EpCAM Expression in Lymph Node and Bone Metastases of Prostate Carcinoma: A Pilot Study
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Went, P.; Vasei, M.; Bubendorf, L.; Terracciano, L.; Tornillo, L.; Riede, U.; Kononen, J.; Simon, R.; Sauter, G.; Baeuerle, P.A. Frequent high-level expression of the immunotherapeutic target Ep-CAM in colon, stomach, prostate and lung cancers. Br. J. Cancer 2006, 94, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Litvinov, S.V.; Velders, M.P.; Bakker, H.A.; Fleuren, G.J.; Warnaar, S.O. Ep-CAM: A human epithelial antigen is a homophilic cell–cell adhesion molecule. J. Cell Biol. 1994, 125, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Went, P.T.; Lugli, A.; Meier, S.; Bundi, M.; Mirlacher, M.; Sauter, G.; Dirnhofer, S. Frequent EpCAM protein expression in human carcinomas. Hum. Pathol. 2004, 35, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Trzpis, M.; McLaughlin, P.M.; de Leij, L.M.; Harmsen, M.C. Epithelial cell adhesion molecule: More than a carcinoma marker and adhesion molecule. Am. J. Pathol. 2007, 171, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Poczatek, R.B.; Myers, R.B.; Manne, U.; Oelschlager, D.K.; Weiss, H.L.; Bostwick, D.G.; Grizzle, W.E. Ep-CAM levels in prostatic adenocarcinoma and prostatic intraepithelial neoplasia. J. Urol. 1999, 162, 1462–1466. [Google Scholar] [CrossRef]
- Zellweger, T.; Ninck, C.; Bloch, M.; Mirlacher, M.; Koivisto, P.A.; Helin, J.H.; Mihatsch, M.J.; Gasser, T.C.; Bubendorf, L. Expression patterns of potential therapeutic targets in prostate cancer. Int. J. Cancer 2005, 113, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Benko, G.; Spajic, B.; Kruslin, B.; Tomas, D. Impact of the EpCAM expression on biochemical recurrence-free survival in clinically localized prostate cancer. Urol. Oncol. 2013, 31, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Leung, K. DiD-Labeled anti-EpCAM-directed NK-92-scFv(MOC31) zeta cells. In Molecular Imaging and Contrast Agent Database (MICAD) Bethesda (MD); National Center for Biotechnology Information (US): Bethesda, MD, USA, 2004. [Google Scholar]
- Hall, M.A.; Pinkston, K.L.; Wilganowski, N.; Robinson, H.; Ghosh, P.; Azhdarinia, A.; Vazquez-Arreguin, K.; Kolonin, A.M.; Harvey, B.R.; Sevick-Muraca, E.M. Comparison of mAbs targeting epithelial cell adhesion molecule for the detection of prostate cancer lymph node metastases with multimodal contrast agents: Quantitative small-animal PET/CT and NIRF. J. Nucl. Med. 2012, 53, 1427–1437. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.A.; Kwon, S.; Robinson, H.; Lachance, P.-A.; Azhdarinia, A.; Ranganathan, R.; Price, R.E.; Chan, W.; Sevick-Muraca, E.M. Imaging prostate cancer lymph node metastases with a multimodality contrast agent. Prostate 2012, 72, 129–146. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Wu, G.; Robinson, H.; Wilganowski, N.; Hall, M.A.; Ghosh, S.C.; Pinkston, K.L.; Azhdarinia, A.; Harvey, B.R.; Sevick-Muraca, E.M. Tumor margin detection using quantitative NIRF molecular imaging targeting EpCAM validated by far red gene reporter iRFP. Mol. Imaging Biol. 2013, 15, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Tavri, S.; Jha, P.; Meier, R.; Henning, T.D.; Müller, T.; Hostetter, D.; Knopp, C.; Johansson, M.; Reinhart, V.; Boddington, S. Optical imaging of cellular immunotherapy against prostate cancer. Mol. Imaging 2009, 8, 15–26. [Google Scholar] [PubMed]
- Pinto, F.; Totaro, A.; Palermo, G.; Calarco, A.; Sacco, E.; D’Addessi, A.; Racioppi, M.; Valentini, A.L.; Gui, B.; Bassi, P.F. Imaging in prostate cancer staging: Present role and future perspectives. Urol. Int. 2012, 88, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Jung, A.J.; Westphalen, A.C. Imaging prostate cancer. Radiol. Clin. N. Am. 2012, 50, 1043–1059. [Google Scholar] [CrossRef] [PubMed]
- Talab, S.S.; Preston, M.A.; Elmi, A.; Tabatabaei, S. Prostate cancer imaging: What the urologist wants to know. Radiol. Clin. N. Am. 2012, 50, 1015–1041. [Google Scholar] [CrossRef] [PubMed]
- Brogsitter, C.; Zophel, K.; Kotzerke, J. 18F-Choline, 11C-choline and 11C-acetate PET/CT: Comparative analysis for imaging prostate cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2013, 40 (Suppl. 1), 18–27. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, M.H.; Bouchelouche, K.; Hoilund-Carlsen, P.F.; Petersen, H.; Gerke, O.; Steffansen, S.I.; Marcussen, N.; Svolgaard, N.; Vach, W.; Geertsen, U.; et al. [18F]fluoromethylcholine (FCH) positron emission tomography/computed tomography (PET/CT) for lymph node staging of prostate cancer: A prospective study of 210 patients. BJU Int. 2012, 110, 1666–1671. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, A.; Ohlmann, C.H.; Polyakov, S. Anatomical extent of pelvic lymphadenectomy in patients undergoing radical prostatectomy. Eur. Urol. 2007, 52, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Briganti, A.; Blute, M.L.; Eastham, J.H.; Graefen, M.; Heidenreich, A.; Karnes, J.R.; Montorsi, F.; Studer, U.E. Pelvic lymph node dissection in prostate cancer. Eur. Urol. 2009, 55, 1251–1265. [Google Scholar] [CrossRef] [PubMed]
- Berney, D.M.; Wheeler, T.M.; Grignon, D.J.; Epstein, J.I.; Griffiths, D.F.; Humphrey, P.A.; van der Kwast, T.; Montironi, R.; Delahunt, B.; Egevad, L.; et al. International Society of Urological Pathology (ISUP) Consensus Conference on Handling and Staging of Radical Prostatectomy Specimens. Working group 4: Seminal vesicles and lymph nodes. Mod. Pathol. 2011, 24, 39–47. [Google Scholar] [CrossRef] [PubMed]
- McGregor, B.; Tulloch, A.G.; Quinlan, M.F.; Lovegrove, F. The role of bone scanning in the assessment of prostatic carcinoma. Br. J. Urol. 1978, 50, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Messiou, C.; Cook, G.; deSouza, N.M. Imaging metastatic bone disease from carcinoma of the prostate. Br. J. Cancer 2009, 101, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Cook, G.J.; Venkitaraman, R.; Sohaib, A.S.; Lewington, V.J.; Chua, S.C.; Huddart, R.A.; Parker, C.C.; Dearnaley, D.D.; Horwich, A. The diagnostic utility of the flare phenomenon on bone scintigraphy in staging prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.H.; Bono, A.; Calais da Silva, F.; Debruyne, F.; Denis, L.; Robinson, P.; Sylvester, R.; Armitage, T.G. Some limitations of the radioisotope bone scan in patients with metastatic prostatic cancer. A subanalysis of EORTC trial 30853. The EORTC Urological Group. Cancer 1990, 66 (Suppl. 5), 1009–1016. [Google Scholar] [PubMed]
- Scher, H.I. Prostate carcinoma: Defining therapeutic objectives and improving overall outcomes. Cancer 2003, 97 (Suppl. 3), 758–771. [Google Scholar] [CrossRef] [PubMed]
- Gastl, G.; Spizzo, G.; Obrist, P.; Dünser, M.; Mikuz, G. Ep-CAM overexpression in breast cancer as a predictor of survival. Lancet 2000, 356, 1981–1982. [Google Scholar] [CrossRef]
- Rybalov, M.; Ananias, H.J.; Hoving, H.D.; van der Poel, H.G.; Rosati, S.; de Jong, I.J. PSMA, EpCAM, VEGF and GRPR as imaging targets in locally recurrent prostate cancer after radiotherapy. Int. J. Mol. Sci. 2014, 15, 6046–6061. [Google Scholar] [CrossRef] [PubMed]
- Spizzo, G.; Fong, D.; Wurm, M.; Ensinger, C.; Obrist, P.; Hofer, C.; Mazzoleni, G.; Gastl, G.; Went, P. EpCAM expression in primary tumour tissues and metastases: An immunohistochemical analysis. J. Clin. Pathol. 2011, 64, 415–420. [Google Scholar] [CrossRef] [PubMed]
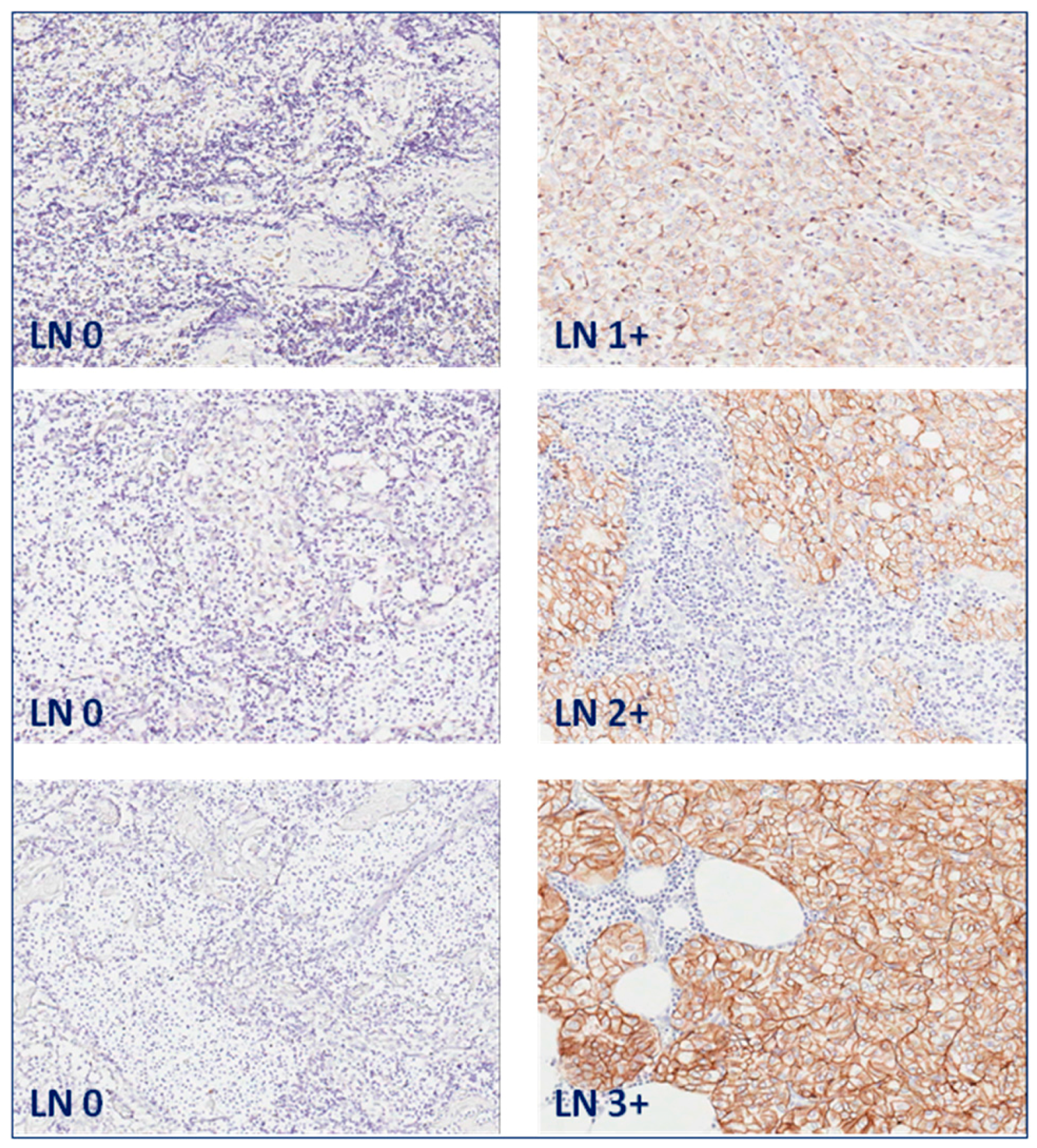
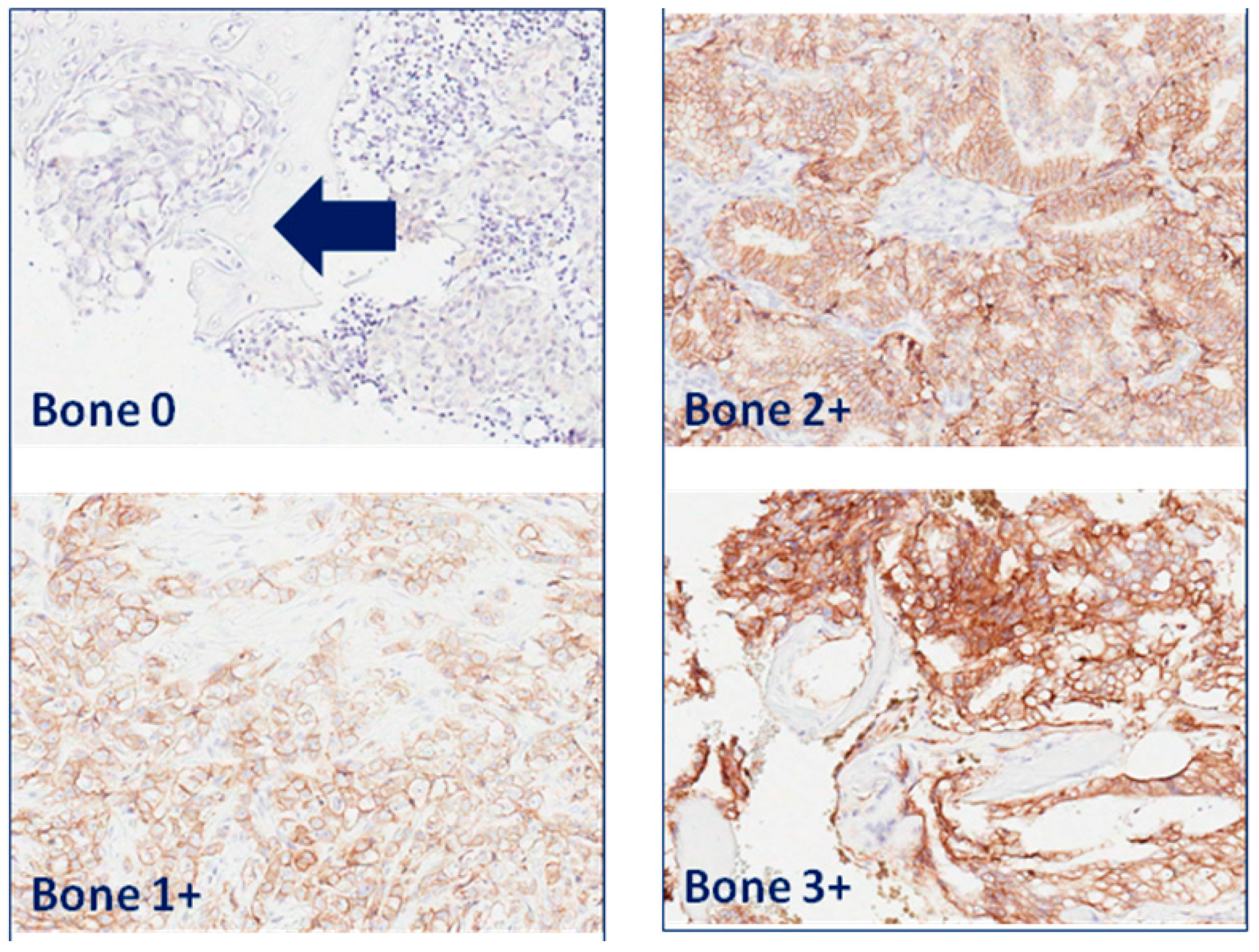
| Patient | Tissue Type | Hormonal Therapy | Radiotherapy | PS | IS | TIS |
|---|---|---|---|---|---|---|
| 1 | Lymph node normal | No | No | 0 | 0 | 0 |
| Lymph node metastasis | No | No | 4 | 2 | 8 | |
| 2 | Lymph node normal | No | No | 0 | 0 | 0 |
| Lymph node normal | No | No | 0 | 0 | 0 | |
| Lymph node metastasis | No | No | 4 | 2 | 8 | |
| Lymph node metastasis | No | No | 4 | 2 | 8 | |
| 3 | Lymph node normal | No | No | 0 | 0 | 0 |
| Lymph node metastasis | No | No | 4 | 3 | 12 | |
| 4 | Lymph node normal | No | No | 0 | 0 | 0 |
| Lymph node metastasis | No | No | 3 | 1 | 3 | |
| 5 | Lymph node normal | No | No | 0 | 0 | 0 |
| Lymph node metastasis | No | No | 4 | 3 | 12 | |
| 6 | Lymph node normal | No | No | 0 | 0 | 0 |
| Lymph node metastasis | No | No | 3 | 3 | 9 | |
| 7 | Lymph node normal | No | No | 0 | 0 | 0 |
| Lymph node metastasis | No | No | 4 | 2 | 8 | |
| 8 | Lymph node normal | No | No | 0 | 0 | 0 |
| Lymph node metastasis | No | No | 4 | 3 | 12 | |
| 9 | Lymph node normal | No | No | 0 | 0 | 0 |
| Lymph node normal | No | No | 0 | 0 | 0 | |
| Lymph node normal | No | No | 0 | 0 | 0 | |
| Lymph node metastasis | No | No | 4 | 2 | 8 | |
| Lymph node metastasis | No | No | 4 | 2 | 8 | |
| Lymph node metastasis | No | No | 4 | 3 | 12 | |
| 10 | Lymph node normal | No | No | 0 | 0 | 0 |
| Lymph node normal | No | No | 0 | 0 | 0 | |
| Lymph node metastasis | No | No | 3 | 2 | 6 | |
| Lymph node metastasis | No | No | 3 | 1 | 3 | |
| 11 | Lymph node normal | No | No | 0 | 0 | 0 |
| Lymph node metastasis | No | No | 3 | 1 | 3 | |
| 12 | Lymph node normal | No | No | 0 | 0 | 0 |
| Lymph node metastasis | No | No | 4 | 3 | 12 | |
| 13 | Lymph node normal | No | No | 0 | 0 | 0 |
| Lymph node metastasis | No | No | 4 | 2 | 8 | |
| 14 | Lymph node normal | No | No | 0 | 0 | 0 |
| Lymph node metastasis | No | No | 3 | 3 | 9 | |
| 15 | Lymph node normal | Yes | Yes | 0 | 0 | 0 |
| Lymph node normal | Yes | Yes | 0 | 0 | 0 | |
| Lymph node metastasis | Yes | Yes | 4 | 3 | 12 | |
| Lymph node metastasis | Yes | Yes | 3 | 3 | 9 | |
| 16 | Lymph node normal | No | No | 0 | 0 | 0 |
| Lymph node metastasis | No | No | 4 | 2 | 8 |
| Patient | Tissue Type | Hormonal Therapy | Radiotherapy | PS | IS | TIS |
|---|---|---|---|---|---|---|
| 1 | Bone metastasis | Unknown | Unknown | 4 | 3 | 12 |
| 2 | Bone metastasis | Unknown | Unknown | 4 | 3 | 12 |
| 3 | Bone metastasis | Yes | No | 4 | 3 | 12 |
| 4 | Bone metastasis | Unknown | Unknown | 4 | 3 | 12 |
| 5 | Bone metastasis | Yes | No | 4 | 3 | 12 |
| 6 | Bone metastasis | No | No | 4 | 2 | 8 |
| 7 | Bone metastasis | Unknown | Unknown | 0 | 0 | 0 |
| 8 | Bone metastasis | Yes | Yes | 4 | 2 | 8 |
| 9 | Bone metastasis | Unknown | Unknown | 4 | 3 | 12 |
| 10 | Bone metastasis | Yes | No | 3 | 2 | 6 |
| 11 | Bone metastasis | Unknown | Yes | 4 | 3 | 12 |
| 12 | Bone metastasis | Yes | Yes | 4 | 3 | 12 |
| 13 | Bone metastasis | Yes | Yes | 4 | 3 | 12 |
| 14 | Bone metastasis | No | No | 4 | 3 | 12 |
| 15 | Bone metastasis | Yes | No | 3 | 3 | 9 |
| 16 | Bone metastasis | Unknown | Yes | 4 | 3 | 12 |
| 17 | Bone metastasis | Unknown | Unknown | 1 | 1 | 1 |
| 18 | Bone metastasis | No | No | 4 | 3 | 12 |
| 19 | Bone metastasis | No | No | 4 | 3 | 12 |
| 20 | Bone metastasis | No | No | 4 | 3 | 12 |
| Patient | Tissue Type | Hormonal Therapy | Radiotherapy | PS | IS | TIS |
|---|---|---|---|---|---|---|
| 1 | Normal bone | Unknown | Unknown | 0 | 0 | 0 |
| 2 | Normal bone | Unknown | Unknown | 0 | 0 | 0 |
| 3 | Normal bone | Unknown | Unknown | 0 | 0 | 0 |
| 4 | Normal bone | Unknown | Unknown | 0 | 0 | 0 |
| 5 | Normal bone | Unknown | Unknown | 0 | 0 | 0 |
| 6 | Normal bone | Unknown | Unknown | 0 | 0 | 0 |
| 7 | Normal bone | Unknown | Unknown | 0 | 0 | 0 |
| 8 | Normal bone | Unknown | Unknown | 0 | 0 | 0 |
| 9 | Normal bone | Unknown | Unknown | 0 | 0 | 0 |
| 10 | Normal bone | Unknown | Unknown | 0 | 0 | 0 |
| 11 | Normal bone | Unknown | Unknown | 0 | 0 | 0 |
| 12 | Normal bone | Unknown | Unknown | 0 | 0 | 0 |
| 13 | Normal bone | Unknown | Unknown | 0 | 0 | 0 |
| 14 | Normal bone | Unknown | Unknown | 0 | 0 | 0 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campos, A.K.; Hoving, H.D.; Rosati, S.; Van Leenders, G.J.L.H.; De Jong, I.J. EpCAM Expression in Lymph Node and Bone Metastases of Prostate Carcinoma: A Pilot Study. Int. J. Mol. Sci. 2016, 17, 1650. https://doi.org/10.3390/ijms17101650
Campos AK, Hoving HD, Rosati S, Van Leenders GJLH, De Jong IJ. EpCAM Expression in Lymph Node and Bone Metastases of Prostate Carcinoma: A Pilot Study. International Journal of Molecular Sciences. 2016; 17(10):1650. https://doi.org/10.3390/ijms17101650
Chicago/Turabian StyleCampos, Anna K., Hilde D. Hoving, Stefano Rosati, Geert J. L. H. Van Leenders, and Igle J. De Jong. 2016. "EpCAM Expression in Lymph Node and Bone Metastases of Prostate Carcinoma: A Pilot Study" International Journal of Molecular Sciences 17, no. 10: 1650. https://doi.org/10.3390/ijms17101650





