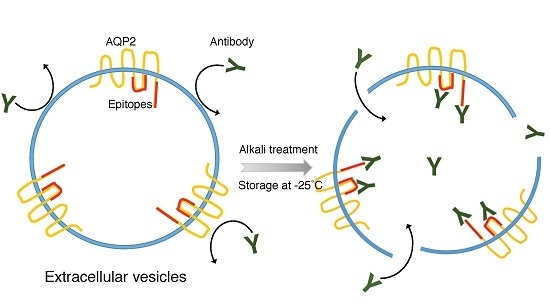
Disruption of Membranes of Extracellular Vesicles Is Necessary for ELISA Determination of Urine AQP2: Proof of Disruption and Epitopes of AQP2 Antibodies
Abstract
:1. Introduction
2. Results
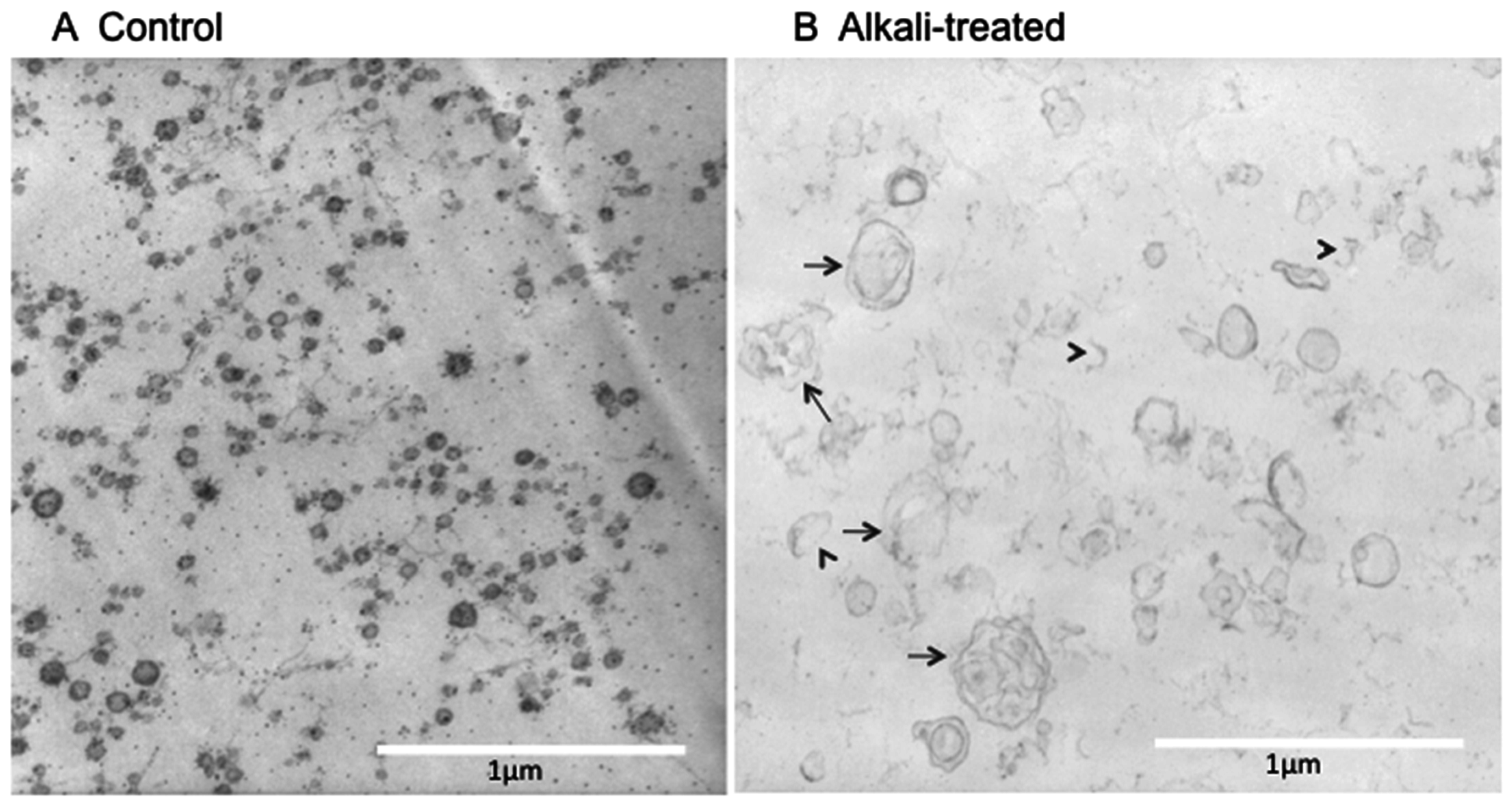
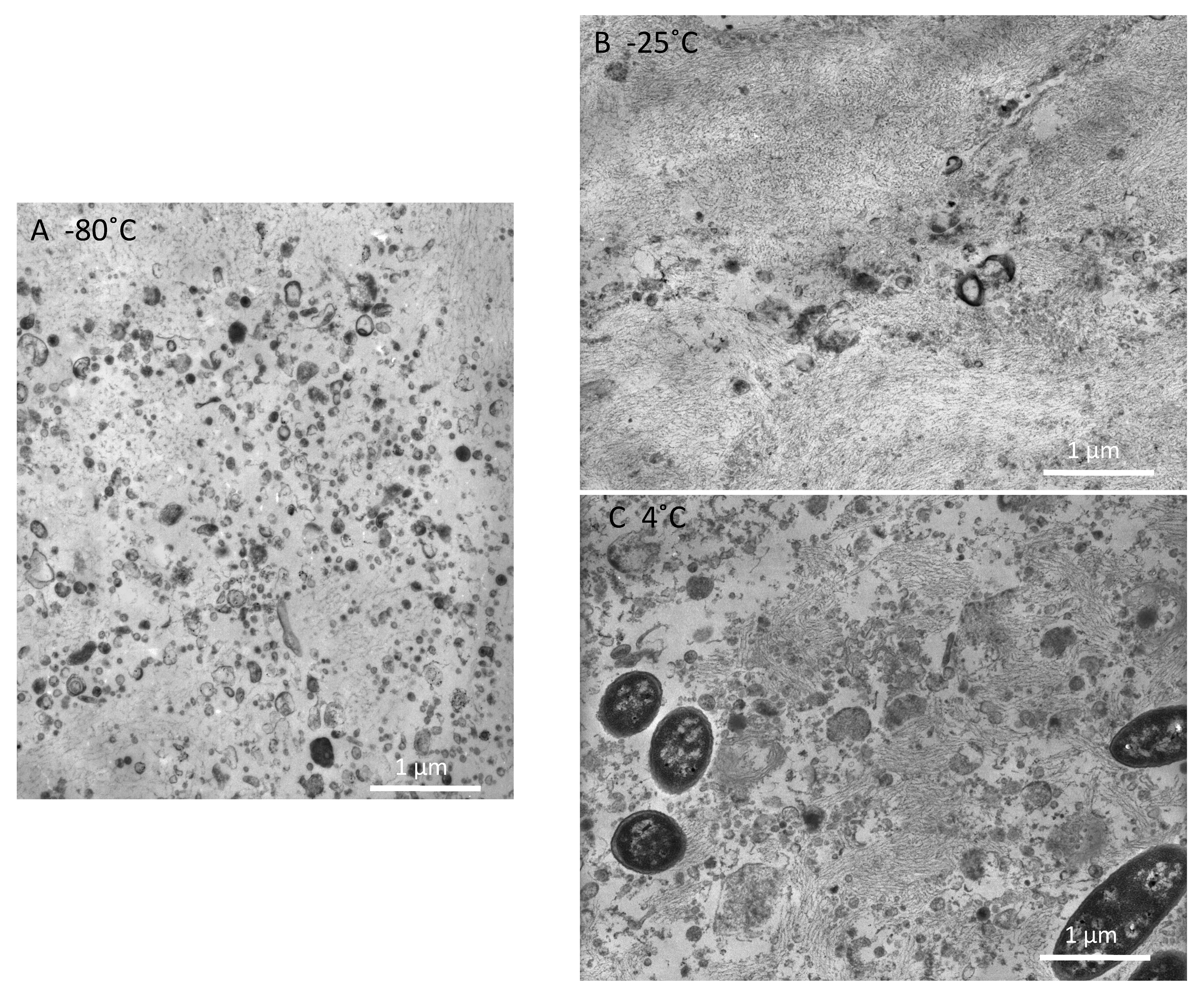
2.1. Electron Microscope (EM) Observation
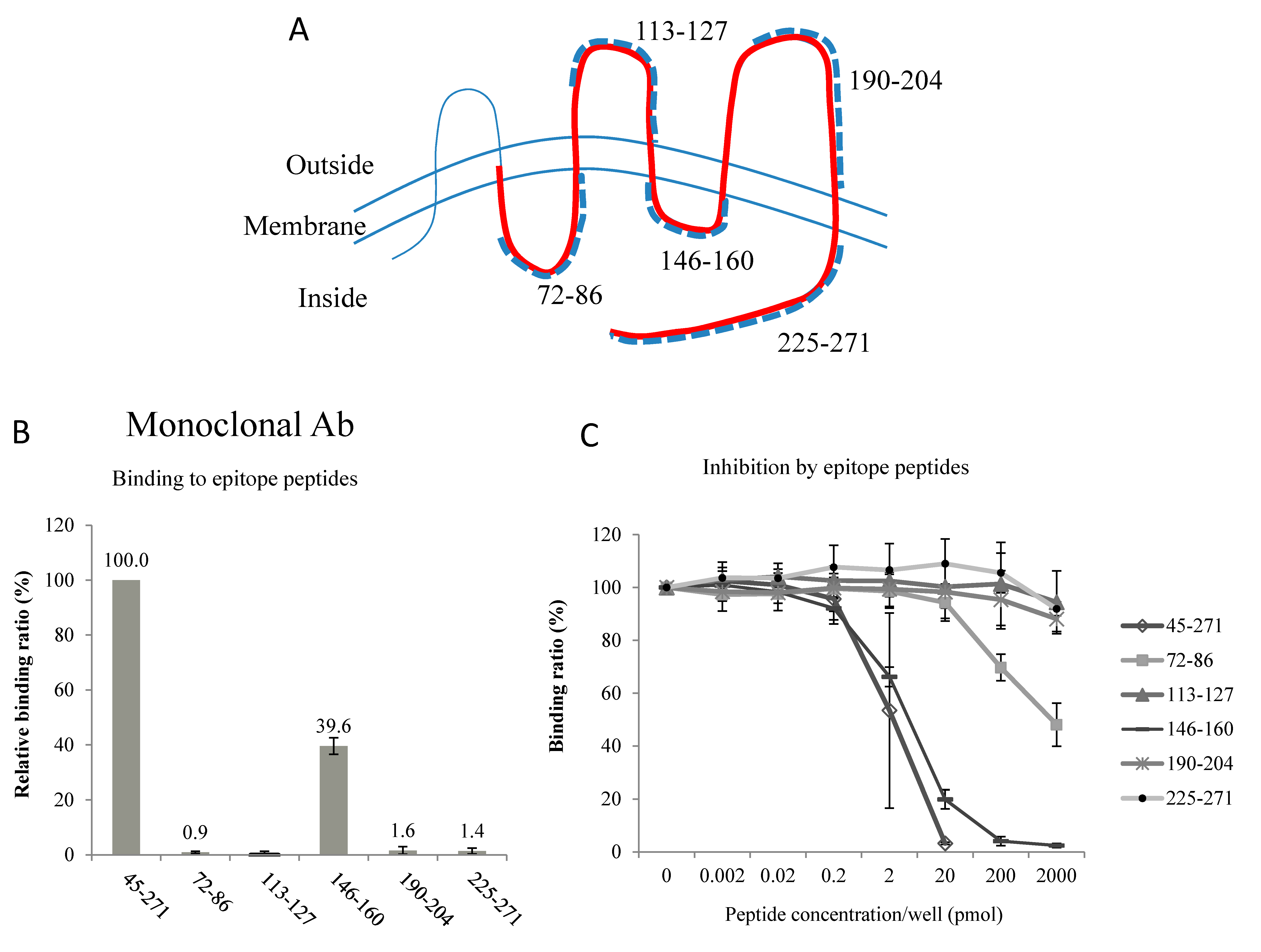
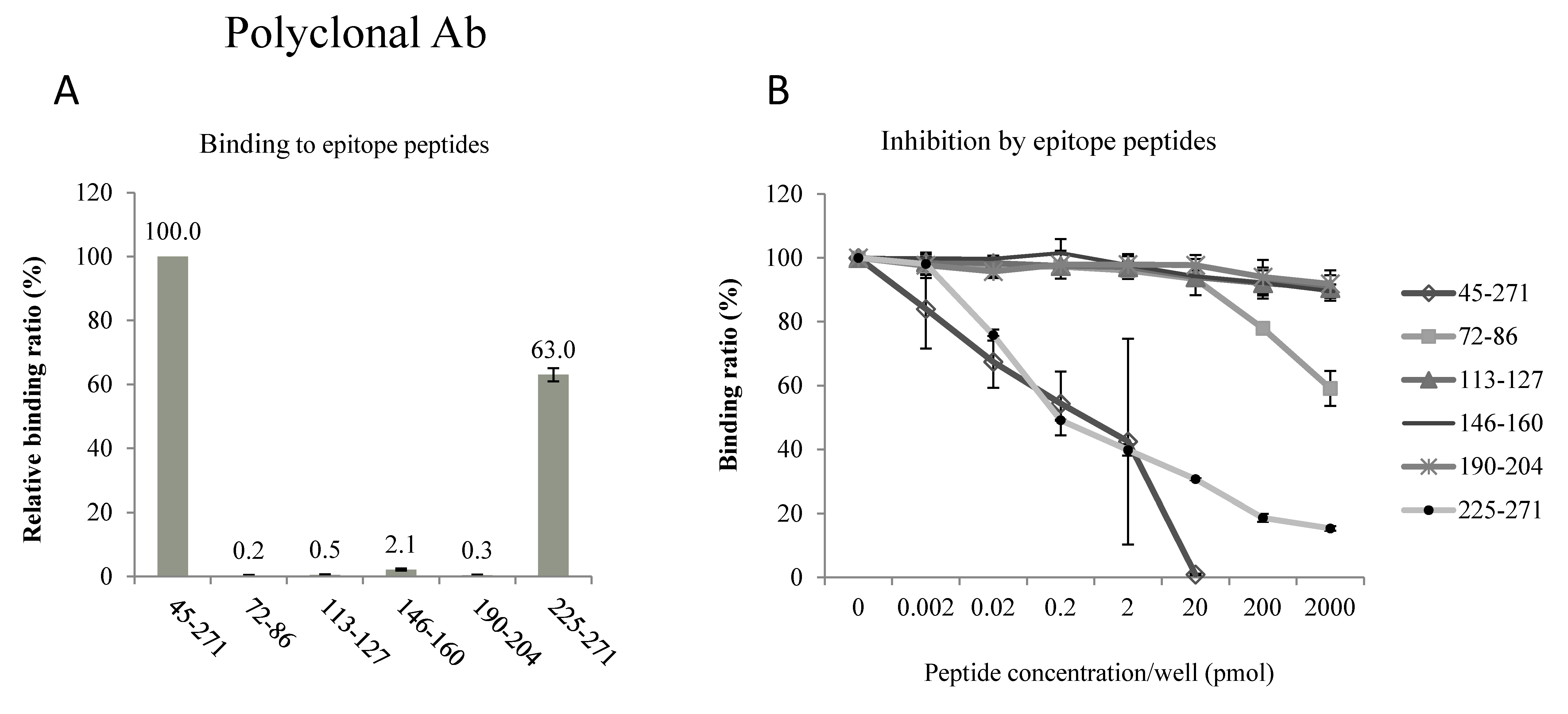
2.2. Epitopes of the AQP2 Antibodies
3. Discussion
4. Materials and Methods
4.1. EM
4.2. Epitopes of the Antibodies
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sasaki, S. Aquaporin 2: From its discovery to molecular structure and medical implications. Mol. Asp. Med. 2012, 33, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Radin, M.J.; Yu, M.J.; Stoedkilde, L.; Miller, R.L.; Hoffert, J.D.; Frokiaer, J.; Pisitkun, T.; Knepper, M.A. Aquaporin-2 regulation in health and disease. Vet. Clin. Pathol. 2012, 41, 455–470. [Google Scholar] [CrossRef] [PubMed]
- Noda, Y.; Sohara, E.; Ohta, E.; Sasaki, S. Aquaporins in kidney pathophysiology. Nat. Rev. Nephrol. 2010, 6, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Kanno, K.; Sasaki, S.; Hirata, Y.; Ishikawa, S.; Fushimi, K.; Nakanishi, S.; Bichet, D.G.; Marumo, F. Urinary excretion of aquaporin-2 in patients with diabetes insipidus. N. Engl. J. Med. 1995, 332, 1540–1545. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Frokiaer, J.; Kwon, T.H.; Nielsen, S. Urinary excretion of aquaporin-2 in rat is mediated by a vasopressin-dependent apical pathway. J. Am. Soc. Nephrol. 1999, 10, 1416–1429. [Google Scholar] [PubMed]
- Pisitkun, T.; Shen, R.F.; Knepper, M.A. Identification and proteomic profiling of exosomes in human urine. Proc. Natl. Acad. Sci. USA 2004, 101, 13368–13373. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, S.E.; Schrier, R.W. Pathophysiological roles of arginine vasopressin and aquaporin-2 in impaired water excretion. Clin. Endocrinol. 2003, 58, 1–17. [Google Scholar] [CrossRef]
- Kortenoeven, M.L.; Fenton, R.A. Renal aquaporins and water balance disorders. Biochim. Biophys. Acta 2014, 1840, 1533–1549. [Google Scholar] [CrossRef] [PubMed]
- Imamura, T.; Kinugawa, K. Urine Aquaporin-2: A promising marker of response to the arginine vasopressin type-2 antagonist, tolvaptan in patients with congestive heart failure. Int. J. Mol. Sci. 2016, 14, 17. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, H.; Kurosaki, M.; Hosokawa, T.; Takahashi, Y.; Itakura, J.; Suzuki, S.; Yasui, Y.; Tamaki, N.; Nakakuki, N.; Takada, H.; et al. Urinary excretion of the water channel aquaporin 2 correlated with the pharmacological effect of tolvaptan in cirrhotic patients with ascites. J. Gastroenterol. 2016, 51, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, S.; Saijo, Y.; Ohmoto, Y.; Iwata, F.; Koga, D.; Katsuragi, K. Alkali treatment stabilizes fluctuations of urine AQP2 values measured by ELISA. Clin. Exp. Nephrol. 2015, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kowal, J.; Tkach, M.; Théry, C. Biogenesis and secretion of exosomes. Curr. Opin. Cell Biol. 2014, 29, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Salih, M.; Zietse, R.; Hoorn, E.J. Urinary extracellular vesicles and the kidney: Biomarkers and beyond. Am. J. Physiol. Ren. Physiol. 2014, 306, F1251–F1259. [Google Scholar] [CrossRef] [PubMed]
- Salih, M.; Fenton, R.A.; Knipscheer, J.; Janssen, J.W.; Vredenbregt-van den Berg, M.S.; Jenster, G.; Zietse, R.; Hoorn, E.J. An immunoassay for urinary extracellular vesicles. Am. J. Physiol. Ren. Physiol. 2016, 310, 796–801. [Google Scholar] [CrossRef] [PubMed]
- Imamura, T.; Kinugawa, K.; Komuro, I. Tolvaptan prolongs blockage of the vasopressin Type II receptor over 24 hours in responders with stage D heart failure. Int. Heart J. 2016, 57, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Frick, A.; Eriksson, U.K.; de Mattia, F.; Oberg, F.; Hedfalk, K.; Neutze, R.; de Grip, W.J.; Deen, P.M.; Törnroth-Horsefield, S. X-ray structure of human aquaporin 2 and its implications for nephrogenic diabetes insipidus and trafficking. Proc. Natl. Acad. Sci. USA 2014, 111, 6305–6310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasaki, S.; Ohmoto, Y.; Mori, T.; Iwata, F.; Muraguchi, M. Daily variance of urinary excretion of AQP2 determined by sandwich ELISA method. Clin. Exp. Nephrol. 2012, 16, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Yuen, P.S.; Pisitkun, T.; Gonzales, P.A.; Yasuda, H.; Dear, J.W.; Gross, P.; Knepper, M.A.; Star, R.A. Collection, storage, preservation, and normalization of human urinary exosomes for biomarker discovery. Kidney Int. 2006, 69, 1471–1476. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nameta, M.; Saijo, Y.; Ohmoto, Y.; Katsuragi, K.; Yamamoto, K.; Yamamoto, T.; Ishibashi, K.; Sasaki, S. Disruption of Membranes of Extracellular Vesicles Is Necessary for ELISA Determination of Urine AQP2: Proof of Disruption and Epitopes of AQP2 Antibodies. Int. J. Mol. Sci. 2016, 17, 1634. https://doi.org/10.3390/ijms17101634
Nameta M, Saijo Y, Ohmoto Y, Katsuragi K, Yamamoto K, Yamamoto T, Ishibashi K, Sasaki S. Disruption of Membranes of Extracellular Vesicles Is Necessary for ELISA Determination of Urine AQP2: Proof of Disruption and Epitopes of AQP2 Antibodies. International Journal of Molecular Sciences. 2016; 17(10):1634. https://doi.org/10.3390/ijms17101634
Chicago/Turabian StyleNameta, Masaaki, Yoko Saijo, Yasukazu Ohmoto, Kiyonori Katsuragi, Keiko Yamamoto, Tadashi Yamamoto, Kenichi Ishibashi, and Sei Sasaki. 2016. "Disruption of Membranes of Extracellular Vesicles Is Necessary for ELISA Determination of Urine AQP2: Proof of Disruption and Epitopes of AQP2 Antibodies" International Journal of Molecular Sciences 17, no. 10: 1634. https://doi.org/10.3390/ijms17101634