Protein/CaCO3/Chitin Nanofiber Complex Prepared from Crab Shells by Simple Mechanical Treatment and Its Effect on Plant Growth
Abstract
:1. Introduction
2. Results and Discussion
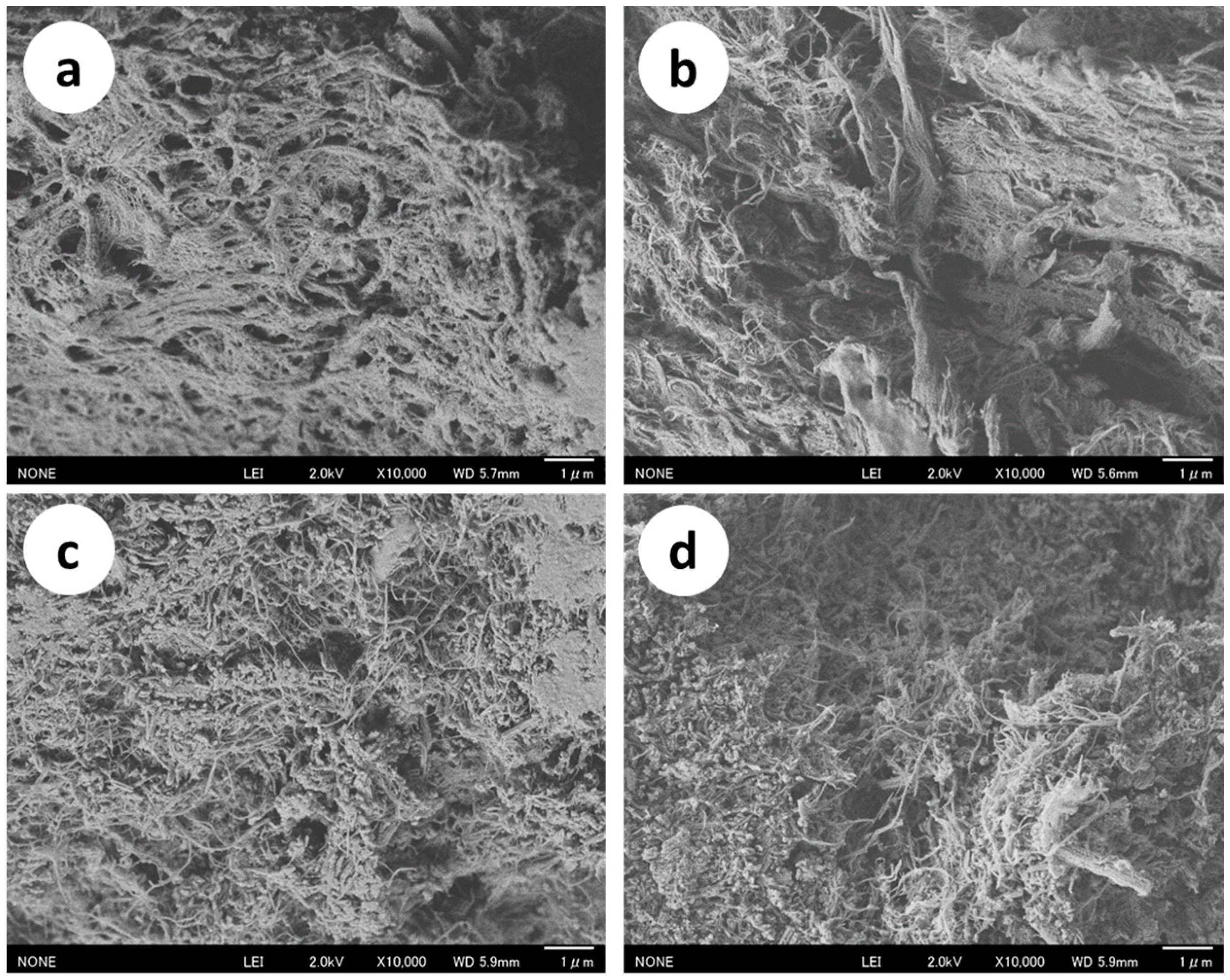
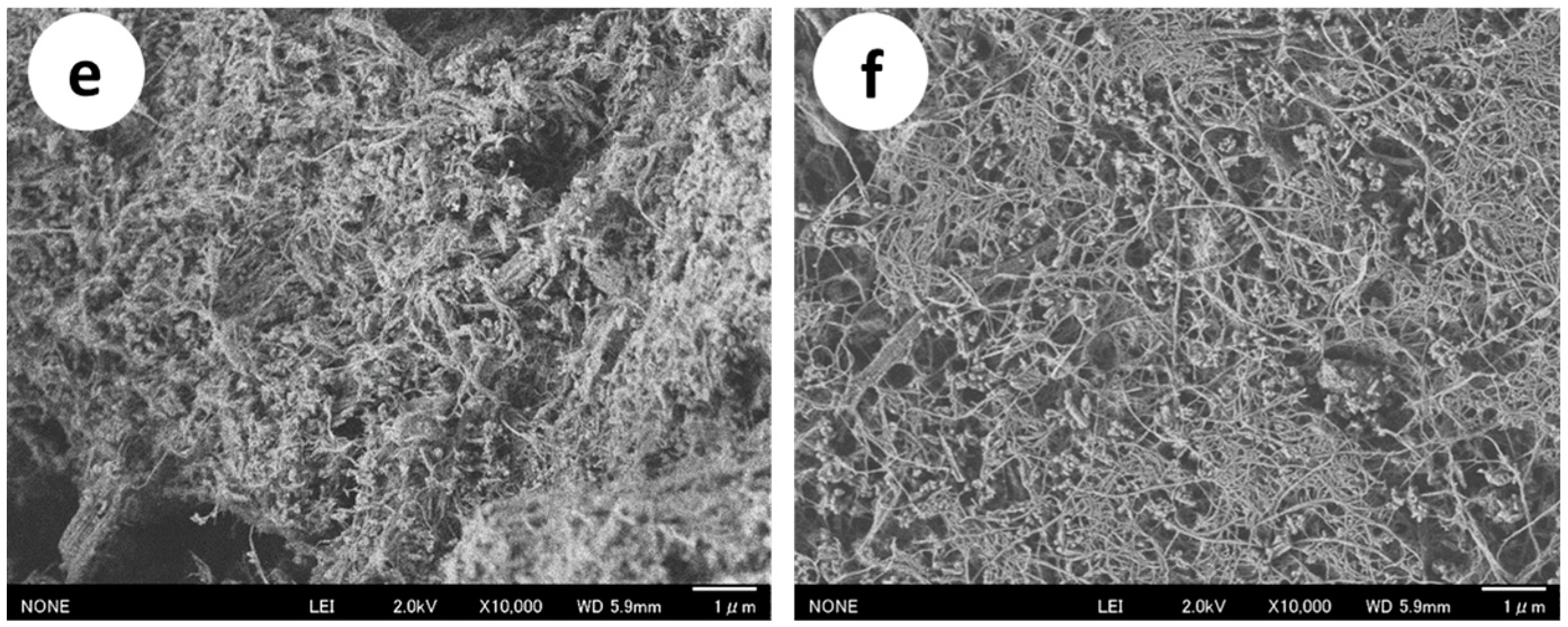
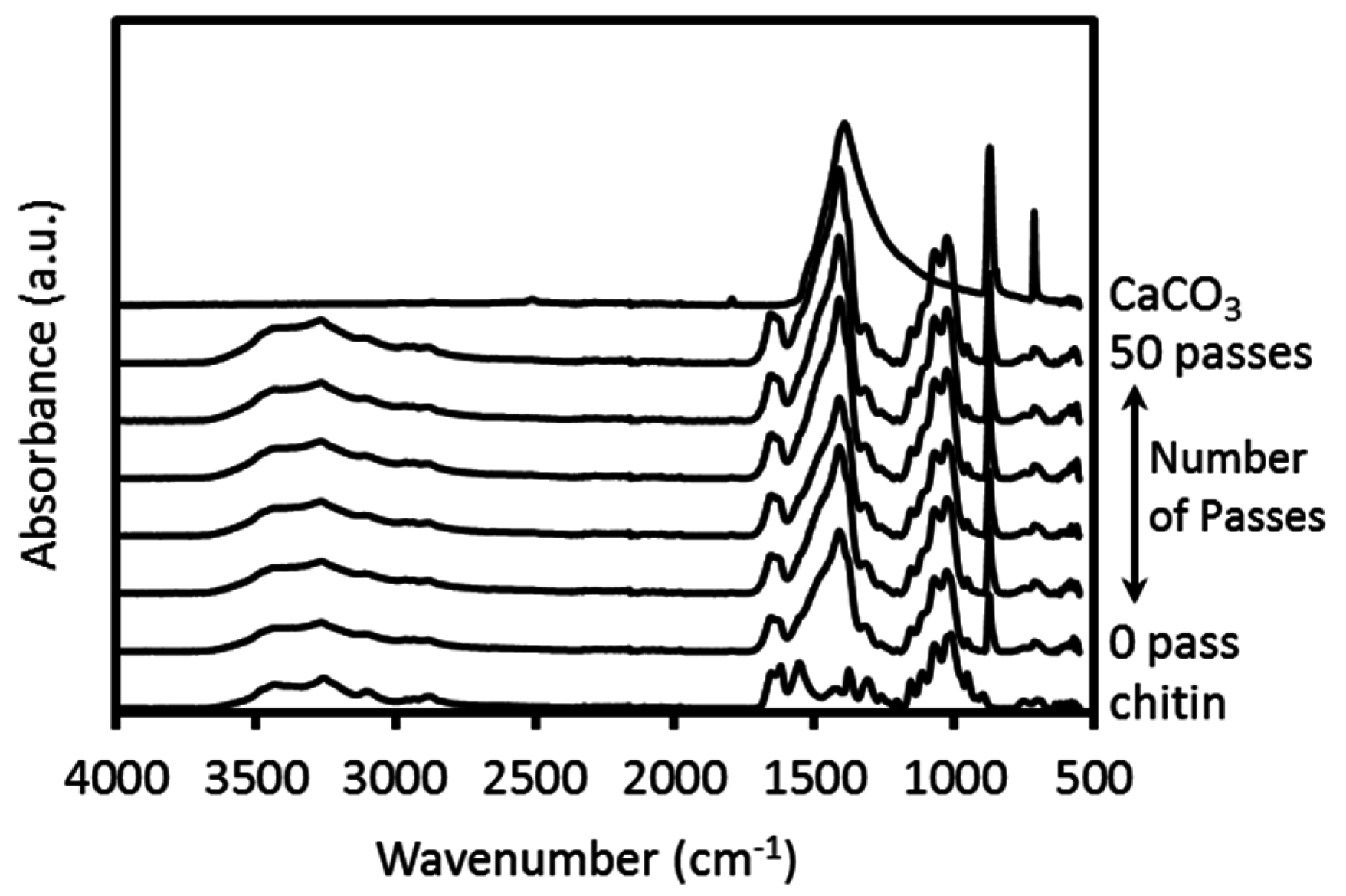
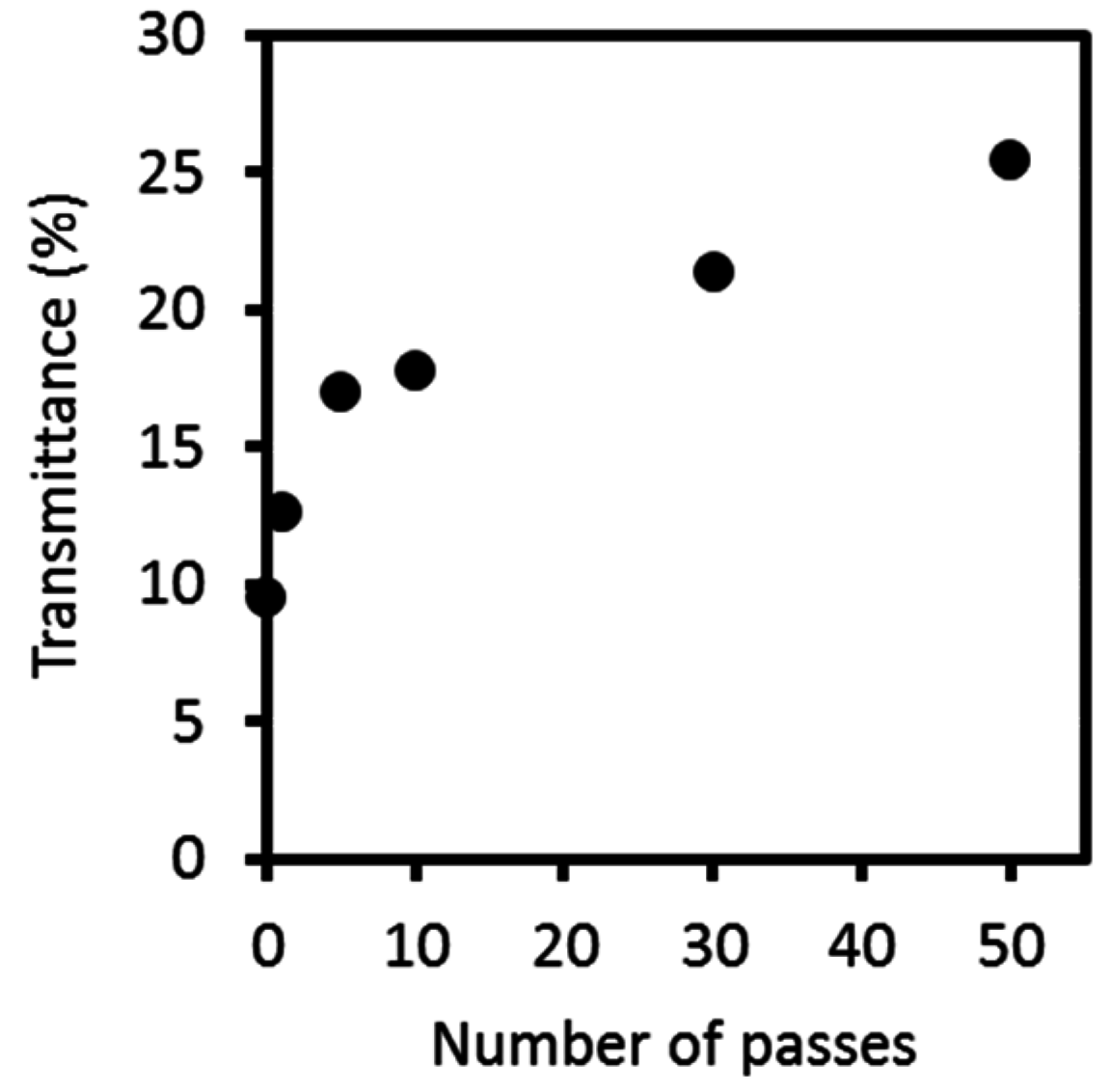
2.1. Preparation and Characterization of the Protein/CaCO3/Chitin Nanofiber Complex
2.2. Plant Growth Effect of the Protein/CaCO3/Chitin Nanofiber Complex
3. Materials and Methods
3.1. Materials
3.2. Preparation of the Protein/CaCO3/Chitin Nanofiber Complex
3.3. Preparation of the Protein/Chitin Nanofiber Complex and Pure Chitin Nanofibers
3.4. Characterization
3.5. Plant Material
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Raabe, D.; Sachs, C.; Romano, P. The crustacean exoskeleton as an example of a structurally and mechanically graded biological nanocomposite material. Acta Mater. 2005, 53, 4281–4292. [Google Scholar] [CrossRef]
- Ifuku, S.; Nogi, M.; Abe, K.; Yoshioka, M.; Morimoto, M.; Saimoto, H.; Yano, H. Preparation of chitin nanofibers with a uniform width as α-chitin from crab shells. Biomacromolecules 2009, 10, 1584–1588. [Google Scholar] [CrossRef] [PubMed]
- Ifuku, S.; Nogi, M.; Yoshioka, M.; Morimoto, M.; Yano, H.; Saimoto, H. Fibrillation of dried chitin into 10–20 nm nanofibers by a simple method under acidic conditions. Carbohydr. Polym. 2010, 81, 134–139. [Google Scholar] [CrossRef]
- Tsutsumi, Y.; Koga, H.; Qi, Z.; Saito, T.; Isogai, A. Nanofibrillar chitin aerogels as renewable base catalysts. Biomacromolecules 2014, 15, 4314–4319. [Google Scholar] [CrossRef] [PubMed]
- Ifuku, S.; Morooka, S.; Nakagaito, A.N.; Morimoto, M.; Saimoto, H. Preparation and characterization of optically transparent chitin nanofiber/(meth)acrylic resin composites. Green Chem. 2011, 13, 1708–1711. [Google Scholar] [CrossRef]
- Ifuku, S.; Ikuta, A.; Egusa, M.; Kaminaka, H.; Izawa, H.; Morimoto, M.; Saimoto, H. Preparation of high-strength transparent chitosan film reinforced with surface-deacetylated chitin nanofibers. Carbohydr. Polym. 2013, 98, 1198–1202. [Google Scholar] [CrossRef] [PubMed]
- Azuma, K.; Ifuku, S.; Osaki, T.; Okamoto, Y.; Minami, S. Preparation and biomedical applications of chitin and chitosan nanofibers. J. Biomed. Nanotechnol. 2014, 10, 2891–2920. [Google Scholar] [CrossRef] [PubMed]
- Azuma, K.; Izumi, R.; Kawata, M.; Nagae, T.; Osaki, T.; Murahata, Y.; Tsuka, T.; Imagawa, T.; Ito, T.; Okamoto, Y.; et al. Effects of oral administration of chitin nanofiber on plasma metabolites and gut microorganisms. Int. J. Mol. Sci. 2015, 16, 21931–21949. [Google Scholar] [CrossRef] [PubMed]
- Izumi, R.; Komada, S.; Ochi, K.; Karasawa, L.; Osaki, T.; Murahata, Y.; Tsuka, T.; Imagawa, T.; Itoh, N.; Okamoto, Y.; et al. Favorable effects of superficially deacetylated chitin nanofibrils on the wound healing process. Carbohydr. Polym. 2015, 123, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Izumi, R.; Azuma, K.; Izawa, H.; Morimoto, M.; Nagashima, M.; Osaki, T.; Tsuka, T.; Imagawa, T.; Ito, N.; Okamoto, Y.; et al. Chitin nanofibrils suppress skin inflammation in atopic dermatitis-like skin lesions in NC/Nga mice. Carbohydr. Polym. 2016, 146, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Gopalan, N.K.; Dufresne, A. Crab shell chitin whisker reinforced natural rubber nanocomposites. 1. Processing and swelling behavior. Biomacromolecules 2003, 4, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Ifuku, S.; Urakami, T.; Izawa, H.; Morimoto, M.; Saimoto, H. Preparation of a protein-chitin nanofiber complex from crab shells and its application as a reinforcement filler or substrate for biomineralization. RSC Adv. 2015, 5, 64196–64201. [Google Scholar] [CrossRef]
- Wei, H.; Shena, Q.; Zhao, Y.; Wang, D.J.; Xu, D.F. Influence of polyvinylpyrrolidone on the precipitation of calcium carbonate and on the transformation of vaterite to calcite. J. Cryst. Growth 2003, 250, 516–524. [Google Scholar] [CrossRef]
- Fan, Y.; Saito, T.; Isogai, A. Individual chitin nano-whiskers prepared from partially deacetylated α-chitin by fibril surface cationization. Carbohydr. Polym. 2010, 79, 1046–1051. [Google Scholar] [CrossRef]
- Dutta, A.K.; Yamada, K.; Izawa, H.; Morimoto, M.; Saimoto, H.; Ifuku, S. Preparation of chitin nanofibers from dry chitin powder by star burst system: dependence on number of passes. J. Chitin Chitosan Sci. 2013, 1, 59–64. [Google Scholar] [CrossRef]
- Ifuku, S.; Yamada, K.; Morimoto, M.; Saimoto, H. Nano-fibrillation of dry chitin powder by Star Burst system. J. Nanomater. 2012, 2012, 1–7. [Google Scholar] [CrossRef]
- Shimahara, K.; Takiguchi, Y. Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1998; Volume 161, pp. 417–423. [Google Scholar]


| Day after Treatment | Treatment | Number of Passes | Number of Leaves | Stem Diameter (mm) | Plant Height (cm) |
|---|---|---|---|---|---|
| 5 weeks | Distilled water | - | 3.2 ± 0.1 a | 1.4 ± 0.0 a | 0.9 ± 0.1 a |
| HYPONeX | - | 8.3 ± 0.2 b | 3.0 ± 0.2 b | 11.6 ± 0.8 b | |
| Crab shell | - | 6.0 ± 0.3 c | 2.0 ± 0.1 c | 4.3 ± 0.2 c,d,e | |
| Protein/CaCO3/chitin nanofiber | 0 | 6.3 ± 0.2 c | 2.2 ± 0.1 c | 6.8 ± 1.9 c | |
| 5 | 5.8 ± 0.3 c | 2.0 ± 0.1 c | 4.4 ± 0.4 c,d | ||
| 50 | 6.7 ± 0.2 c | 2.2 ± 0.1 c | 5.0 ± 0.1 c | ||
| Protein/chitin nanofiber | 0 | 3.1 ± 0.2 a | 1.4 ± 0.0 a | 1.0 ± 0.1 a | |
| 5 | 3.5 ± 0.2 a | 1.5 ± 0.0 a | 1.4 ± 0.1 a,d,e | ||
| 50 | 3.6 ± 0.2 a | 1.5 ± 0.0 a | 1.2 ± 0.1 a,e | ||
| Chitin nanofiber | 0 | 3.2 ± 0.1 a | 1.4 ± 0.0 a | 1.0 ± 0.1 a | |
| 5 | 3.0 ± 0.3 a | 1.4 ± 0.0 a | 1.0 ± 0.1 a | ||
| 50 | 3.0 ± 0.1 a | 1.4 ± 0.0 a | 0.9 ± 0.0 a | ||
| 9 weeks | Distilled water | - | ND | ||
| HYPONeX | - | 9.7 ± 0.4 a | 3.3 ± 0.1 a | 16.6 ± 0.5 a | |
| Crab shell | - | 8.4 ± 0.5 a | 2.1 ± 0.1 b | 7.0 ± 0.9 b | |
| Protein/CaCO3/chitin nanofiber | 0 | 9.1 ± 0.3 a | 2.2 ± 0.1 b | 9.1 ± 0.6 b | |
| 5 | 8.6 ± 0.3 a | 2.2 ± 0.1 b | 7.8 ± 0.6 b | ||
| 50 | 9.2 ± 0.3 a | 2.3 ± 0.1 b | 9.3 ± 0.5 b | ||
| Protein/chitin nanofiber | 0 | ND | |||
| 5 | ND | ||||
| 50 | ND | ||||
| Chitin nanofiber | 0 | ND | |||
| 5 | ND | ||||
| 50 | ND | ||||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aklog, Y.F.; Egusa, M.; Kaminaka, H.; Izawa, H.; Morimoto, M.; Saimoto, H.; Ifuku, S. Protein/CaCO3/Chitin Nanofiber Complex Prepared from Crab Shells by Simple Mechanical Treatment and Its Effect on Plant Growth. Int. J. Mol. Sci. 2016, 17, 1600. https://doi.org/10.3390/ijms17101600
Aklog YF, Egusa M, Kaminaka H, Izawa H, Morimoto M, Saimoto H, Ifuku S. Protein/CaCO3/Chitin Nanofiber Complex Prepared from Crab Shells by Simple Mechanical Treatment and Its Effect on Plant Growth. International Journal of Molecular Sciences. 2016; 17(10):1600. https://doi.org/10.3390/ijms17101600
Chicago/Turabian StyleAklog, Yihun Fantahun, Mayumi Egusa, Hironori Kaminaka, Hironori Izawa, Minoru Morimoto, Hiroyuki Saimoto, and Shinsuke Ifuku. 2016. "Protein/CaCO3/Chitin Nanofiber Complex Prepared from Crab Shells by Simple Mechanical Treatment and Its Effect on Plant Growth" International Journal of Molecular Sciences 17, no. 10: 1600. https://doi.org/10.3390/ijms17101600








