Characteristics of Antisense Transcript Promoters and the Regulation of Their Activity
Abstract
:1. Introduction
2. Location of Antisense Promoters (ASPs)
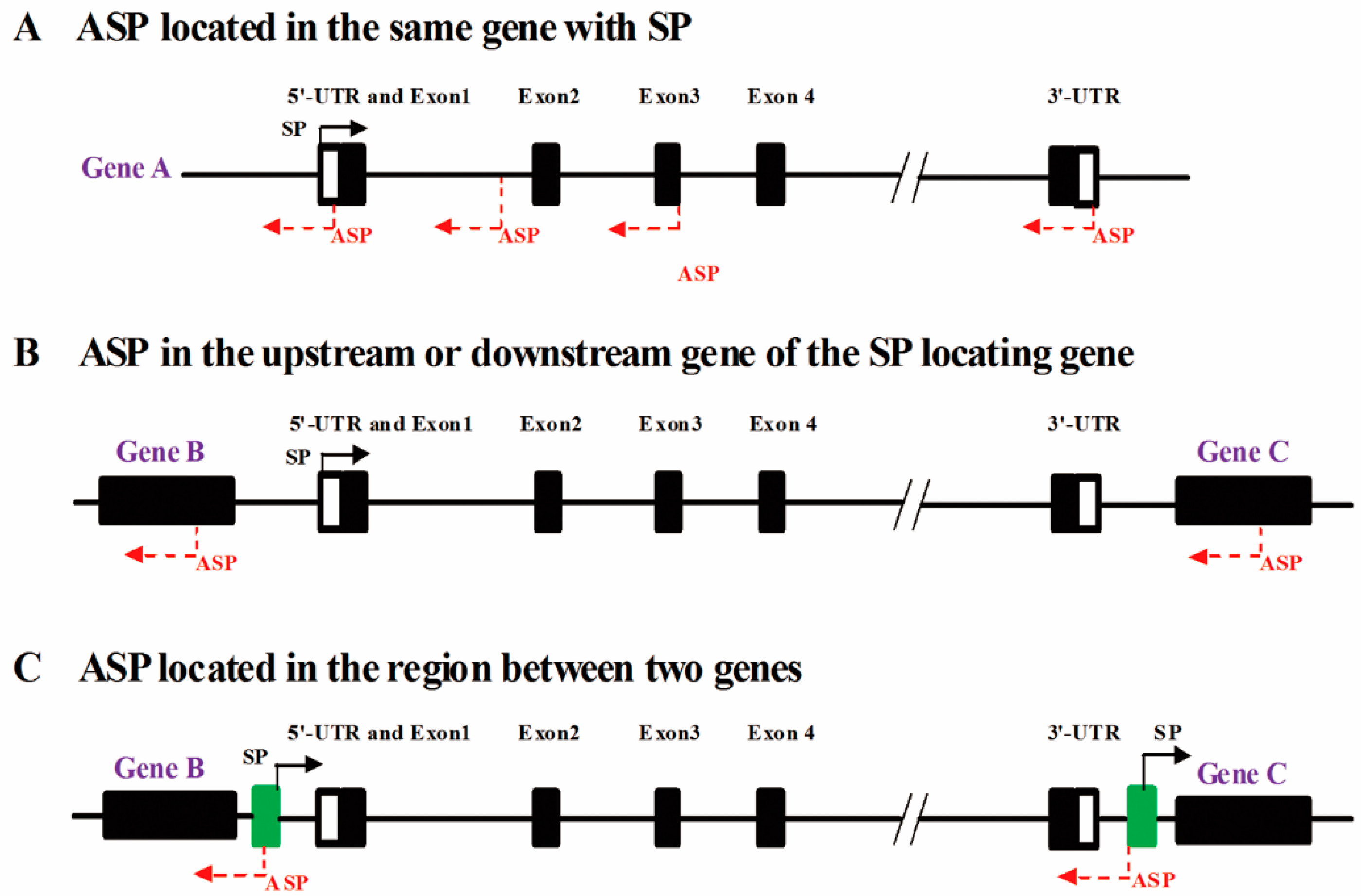
2.1. Both ASPs and Sense Promoters (SPs) Can Be Located in the Same Gene
2.1.1. ASPs and SPs Are Located in the 5′-UTR of the Same Gene
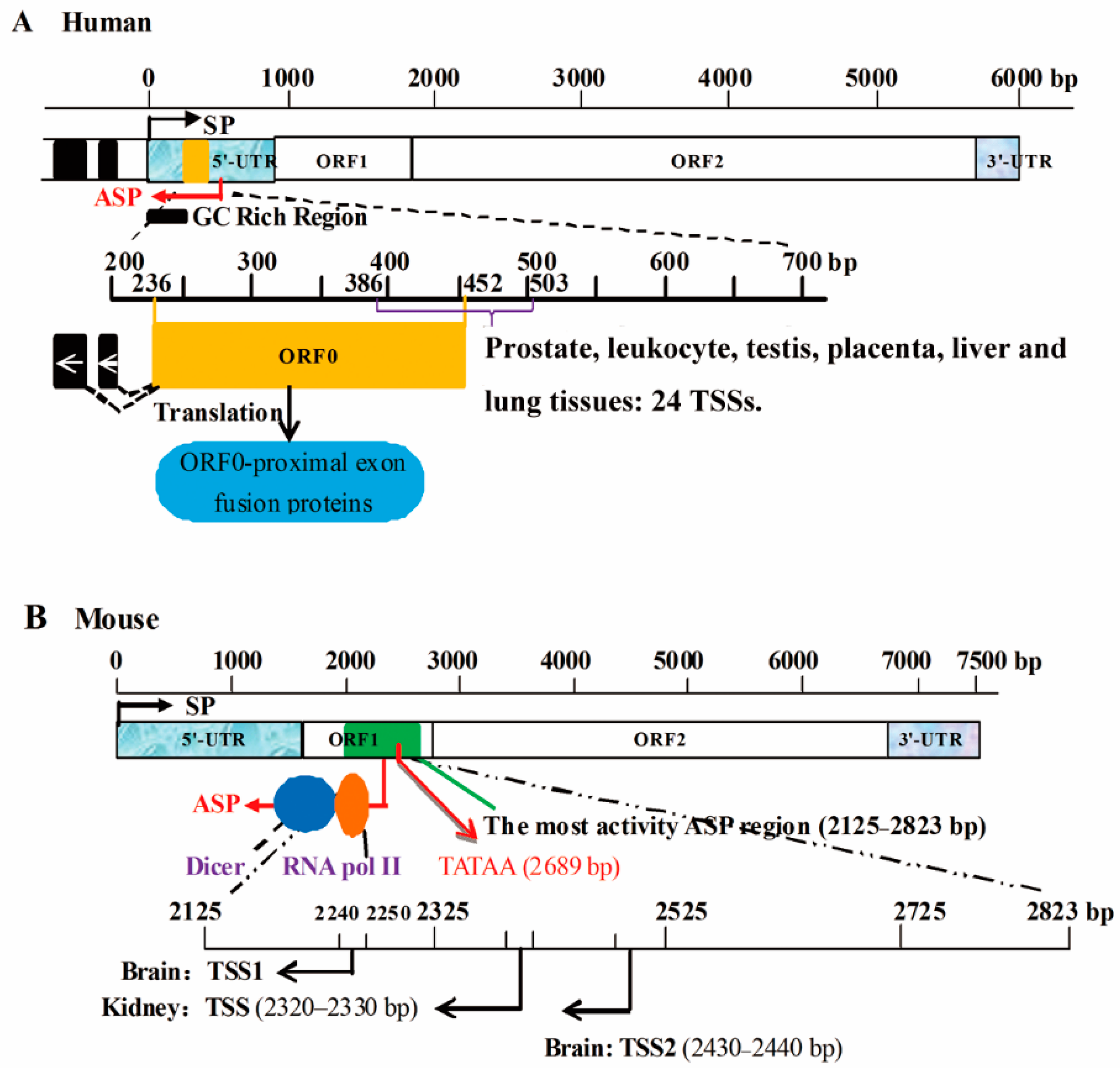
2.1.2. ASPs Are Located in Exons
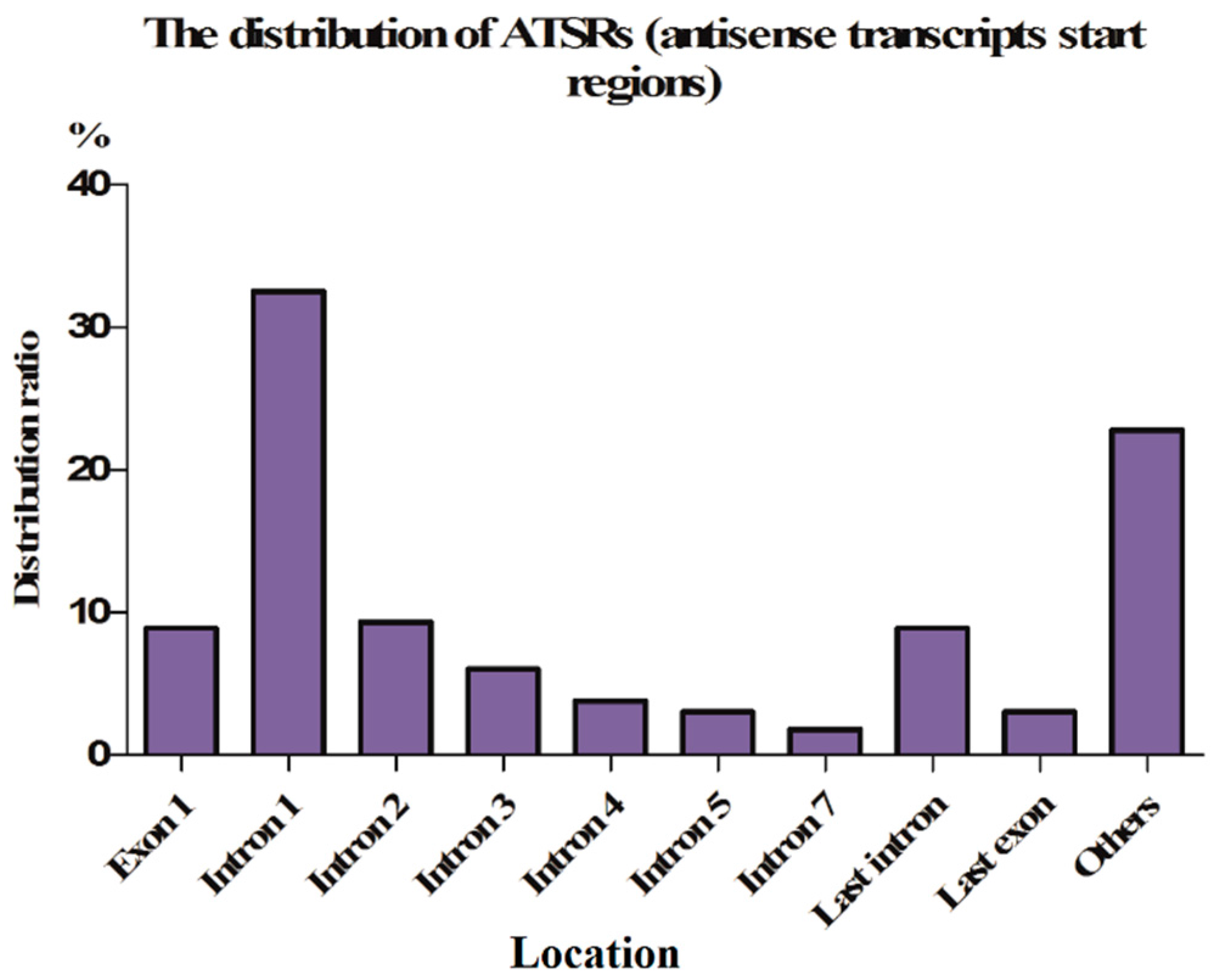
2.1.3. ASPs Are Located in Introns
2.1.4. ASPs Are Located in the 3′-UTR
2.2. ASPs Are Located in Adjacent Genes
2.3. ASPs Are Located in Intergenic Regions
3. Characteristics of ASPs
3.1. ASPs Are RNA Pol II Promoters
3.2. A TATA Box Is Not a Necessary Component of ASPs
| TATA-Less or TATA-Containing Promoters | TF Name | Location | Sequence (5′–3′) | Bidirectional Promoter | Unidirectional Promoter | Mechanism |
|---|---|---|---|---|---|---|
| TATA-box lacking promoters | INR | −3–+5 | YYANWYY | 25.30% [7,59] | 30.80% [7,59] | It can function independently, and together with the TATA box or DPE [7,44,45,59]. |
| BRE | −37–−32 | SSRCGCC | 16.50% [7,59] | 11.10% [7,59] | TFIIB–BRE interaction may play a dominant role in the assembly of the pre-initiation complex and transcription initiation [7,58,59]. | |
| CCAAT | −75–−80 | GGCCAATCT | 12.9% [7,59] | 6.90% [7,59] | Enhances the transcriptional rate [7]. | |
| DPE | +28–+32 | DSWYVY(T) | 46.65% [7,59] | 50.60% [7,59] | It can be recognized by TBP-associated factors (TAFs), such as TAF6 and TAF9 [58,59]. | |
| TATA-box containing promoters | Sp1 | Ubiquitously located near the TSS [66] | GGGGCGG | / | / | Sp1 acts through its zinc finger domain at the carboxyl end to interact with GC rich sequences of downstream target genes [56,57,67]. It binds to the GC-rich box and recruits TFIID to regulate the bidirectional promoter HIV-1 LTR [66,68]. Recognizes the core sequence 5-GGGGCGGG-3 of the target promoter. |
| NF-κB | / | / | / | / | It binds to the DNA sequence: 5-GGGRNYYY-3 [68,69,70]. | |
| TATA | −25–−30 | TATAAA | 9% [59] | 29% [59] | TATA box and INR interact synergistically when they are separated by 25–30 bp [7,44,59,64]. | |
| CpGI | 5′-regions of housekeeping genes and many tissues-specific genes [66] | size: 0.5–2 kb in length | 77% [7,71], enriched binding sites of many transcription factors: Sp1, GABPA, MYC, E2F1, E2F4, Nrf1, YY1, NF-Y [42,72,73]; 86.37% [72]. | 56% [43]; 38%, compared with a bidirectional promoter, more lack GC-pairs [7,71]; 28.48% [72]. | Hypermethylation of a CpGI in the promoter region usually suppresses gene expression [74,75,76], and the promoters of some tumor suppressor genes are hypermethylated in cancer [7,71,77]. Bi-directional genes tend to relate to housekeeping functions in metabolism pathways and nuclear processes [66,72]. |
3.3. ASPs Contain Specific TF Binding Sites (TFBSs)
3.4. ASPs Are Selected by Evolution and Possess Lower Activity than SPs
4. Factors Affecting the Activity of ASPs
4.1. The Activity of ASPs Is Closely Related to the Functions of Different Genes
4.2. ASP Activity Is Associated with the Distribution Density of TSSs
4.3. ASP Activity Is Associated with the Degree of Methylation of CpGIs
4.4. ASP Promoter Activity Is Strongly Regulated by TFs
4.5. The U1 Small Nuclear Ribonucleoprotein (U1 snRNP) Affects ASP Promoter Activity
4.6. Polyadenylation Site Signals Are Implicated in ASP Activity
4.7. Stimulation by Material Outside of Cells Influences the Activity of ASPs
4.8. ASP Activity Is Influenced by Chromatin and Histone Modifications
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, J.; Sun, M.; Kent, W.J.; Huang, X.; Xie, H.; Wang, W.; Zhou, G.; Shi, R.Z.; Rowley, J.D. Over 20% of human transcripts might form sense-antisense pairs. Nucleic Acids Res. 2004, 32, 4812–4820. [Google Scholar] [CrossRef] [PubMed]
- Engstrom, P.G.; Suzuki, H.; Ninomiya, N.; Akalin, A.; Sessa, L.; Lavorgna, G.; Brozzi, A.; Luzi, L.; Tan, S.L.; Yang, L.; et al. Complex Loci in human and mouse genomes. PLoS Genet. 2006, 2, e47. [Google Scholar] [CrossRef] [PubMed]
- Katayama, S.; Tomaru, Y.; Kasukawa, T.; Waki, K.; Nakanishi, M.; Nakamura, M.; Nishida, H.; Yap, C.C.; Suzuki, M.; Kawai, J.; et al. Revisiting the evolution of mouse LINE-1 in the genomic era. Mob. DNA 2013, 4, 3. [Google Scholar] [CrossRef]
- Mahmoudi, S.; Henriksson, S.; Corcoran, M.; Méndez-Vidal, C.; Wiman, K.G.; Farnebo, M. Wrap53, a natural p53 antisense transcript required for p53 induction upon DNA damage. Mol. Cell 2009, 33, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Gius, D.; Onyango, P.; Muldoon-Jacobs, K.; Karp, J.; Feinberg, A.P.; Cui, H. Epigenetic silencing of tumour suppressor gene p15 by its antisense RNA. Nature 2008, 451, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Ahlenstiel, C.; Marks, K.; Kelleher, A.D. Promoter targeting RNAs: Unexpected contributors to the control of HIV-1 transcription. Mol. Ther. Nucl. Acids 2015, 4, e222. [Google Scholar] [CrossRef] [PubMed]
- Orekhova, A.S.; Rubtsov, P.M. Bidirectional promoters in the transcription of mammalian genomes. Biochemistry 2013, 78, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Kaikkonen, M.U.; Lam, M.T.; Glass, C.K. Non-coding RNAs as regulators of gene expression and epigenetics. Cardiovasc. Res. 2011, 90, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Rezaeian, A.H.; Nishibori, M.; Hiraiwa, N.; Yoshizawa, M.; Yasue, H. Expression profile and localization of mouse calcitonin (CT) sense/antisense transcripts in pre- and postnatal tissue development. J. Vet. Med. Sci. 2009, 71, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Chowdhury, T.; Vakeel, P.; Koceja, C.; Sampath, V.; Ramchandran, R. δ-Like 4 mRNA is regulated by adjacent natural antisense transcripts. Vasc. Cell 2015, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Chiba, M. Differential expression of natural antisense transcripts during liver development in embryonic mice. Biomed. Rep. 2014, 2, 918–922. [Google Scholar] [CrossRef] [PubMed]
- Mätlik, K.; Redik, K.; Speek, M. L1 antisense promoter drives tissue-specific transcription of human genes. J. Biomed. Biotechnol. 2006, 2006, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.D.; Chen, W.M.; Qi, F.Z.; Xia, R.; Sun, M.; Xu, T.P.; Yin, L.; Zhang, E.B.; De, W.; Shu, Y.Q. Long non-coding RNA ANRIL is upregulated in hepatocellular carcinoma and regulates cell apoptosis by epigenetic silencing of KLF2. J. Hematol. Oncol. 2015, 8, 50. [Google Scholar] [CrossRef] [PubMed]
- Xi, Q.; Gao, N.; Zhang, X.; Zhang, B.; Ye, W.; Wu, J.; Zhang, X. A natural antisense transcript regulates acetylcholinesterase gene expression via epigenetic modification in Hepatocellular Carcinoma. Int. J. Biochem. Cell Biol. 2014, 55, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Wight, M.; Werner, A. The functions of natural antisense transcripts. Essays Biochem. 2013, 54, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Rinn, J.L.; Chang, H.Y. Genome regulation by long noncoding RNAs. Annu. Rev. Biochem. 2012, 81, 145–166. [Google Scholar] [CrossRef] [PubMed]
- Wery, M.; Kwapisz, M.; Morillon, A. Noncoding RNAs in gene regulation. Rev. Syst. Biol. Med. 2011, 3, 728–738. [Google Scholar] [CrossRef] [PubMed]
- Au, P.C.K.; Zhu, Q.H.; Dennis, E.S.; Wang, M.B. Long non-coding RNA-mediated mechanisms independent of the RNAi pathway in animals and plants. RNA Biol. 2010, 8, 404–414. [Google Scholar] [CrossRef]
- Aartsma-Rus, A.; van-Ommen, G.J. Progress in therapeutic antisense applications for neuromuscular disorders. Eur. J. Hum. Genet. 2010, 18, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Xiong, H.; Fang, J. Natural antisense transcripts regulate gene expression in an epigenetic manner. Biochem. Biophys. Res. Commun. 2010, 396, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Faghihi, M.A.; Wahlestedt, C. Regulatory roles of natural antisense transcripts. Nat. Rev. Mol. Cell Biol. 2009, 10, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Grigoriadis, A.; Oliver, G.R.; Tanney, A.; Kendrick, H.; Smalley, M.J.; Jat, P.; Neville, A.M. Identification of differentially expressed sense and antisense transcript pairs in breast epithelial tissues. BMC Genom. 2009, 10, 324. [Google Scholar] [CrossRef] [PubMed]
- Beckedorff, F.C.; Ayupe, A.C.; Crocci-Souza, R.; Amaral, M.S.; Nakaya, H.I.; Soltys, D.T.; Menck, C.F.; Reis, E.M.; Verjovski-Almeida, S. The intronic long noncoding RNA ANRASSF1 recruits PRC2 to the RASSF1A promoter, reducing the expression of RASSF1A and increasing cell proliferation. PLoS Genet. 2013, 9, e1003705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatterjee, A.; Drews, L.; Mehra, S.; Takano, E.; Kaznessis, Y.N.; Hu, W.S. Convergent transcription in the butyrolactone regulon in Streptomyces coelicolor confers a bistable genetic switch for antibiotic biosynthesis. PLoS ONE 2011, 6, e21974. [Google Scholar] [CrossRef] [PubMed]
- Denli, A.M.; Narvaiza, I.; Kerman, B.E.; Pena, M.; Benner, C.; Marchetto, M.C.; Diedrich, J.K.; Aslanian, A.; Ma, J.; Moresco, J.J.; et al. Primate-specific ORF0 contributes to retrotransposon-mediated diversity. Cell 2015, 163, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Saldaña-Meyer, R.; González-Buendía, E.; Guerrero, G.; Narendra, V.; Bonasio, R.; Recillas-Targa, F.; Reinberg, D. CTCF regulates the human p53 gene through direct interaction with its natural antisense transcript, Wrap53. Genes Dev. 2014, 28, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, S.; Henriksson, S.; Weibrecht, I.; Smith, S.; Söderberg, O.; Strömblad, S.; Wiman, K.G.; Farnebo, M. WRAP53 is essential for Cajal body formation and for targeting the survival of motor neuron complex to Cajal bodies. PLoS Biol. 2010, 8, e1000521. [Google Scholar] [CrossRef] [PubMed]
- Werner, A.; Cockell, S.; Falconer, J.; Carlile, M.; Alnumeir, S.; Robinson, J. Contribution of natural antisense transcription to an endogenous siRNA signature in human cells. BMC Genom. 2014, 15, 19. [Google Scholar] [CrossRef] [PubMed]
- Peters, N.T.; Rohrbach, J.A.; Zalewski, B.A.; Byrkett, C.M.; Vaughn, J.C. RNA editing and regulation of Drosophila 4f-rnp expression by sas-10 antisense readthrough mRNA transcripts. RNA 2003, 9, 698–710. [Google Scholar] [CrossRef] [PubMed]
- Rong, J.; Yin, J.; Su, Z. Natural antisense RNAs are involved in the regulation of CD45 expression in autoimmune diseases. Lupus 2015, 24, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Durairaj, G.; Bhaumik, S.R. Mechanisms of antisense transcription initiation from the 3′-end of the GAL10 coding sequence in vivo. Mol. Cell. Biol. 2013, 33, 3549–3567. [Google Scholar] [CrossRef] [PubMed]
- Beltran, M.; Aparicio-Prat, E.; Mazzolini, R.; Millanes-Romero, A.; Massó, P.; Jenner, R.G.; Díaz, V.M.; Peiró, S.; de Herreros, A.G. Splicing of a non-coding antisense transcript controls LEF1 gene expression. Nucleic Acids Res. 2015, 43, 5785–5797. [Google Scholar] [CrossRef] [PubMed]
- Finocchiaro, G.; Carro, M.S.; Francois, S.; Parise, P.; DiNinni, V.; Muller, H. Localizing hotspots of antisense transcription. Nucleic Acids Res. 2007, 35, 1488–1500. [Google Scholar] [CrossRef] [PubMed]
- Boque-Sastre, R.; Soler, M.; Oliveira-Mateos, C.; Portela, A.; Moutinho, C.; Sayols, S.; Villanueva, A.; Esteller, M.; Guil, S. Head-to-head antisense transcription and R-loop formation promotes transcriptional activation. Proc. Natl. Acad. Sci. USA 2015, 112, 5785–5790. [Google Scholar] [CrossRef] [PubMed]
- Lau, S.L. Molecular Characterization of the Chicken Growth Hormone Receptor Gene. Ph.D. Thesis, University of Hong Kong, Hong Kong, China, September 2005. [Google Scholar]
- Cruickshanks, H.A.; Tufarelli, C. Isolation of cancer-specific chimeric transcripts induced by hypomethylation of the LINE-1 antisense promoter. Genomics 2009, 94, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kannan, M.; Trivett, A.L.; Liao, H.; Wu, X.; Akagi, K.; Symer, D.E. An antisense promoter in mouse L1 retrotransposon open reading frame-1 initiates expression of diverse fusion transcripts and limits retrotransposition. Nucleic Acids Res. 2014, 42, 4546–4562. [Google Scholar] [CrossRef] [PubMed]
- Nigumann, P.; Redik, K.; Mätlik, K.; Speek, M. Many human genes are transcribed from the antisense promoter of L1 retrotransposon. Genomics 2002, 79, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.L.; Miller, G.M. 5′-Untranslated region of the tryptophan hydroxylase-2 gene harbors an asymmetric bidirectional promoter but not internal ribosome entry site in vitro. Gene 2009, 435, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Gotea, V.; Petrykowska, H.M.; Elnitski, L. Bidirectional promoters as important drivers for the emergence of species-specific transcripts. PLoS ONE 2013, 8, e57323. [Google Scholar] [CrossRef] [PubMed]
- Batagov, A.O.; Yarmishyn, A.A.; Jenjaroenpun, P.; Tan, J.Z.; Nishida, Y.; Kurochkin, I.V. Role of genomic architecture in the expression dynamics of long noncoding RNAs during differentiation of human neuroblastoma cells. BMC Syst. Biol. 2013, 7, S11. [Google Scholar] [CrossRef] [PubMed]
- Lepoivre, C.; Belhocine, M.; Bergon, A.; Griffon, A.; Yammine, M.; Vanhille, L.; Zacarias-Cabeza, J.; Garibal, M.A.; Koch, F.; Maqbool, M.A.; et al. Divergent transcription is associated with promoters of transcriptional regulators. BMC Genom. 2013, 14, 914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hildebrandt, M.; Nellen, W. Differential antisense transcription from the dictyostelium EB4 gene locus: Implications on antisense mediated regulation of mRNA stability. Cell 1992, 69, 197–204. [Google Scholar] [CrossRef]
- Silverman, T.A.; Noguchi, M.; Safer, B. Role of sequences within the first intron in the regulation of expression of eukaryotic initiation factor 2α. J. Biol. Chem. 1992, 267, 9738–9742. [Google Scholar] [PubMed]
- Duart-Garcia, C.; Braunschweig, M.H. Functional expression study of Igf2 antisense transcript in mouse. Int. J. Genom. 2014, 2014, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, W.; Sun, Y.; Yu, D.; Wen, X.; Wang, H.; Cui, J.; Wang, G.; Hoffman, A.R.; Hu, J.F. A novel antisense long noncoding RNA within the IGF1R gene locus is imprinted in hematopoietic malignancies. Nucleic Acids Res. 2014, 42, 9588–9601. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Caniggia, I.; Post, M. Hypoxia-inducible regulation of placental BOK expression. Biochem. J. 2014, 461, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Wright, P.W.; Huehn, A.; Cichocki, F.; Li, H.; Sharma, N.; Dang, H.; Lenvik, T.R.; Woll, P.; Kaufman, D.; Miller, J.S.; et al. Identification of a KIR antisense lncRNA expressed by progenitor cells. Genes Immun. 2013, 14, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Spicer, D.B.; Sonenshein, G.E. An antisense promoter of the murine c-myc gene is localized within intron 2. Mol. Cell. Biol. 1992, 12, 1324–1329. [Google Scholar] [CrossRef] [PubMed]
- Ebralidze, A.K.; GuibalF, C.; Steidl, U.; Zhang, P.; Lee, S.; Bartholdy, B.; JordaM, A.; Petkova, V.; Rosenbauer, F.; Huang, G.; et al. PU.1 expression is modulated by the balance of functional sense and antisense RNAs regulated by a shared cis-regulatory element. Genes Dev. 2008, 22, 2085–2092. [Google Scholar] [CrossRef] [PubMed]
- Schultz, B.M.; Gallicio, G.A.; Cesaroni, M.; Lupey, L.N.; Engel, N. Enhancers compete with a long non-coding RNA for regulation of the Kcnq1 domain. Nucleic Acids Res. 2015, 43, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Barann, M.; Esser, D.; Klostermeier, U.C.; Lappalainen, T.; Luzius, A.; Kuiper, J.W.; Ammerpohl, O.; Vater, I.; Siebert, R.; Amstislavskiy, V.; et al. Janus—A comprehensive tool investigating the two faces of transcription. Bioinformatics 2013, 29, 1600–1606. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Vogelstein, B.; Velculescu, V.E.; Papadopoulos, N.; Kinzler, K.W. The antisense transcriptomes of human cells. Science 2008, 322, 1855–1857. [Google Scholar] [CrossRef] [PubMed]
- Lapidot, M.; Pilpel, Y. Genome-wide natural antisense transcription: Coupling its regulation to its different regulatory mechanisms. EMBO Rep. 2006, 7, 1216–1222. [Google Scholar] [CrossRef] [PubMed]
- Dreos, R.; Ambrosini, G.; Cavin-Périer, R.; Bucher, P. EPD and EPDnew, high-quality promoter resources in the next-generation sequencing era. Nucleic Acids Res. 2013, 41, D157–D164. [Google Scholar] [CrossRef] [PubMed]
- Gazon, H.; Lemasson, I.; Polakowski, N.; Césaire, R.; Matsuoka, M.; Barbeau, B.; Mesnard, J.M.; Peloponese, J.M. Human T-cell leukemia virus type 1 (HTLV-1) bZIP factor requires cellular transcription factor JunD to upregulate HTLV-1 antisense transcription from the 3′-long terminal repeat. J. Virol. 2012, 86, 9070–9078. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Kawano, Y.; Sato, K.; Ando, Y.; Aoki, J.; Miura, Y.; Komano, J.; Tanaka, Y.; Koyanagi, Y. A CD63 mutant inhibits T-cell tropic human immunodeficiency virus type 1 entry by disrupting CXCR4 trafficking to the plasma membrane. Traffic 2008, 9, 540–558. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.C.; Pan, Q.H.; Yu, Y.C.; Sun, F.Y. Core promoter of eukaryotic genes. J. Med. Mol. Biol. 2007, 4, 132–135. [Google Scholar]
- Yang, M.Q.; Elnitski, L.L. Diversity of core promoter elements comprising human bidirectional promoters. BMC Genom. 2008, 9, S3. [Google Scholar] [CrossRef] [PubMed]
- Flynn, R.A.; Almada, A.E.; Zamudio, J.R.; Sharp, P.A. Antisense RNA polymerase II divergent transcripts are P-TEFb dependent and substrates for the RNA exosome. Proc. Natl. Acad. Sci. USA 2011, 108, 10460–10465. [Google Scholar] [CrossRef] [PubMed]
- Farrell, C.M.; Lukens, L.N. Naturally occurring antisense transcripts are present in chick embryo chondrocytes simultaneously with the down-regulation of the α1(I) collagen gene. J. Biol. Chem. 1995, 270, 3400–3408. [Google Scholar] [PubMed]
- Trinklein, N.D.; Aldred, S.F.; Hartman, S.J.; Schroeder, D.I.; Otillar, R.P.; Myers, R.M. An abundance of bidirectional promoters in the human genome. Genome Res. 2004, 14, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Putta, P.; Mitra, C.K. Conserved short sequences in promoter regions of human genome. J. Biomol. Struct. Dyn. 2010, 27, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Abe, H.; Gemmell, N.J. Abundance, arrangement, and function of sequence motifs in the chicken promoters. BMC Genom. 2014, 15, 900. [Google Scholar] [CrossRef] [PubMed]
- Lyle, R.; Watanabe, D.; Te Vruchte, D.; Lerchner, W.; Smrzka, O.W.; Wutz, A.; Schageman, J.; Hahner, L.; Davies, C.; Barlow, D.P. The imprinted antisense RNA at the Igf2r locus overlaps but does not imprint Mas1. Nat. Genet. 2000, 25, 19–21. [Google Scholar] [CrossRef] [PubMed]
- Wierstra, I. Sp1: Emerging roles—Beyond constitutive activation of TATA-less housekeeping genes. Biochem. Biophys. Res. Commun. 2008, 372, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zanotto, E.; Lehtonen, V.; Jacobs, H.T. Modulation of Mrps12/Sarsm promoter activity in response to mitochondrial stress. Biochim. Biophys. Acta 2008, 1783, 2352–2362. [Google Scholar] [CrossRef] [PubMed]
- Arpin-André, C.; Laverdure, S.; Barbeau, B.; Gross, A.; Mesnard, J.M. Construction of a reporter vector for analysis of bidirectional transcriptional activity of retrovirus LTR. Plasmid 2014, 74, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Bentley, K.; Deacon, N.; Sonza, S.; Zeichner, S.; Churchill, M. Mutational analysis of the HIV-1 LTR as a promoter of negative sense transcription. Arch. Virol. 2004, 149, 2277–2294. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi-Ishihara, M.; Yamagishi, M.; Hara, T.; Matsuda, Y.; Takahashi, R.; Miyake, A.; Nakano, K.; Yamochi, T.; Ishida, T.; Watanabe, T. HIV-1-encoded antisense RNA suppresses viral replication for a prolonged period. Retrovirology 2012, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Tufarelli, C.; Cruickshanks, H.A.; Meehan, R.R. LINE-1 activation and epigenetic silencing of suppressor genes in cancer: Causally related events? Mob. Genet. Elements 2013, 3, e26832. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Chen, J.; Shen, B. Genome-wide analysis of the transcription factor binding preference of human bi-directional promoters and functional annotation of related gene pairs. BMC Syst. Biol. 2011, 5, S2. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.M.; Collins, P.J.; Trinklein, N.D.; Fu, Y.; Xi, H.; Myers, R.M.; Weng, Z. Transcription factor binding and modified histones in human bidirectional promoters. Genome Res. 2007, 17, 818–827. [Google Scholar] [PubMed]
- Li, Q.; Li, N.; Hu, X.; Li, J.; Du, Z.; Chen, L.; Yin, G.; Duan, J.; Zhang, H.; Zhao, Y.; et al. Genome-wide mapping of DNA methylation in chicken. PLoS ONE 2011, 6, e19428. [Google Scholar] [CrossRef] [PubMed]
- Weber, B.; Kimhi, S.; Howard, G.; Eden, A.; Lyko, F. Demethylation of a LINE-1 antisense promoter in the cMet locus impairs Met signalling through induction of illegitimate transcription. Oncogene 2010, 29, 5775–5784. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.S.; Chai, X.W.; Wang, Z.F.; Nie, Q.H.; Zhang, X.Q. Impact of GC content on gene expression pattern in chicken. Genet. Sel. Evol. 2013, 45, 9. [Google Scholar] [CrossRef] [PubMed]
- Shu, J.; Jelinek, J.; Chang, H.; Shen, L.; Qin, T.; Chung, W.; Oki, Y.; Issa, J.P. Silencing of bidirectional promoters by DNA methylation in tumorigenesis. Cancer Res. 2006, 66, 5077–5084. [Google Scholar] [CrossRef] [PubMed]
- Mueller, B.U.; Pabst, T.; Osato, M.; Asou, N.; Johansen, L.M.; Minden, M.D.; Behre, G.; Hiddemann, W.; Ito, Y.; Tenen, D.G. Heterozygous PU.1 mutations are associated with acute myeloid leukemia. Blood 2003, 101, 2074. [Google Scholar] [CrossRef] [PubMed]
- Rosenbauer, F.; Owens, B.M.; Yu, L.; Tumang, J.R.; Steidl, U.; Kutok, J.L.; Clayton, L.K.; Wagner, K.; Scheller, M.; Iwasaki, H.; et al. Lymphoid cell growth and transformation are suppressed by a key regulatory element of the gene encoding PU.1. Nat. Genet. 2006, 38, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Zhang, P.; Hirai, H.; Elf, S.; Yan, X.; Chen, Z.; Koschmieder, S.; Okuno, Y.; Dayaram, T.; Growney, J.D. PU.1 is a major downstream target of AML1 (RUNX1) in adult mouse hematopoiesis. Nat. Genet. 2008, 40, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ray-Gallet, D.; Zhang, P.; Hetherington, C.J.; Gonzalez, D.A.; Zhang, D.E.; Moreau-Gachelin, F.; Tenen, D.G. PU.1 (Spi-1) autoregulates its expression in myeloid cells. Oncogene 1995, 11, 1549–1560. [Google Scholar] [PubMed]
- Okuno, Y.; Huang, G.; Rosenbauer, F.; Evans, E.K.; Radomska, H.S.; Iwasaki, H.; Akashi, K.; Moreau-Gachelin, F.; Li, Y.; Zhang, P.; et al. Potential autoregulation of transcription factor PU.1 by an upstream regulatory element. Mol. Cell. Biol. 2005, 25, 2832–2845. [Google Scholar] [CrossRef] [PubMed]
- Rahl, P.B.; Lin, C.Y.; Seila, A.C.; Flynn, R.A.; McCuine, S.; Burge, C.B.; Sharp, P.A.; Young, R.A. c-Myc regulates transcriptional pause release. Cell 2010, 141, 432–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, C.; Chen, J.; Shen, B. The preservation of bidirectional promoter architecture in eukaryotes: What is the driving force? BMC Syst. Biol. 2012, 6, S21. [Google Scholar] [CrossRef] [PubMed]
- Almada, A.E.; Wu, X.B.; Kriz, A.J.; Burge, C.B.; Sharp, P.A. Promoter directionality is controlled by U1 snRNP and polyadenylation signals. Nature 2013, 499, 360–363. [Google Scholar] [CrossRef] [PubMed]
- Uesaka, M.; Nishimura, O.; Go, Y.; Nakashima, K.; Agata, K.; Imamura, T. Bidirectional promoters are the major source of gene activation-associated non-coding RNAs in mammals. BMC Genom. 2014, 15, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conley, A.B.; Miller, W.J.; Jordan, I.K. Human cis natural antisense transcripts initiated by transposable elements. Trends Genet. 2008, 24, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Carninci, P.; Sandelin, A.; Lenhard, B.; Katayama, S.; Shimokawa, K.; Ponjavic, J.; Semple, C.A.; Taylor, M.S.; Engström, P.G.; Frith, M.C.; et al. Genome-wide analysis of mammalian promoter architecture and evolution. Nat. Genet. 2006, 38, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Osato, N.; Suzuki, Y.; Ikeo, K.; Gojobori, T. Transcriptional interferences in cis natural antisense transcripts of humans and mice. Genetics 2007, 176, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- Klose, R.J.; Bird, A.P. Genomic DNA methylation: The mark and its mediators. Trends Biochem. Sci. 2006, 31, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Bibikova, M.; Lin, Z.; Zhou, L.; Chudin, E.; Garcia, E.W.; Wu, B.; Doucet, D.; Thomas, N.J.; Wang, Y.; Vollmer, E.; et al. High-throughput DNA methylation profiling using universal bead arrays. Genome Res. 2006, 16, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Young, J.M.; Whiddon, J.L.; Yao, Z.; Kasinathan, B.; Snider, L.; Geng, L.N.; Balog, J.; Tawil, R.; vander-Maarel, S.M.; Tapscott, S.J. DUX4 binding to retroelements creates promoters that are active in FSHD muscle and testis. PLoS Genet. 2013, 9, e1003947. [Google Scholar] [CrossRef] [PubMed]
- Vanhée-Brossollet, C.; Vaquero, C. Do natural antisense transcripts make sense in eukaryotes? Gene 1998, 211, 1–9. [Google Scholar] [CrossRef]
- Burns, K.H.; Boeke, J.D. Human transposon tectonics. Cell 2012, 149, 740–752. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.L.; Cruceanu, M.; Branciforte, D.; Wai-Lun Li, P.; Kwok, S.C.; Hodges, R.S.; Williams, M.C. LINE-1 retrotransposition requires the nucleic acid chaperone activity of the ORF1 protein. J. Mol. Biol. 2005, 348, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Carey, M.; Workman, J.L. The role of chromatin during transcription. Cell 2007, 128, 707–719. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, A.D.; Allis, C.D.; Bernstein, E. Epigenetics: A landscape takes shape. Cell 2007, 128, 635–638. [Google Scholar] [CrossRef] [PubMed]
- Murray, S.C.; Haenni, S.; Howe, F.S.; Fischl, H.; Chocian, K.; Nair, A.; Mellor, J. Sense and antisense transcription are associated with distinct chromatin architectures across genes. Nucleic Acids Res. 2015, 43, 7823–7837. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, S.; Zhang, L.; Luo, W.; Zhang, X. Characteristics of Antisense Transcript Promoters and the Regulation of Their Activity. Int. J. Mol. Sci. 2016, 17, 9. https://doi.org/10.3390/ijms17010009
Lin S, Zhang L, Luo W, Zhang X. Characteristics of Antisense Transcript Promoters and the Regulation of Their Activity. International Journal of Molecular Sciences. 2016; 17(1):9. https://doi.org/10.3390/ijms17010009
Chicago/Turabian StyleLin, Shudai, Li Zhang, Wen Luo, and Xiquan Zhang. 2016. "Characteristics of Antisense Transcript Promoters and the Regulation of Their Activity" International Journal of Molecular Sciences 17, no. 1: 9. https://doi.org/10.3390/ijms17010009






