endocrinol. 2011, 172, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Yokota, H.; Eguchi, S.; Nakai, M. Development of an in vitro binding assay for ecdysone receptor of mysid shrimp (Americamysis bahia). Aquat. Toxicol. 2011, 105, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Tarrant, A.M.; Behrendt, L.; Stegeman, J.J.; Verslycke, T. Ecdysteroid receptor from the american lobster Homarus americanus: EcR/RXR isoform cloning and ligand-binding properties. Gen. Comp. Endocrinol. 2011, 173, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Gaertner, K.; Chandler, G.T.; Quattro, J.; Ferguson, P.L.; Sabo-Attwood, T. Identification and expression of the ecdysone receptor in the harpacticoid copepod, amphiascus tenuiremis, in response to fipronil. Ecotoxicol. Environ. Saf. 2012, 76, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Techa, S.; Chung, J.S. Ecdysone and retinoid-X receptors of the blue crab, Callinectes sapidus: Cloning and their expression patterns in eyestalks and Y-organs during the molt cycle. Gene 2013, 527, 139–153. [Google Scholar] [CrossRef] [PubMed]
- De Wilde, R.; Swevers, L.; Soin, T.; Christiaens, O.; Rouge, P.; Cooreman, K.; Janssen, C.R.; Smagghe, G. Cloning and functional analysis of the ecdysteroid receptor complex in the opossum shrimp neomysis integer (leach, 1814). Aquat. Toxicol. 2013, 130–131, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Zhou, X.; Bai, A.; Ren, X.; Zhang, Y. Ecdysone receptor gene from the freshwater prawn macrobrachium nipponense: Identification of different splice variants and sexually dimorphic expression, fluctuation of expression in the molt cycle and effect of eyestalk ablation. Gen. Comp. Endocrinol. 2013, 193, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Zhou, X.; Bai, A.; Ren, X. cDNA cloning of retinoid-X receptor gene from macrobrachium nipponense and different responses of two splice variants during the molting cycle. CRUS 2013, 86, 1586–1604. [Google Scholar] [CrossRef]
- Shen, H.; Hu, Y.; Zhou, X. Cloning of ecdysone receptor gene from the Chinese Mitten Crab, Eriocheir sinensis and sexually dimorphic expression of two splice variants. J. World Aquac. Soc. 2015. [Google Scholar] [CrossRef]
- Uawisetwathana, U.; Leelatanawit, R.; Klanchui, A.; Prommoon, J.; Klinbunga, S.; Karoonuthaisiri, N. Insights into eyestalk ablation mechanism to induce ovarian maturation in the black tiger shrimp. PLoS ONE 2011, 6, e24427. [Google Scholar] [CrossRef] [PubMed]
- McDonald, A.A.; Chang, E.S.; Mykles, D.L. Cloning of a nitric oxide synthase from green shore crab, carcinus maenas: A comparative study of the effects of eyestalk ablation on expression in the molting glands (Y-organs) of C. maenas, and blackback land crab, Gecarcinus lateralis. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2011, 158, 150–162. [Google Scholar] [CrossRef] [PubMed]
- Sroyraya, M.; Chotwiwatthanakun, C.; Stewart, M.J.; Soonklang, N.; Kornthong, N.; Phoungpetchara, I.; Hanna, P.J.; Sobhon, P. Bilateral eyestalk ablation of the blue swimmer crab, portunus pelagicus, produces hypertrophy of the androgenic gland and an increase of cells producing insulin-like androgenic gland hormone. Tissue Cell 2010, 42, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Okumura, T. Effects of bilateral and unilateral eyestalk ablation on vitellogenin synthesis in immature female kuruma prawns, marsupenaeus japonicus. Zool. Sci. 2007, 24, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Venkitraman, P.R.; Jayalakshmy, K.V.; Balasubramanian, T.; Nair, M.; Nair, K.K. Effects of eyestalk ablation on moulting and growth of penaeid prawn metapenaeus dobsoni (de Man). Indian J. Exp. Biol. 2004, 42, 403–412. [Google Scholar] [PubMed]
- Zhang, Y.; Sun, Y.; Liu, Y.; Geng, X.; Wang, X.; Wang, Y.; Sun, J.; Yang, W. Molt-inhibiting hormone from Chinese mitten crab (Eriocheir sinensis): Cloning, tissue expression and effects of recombinant peptide on ecdysteroid sEcRetion of YOs. Gen. Comp. Endocrinol. 2011, 173, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Webster, S.G.; Keller, R.; Dircksen, H. The CHH-superfamily of multifunctional peptide hormones controlling crustacean metabolism, osmoregulation, moulting, and reproduction. Gen. Comp. Endocrinol. 2012, 175, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Sedlmeier, D. The mode of action of the crustacean neurosEcRetory hyperglycemic hormone (CHH): II. Involvement of glycogen synthase. Gen. Comp. Endocrinol. 1982, 47, 426–432. [Google Scholar] [CrossRef]
- Soyez, D.; Le Caer, J.P.; Noel, P.Y.; Rossier, J. Primary structure of two isoforms of the vitellogenesis inhibiting hormone from the lobster Homarus americanus. Neuropeptides 1991, 20, 25–32. [Google Scholar] [CrossRef]
- Spanings-Pierrot, C.; Soyez, D.; van Herp, F.; Gompel, M.; Skaret, G.; Grousset, E.; Charmantier, G. Involvement of crustacean hyperglycemic hormone in the control of gill ion transport in the crab Pachygrapsus marmoratus. Gen. Comp. Endocrinol. 2000, 119, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Yin, H. Effects of eyestalk ablation on the molting, growth and gonad development of Procambarus clarkia. J. Anhui Agric. Sci. 2007, 35, 16. [Google Scholar]





| Primer | Sequence (5′ to 3′) | Primer Description |
|---|---|---|
| PcEcR-3a-Outer | CTCACAGAATTGCGAACCCTT | 3′ RACE Primer for first round |
| PcEcR-3a-Inner | CACCCAGGACCCACTTTCAG | 3′ RACE Primer for second round |
| PcEcR-5a-Outer | GAGATGTTACTGCTTCCCAC | 5′ RACE Primer for first round |
| PcEcR-5a-Inner | ATCCTTTGGGTTTACAATCA | 5′ RACE Primer for second round |
| PcRXR-3a-Outer | CAATACTGGCATCGGTTCCT | 3′ RACE Primer for first round |
| PcRXR-3a-Inner | CCGCCATTGGTGGTGGAGAA | 3′ RACE Primer for second round |
| PcRXR-5a-Outer | AAAACAAGGAAGTAGTTGGC | 5′ RACE Primer for first round |
| PcRXR-5a-Inner | TAAAACTCAAGGAAACTGATG | 5′ RACE Primer for second round |
| Rt-PcEcR-F | CCTGTGAGGGATGCAAAGGT | FWD primer for EsEcR expression |
| Rt-PcEcR-R | GCATTGAGACTCGGGAACCA | RVS primer for EsEcR expression |
| Rt-PcRXR-F | CCTTCACCATTGGGTCGAGT | FWD primer for EsEcR expression |
| Rt-PcRXR-R | AGCTGTAGACGCCATAGTGC | RVS primer for EsEcR expression |
| Pc18S-F | ATCACGTCTCTGACCGCAAG | FWD primer for 18S expression |
| Pc18S-R | GACACTTGAAAGATGCGGCG | RVS primer for 18S expression |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, T.-H.; Sserwadda, A.; Song, K.; Zang, Y.-N.; Shen, H.-S. Cloning and Expression of Ecdysone Receptor and Retinoid X Receptor from Procambarus clarkii: Induction by Eyestalk Ablation. Int. J. Mol. Sci. 2016, 17, 1739. https://doi.org/10.3390/ijms17101739
Dai T-H, Sserwadda A, Song K, Zang Y-N, Shen H-S. Cloning and Expression of Ecdysone Receptor and Retinoid X Receptor from Procambarus clarkii: Induction by Eyestalk Ablation. International Journal of Molecular Sciences. 2016; 17(10):1739. https://doi.org/10.3390/ijms17101739
Chicago/Turabian StyleDai, Tian-Hao, Ali Sserwadda, Kun Song, Ya-Nan Zang, and Huai-Shun Shen. 2016. "Cloning and Expression of Ecdysone Receptor and Retinoid X Receptor from Procambarus clarkii: Induction by Eyestalk Ablation" International Journal of Molecular Sciences 17, no. 10: 1739. https://doi.org/10.3390/ijms17101739





