Development of 12 Microsatellite Markers in Dorcus titanus castanicolor (Motschulsky, 1861) (Lucanidae, Coleoptera) from Korea Using Next-Generation Sequencing
Abstract
:1. Introduction
2. Results and Discussion
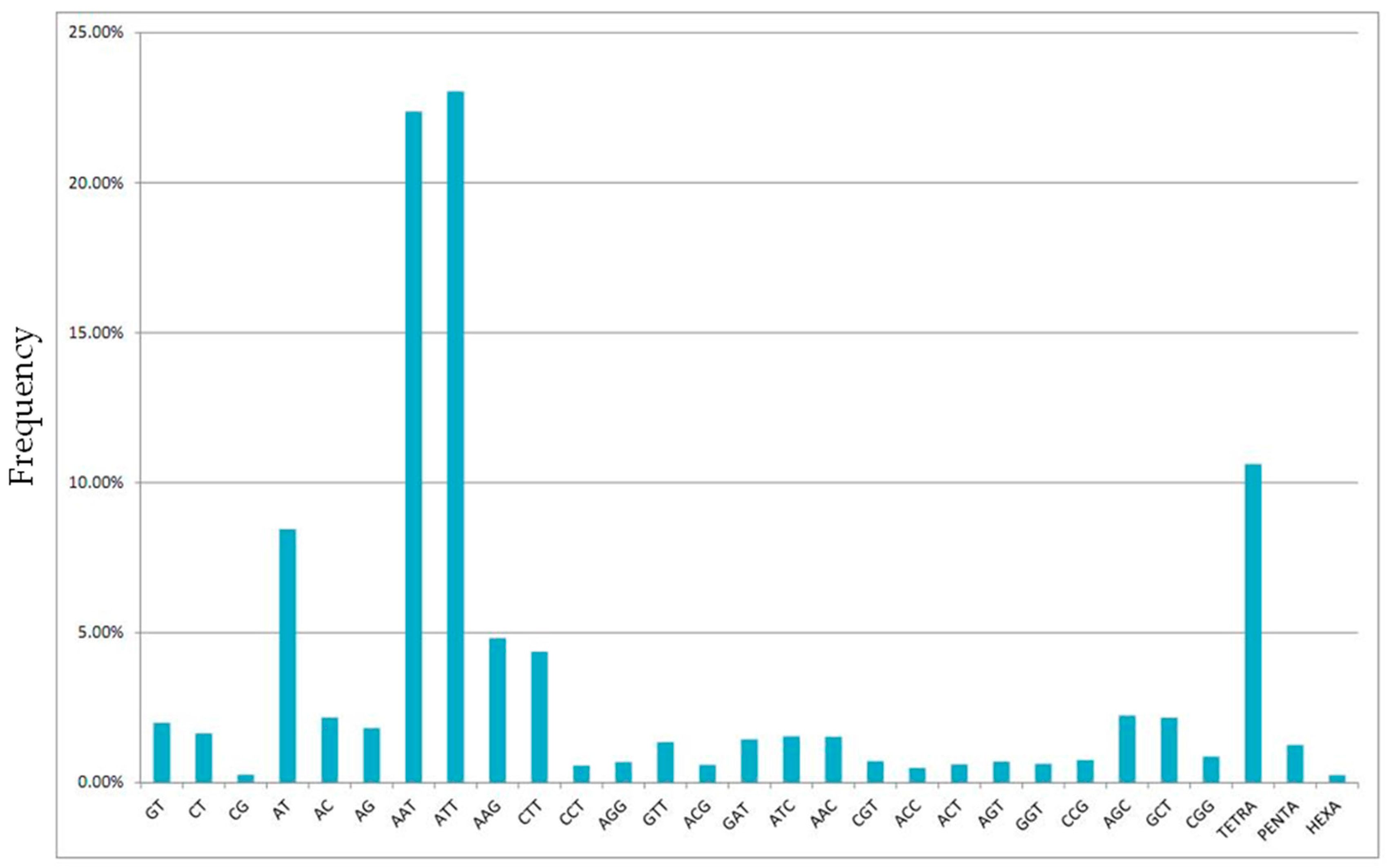
2.1. Next-Generation Sequencing and Microsatellite Loci Identification
2.2. Microsatellite Marker Assessment
2.3. Genetic Diversity of the Korean Dorcus titanus castanicolor Population
3. Materials and Methods
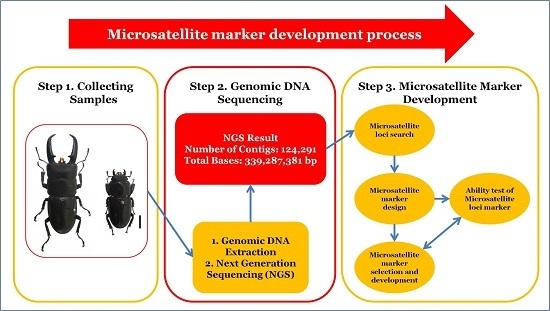
3.1. Sample Collection and Genomic DNA Extraction
3.2. Next-Generation Sequencing, Microsatellite Loci Identification, Marker Selection, and Genotyping
3.3. Data Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Motschulsky, V. Insectes du Japon (continuation). Etudes Ent. 1861, 10, 3–24. [Google Scholar]
- Mizunuma, T.; Nagai, S. The Lucanid Beetles of the World; Mushi-sha: Tokyo, Japan, 1994; pp. 47–202. [Google Scholar]
- Kim, S.I.; Kim, J.I. Review of family Lucanidae (Insecta: Coleoptera) in Korea with the description of one new species. Entomol. Res. 2010, 40, 55–81. [Google Scholar] [CrossRef]
- Kim, S.I.; Kim, J.I. Insect Fauna of Korea. In Arthropoda: Insect: Coleoptera: Scarabaeoidea: Lucanidae and Passalidae; National Institute of Biological Resources: Incheon, Korea, 2014; pp. 1–58. [Google Scholar]
- Choi, Y.C.; Kim, N.J.; Park, I.K.; Lee, S.B.; Hwang, J.S. RDA interrobang IV; Rural Development Administration (in Korean): Suwon, Korea, 2011; pp. 1–20.
- Kang, T.H.; Han, S.H.; Weon, H.Y.; Lee, Y.B.; Kim, N.; Nam, S.H.; Park, H.C. Purification and identification of Paenibacillus sp., isolated from diseased larvae of Allomyrina dichotoma (Linnaeus, 1771) (Coleoptera: Scarabaeidae) in Insect Farms. Int. J. Ind. Entomol. 2012, 25, 195–203. [Google Scholar] [CrossRef]
- Bae, S.M.; Jun, Y.S.; Shin, T.Y.; Woo, S.D. An analysis of likability of insects in Korea. Int. J. Ind. Entomol. 2014, 29, 193–197. [Google Scholar] [CrossRef]
- Kim, J. Regional Variation of Dorcus titanus castanicolor (Motschulsky, 1861) (Coleoptera: Lucanidae) in Northern and Southern South Korea. Scarabs 2014, 75, 1–6. [Google Scholar]
- Goka, K.; Kojima, H.; Okabe, K. Biological invasion caused by commercialization of Stag Beetles in Japan. Glob. Environ. Res. 2004, 8, 67–74. [Google Scholar]
- Saunders, W.W. Characters of undescribed Lucanidae, collected in China, by R. Fortune, Esq. Trans. Entomol. Soc. Lond. 1854, 3, 45–55. [Google Scholar] [CrossRef]
- Kang, T.H.; Han, S.H.; Park, S.J. Development of seven microsatellite markers using next generation sequencing for the conservation on the Korean population of Dorcus hopei (E. Saunders, 1854) (Coleoptera, Lucanidae). Int. J. Mol. Sci. 2015, 16, 21330–21341. [Google Scholar] [CrossRef] [PubMed]
- Koressaar, T.; Remm, M. Enhancements and modifications of primer design program Primer3. Bioinformatics 2007, 23, 1289–1291. [Google Scholar] [CrossRef] [PubMed]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3—New capabilities and interfaces. Nucleic Acids Res. 2012. [Google Scholar] [CrossRef] [PubMed]
- Schuelke, M. An economic method for the fluorescent labeling of PCR fragments. Nat. Biotechnol. 2000, 18, 233–234. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Muse, S.V. PowerMarker: Integrated analysis environment for genetic marker data. Bioinformatics 2005, 21, 2128–2129. [Google Scholar] [CrossRef] [PubMed]
- Leese, F.; Mayer, C.; Held, C. Isolation of microsatellites from unknown genomes using known genomes as enrichment templates. Limnol. Oceanogr. Method 2008, 7, 412–426. [Google Scholar] [CrossRef]
- Mayer, C.; Leese, F.; Tollrian, R. Genome-wide analysis of tandem repeats in Daphnia pulex—A comparative approach. BMC Genom. 2010, 11, 277–305. [Google Scholar] [CrossRef] [PubMed]
- Oosterhout, C.V.; Hutchinson, W.F.; Wills, D.P.M.; Shipley, P. MICRO-CHECKER: Software for identifying and correcting genotyping errors in microsatellite data. Mol. Ecol. Notes 2004, 4, 535–538. [Google Scholar] [CrossRef]
- Excoffier, L.; Laval, G.; Schneider, S. Arlequin version 3.1: An integrated software package for population-genetics data analysis. Evol. Bioinform. 2005, 1, 47–50. [Google Scholar]
| Number of Contigs (>1000 bp) | Total Bases | Maximum Length | N50 Length | Average Contig Length | GC Content |
|---|---|---|---|---|---|
| 124,291 | 339,287,381 | 103,231 | 3258 | 2729 | 33.98% |
| MSL | Forward Sequence | Reverse Sequence | RM | SS | MN | NAn |
|---|---|---|---|---|---|---|
| 31,870 | CGACACTCACACGCAATAAATAG | GCCATTCCATCGGTGAATTTTTA | (CGTT)6 | 214 bp | DT01 | KX268665 |
| 13,508 | TGAAGTTTCAGAAGGAGTTTGGA | CAATCAACTCCGGATAGAGTGAA | (ACAT)6 | 264 bp | DT02 | KX268666 |
| 78,687 | AGAACGCGTGAACTTGTTTATTT | TTGTCACGTTCTAAAGCGTTTTT | (AAG)8 | 244 bp | DT03 | KX268667 |
| 9989 | CCCCACGAACAATAAATCAGAAC | TACTTCCGCTTACAGCAGTAAAT | (CGT)8 | 202 bp | DT05 | KX268668 |
| 9645 | TTATGACGAACTTGTTTTGGCAG | TGTGGAATTTTGTTTCGGATGTT | (AC)12 | 298 bp | DT06 | KX268669 |
| 8497 | GTCATCTTACTAGAGGTTGGTCG | CAACACTTCTTCTAATGCACTCG | (AAG)17 | 205 bp | DT08 | KX268670 |
| 15,216 | ATTACGTAGACATGGCTTAAGCA | TCCTCATGCCAAATATGATCCAA | (AACT)10 | 245 bp | DT11 | KX268671 |
| 29,993 | TAATGACGCCATTCACACATTTC | GGTCTTTAAACAGTCTGCCAAAA | (GT)20 | 239 bp | DT12 | KX268672 |
| 12,743 | ACTTGACTGTCACATACACACAT | TTGTATGTCTGGCTTTGGTTACT | (CT)19 | 212 bp | DT13 | KX268673 |
| 30,799 | AAGACGATCGGCAATTTCAAAAT | TAGACCAGAGTCCTTCATTACCA | (AG)17 | 209 bp | DT15 | KX268675 |
| 24,714 | GAGCACCTAGATAATCAACCCAA | GATTTGGTTTCTTGCATTGTCCT | (AAG)11 | 230 bp | DT17 | KX268676 |
| 3144 | TCATTTGGACGTAGCAAATGTTC | TGCATGTTGAAAGGTACCAAGTA | (AAC)10 | 260 bp | DT24 | KX675371 |
| 3488 | TAATCCTTGCATGCAGCCTAATA | AGTTTGTGTTTGTGATGAAGTGG | (AGC)10 | 298 bp | DT25 | KX675372 |
| 74,360 | GTTATGGTGTGAGCTGGAATTTC | ATTTACATCAAGATTCGCCGTTG | (GTT)10 | 271 bp | DT26 | KX675373 |
| 557 | AACAAATAACGTGTTGGAGTTCG | AGTAGATGAGAACTGTGTTTGGG | (ACAT)7 | 284 bp | DT27 | KX675374 |
| 4515 | TCCACGTTATCTTTCAAAACACG | TTCAAGTTTGGGTGAAACAATCG | (CT)14 | 226 bp | DT28 | KX675375 |
| 2260 | AACATGACCCACTAAGGTATCAA | CTAACAAGAAGTACCACCACCAT | (GCT)9 | 300 bp | DT32 | KX675376 |
| 16,503 | ACGCTACTGGCAATCAAATAAAC | GCACCCACAAACAACATACATAA | (ACT)9 | 284 bp | DT33 | KX675377 |
| 426 | GCACGTCACGACTATTTTGATTT | TAACTCGTTAATGAACATGTGCG | (AC)13 | 210 bp | DT35 | KX675378 |
| MSL | DT1 | DT2 | DT3 | DT5 | DT6 | DT8 | DT11 | DT12 | DT13 | DT15 |
| NP | no | no | no | no | no | no | no | no | no | no |
| NFreq | −0.1909 | −0.1408 | −0.3352 | −0.3970 | −0.1199 | −0.0986 | −0.1269 | −0.0874 | −0.2445 | −0.0930 |
| MSL | DT17 | DT24 | DT25 | DT26 | DT27 | DT28 | DT32 | DT33 | DT35 | – |
| NP | no | no | no | no | no | no | no | no | no | – |
| NFreq | −0.1109 | 0.0250 | −0.0022 | −0.6180 | −0.0642 | −0.0800 | 0.0171 | −0.0144 | −0.1185 | – |
| MSL | MAF | Gn | SS | An | He | Ho | PIC | HWE |
|---|---|---|---|---|---|---|---|---|
| DT1 | 0.8182 | 3 | 22 | 3 | 0.3140 | 0.3636 | 0.2918 | 1.0000 |
| DT2 | 0.6591 | 5 | 22 | 6 | 0.5103 | 0.6364 | 0.4619 | 0.1100 |
| DT6 | 0.3409 | 9 | 22 | 6 | 0.7789 | 0.9545 | 0.7466 | 0.0120 |
| DT12 | 0.3409 | 10 | 22 | 6 | 0.7397 | 0.8636 | 0.6968 | 0.5770 |
| DT13 | 0.3864 | 7 | 22 | 6 | 0.7118 | 1.0000 | 0.6647 | 0.0190 |
| DT15 | 0.3636 | 9 | 22 | 7 | 0.7717 | 0.9091 | 0.7404 | 0.0510 |
| DT17 | 0.4091 | 8 | 22 | 9 | 0.7603 | 0.8636 | 0.7330 | 0.0010 |
| DT24 | 0.6818 | 5 | 22 | 4 | 0.4917 | 0.5000 | 0.4507 | 0.1530 |
| DT25 | 0.4318 | 11 | 22 | 6 | 0.7293 | 0.7273 | 0.6939 | 0.4650 |
| DT27 | 0.4545 | 6 | 22 | 4 | 0.6353 | 0.6818 | 0.5632 | 0.0060 |
| DT28 | 0.3182 | 10 | 22 | 6 | 0.7531 | 0.8636 | 0.7109 | 0.5390 |
| DT32 | 0.7045 | 4 | 22 | 3 | 0.4287 | 0.4091 | 0.3543 | 1.0000 |
| DT33 | 0.4773 | 9 | 22 | 6 | 0.6901 | 0.6818 | 0.6499 | 0.4200 |
| Mean | 0.4913 | 7.3846 | 22 | 5.5385 | 0.6396 | 0.7273 | 0.5968 | – |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, T.H.; Han, S.H.; Park, S.J. Development of 12 Microsatellite Markers in Dorcus titanus castanicolor (Motschulsky, 1861) (Lucanidae, Coleoptera) from Korea Using Next-Generation Sequencing. Int. J. Mol. Sci. 2016, 17, 1621. https://doi.org/10.3390/ijms17101621
Kang TH, Han SH, Park SJ. Development of 12 Microsatellite Markers in Dorcus titanus castanicolor (Motschulsky, 1861) (Lucanidae, Coleoptera) from Korea Using Next-Generation Sequencing. International Journal of Molecular Sciences. 2016; 17(10):1621. https://doi.org/10.3390/ijms17101621
Chicago/Turabian StyleKang, Tae Hwa, Sang Hoon Han, and Sun Jae Park. 2016. "Development of 12 Microsatellite Markers in Dorcus titanus castanicolor (Motschulsky, 1861) (Lucanidae, Coleoptera) from Korea Using Next-Generation Sequencing" International Journal of Molecular Sciences 17, no. 10: 1621. https://doi.org/10.3390/ijms17101621