Aneurysm miRNA Signature Differs, Depending on Disease Localization and Morphology
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results
2.1.1. Candidate miRs for Abdominal Aortic Aneurysm (AAA) Formation
2.1.2. Down-Regulation of Certain miRNAs Depends on Inflammatory Activity
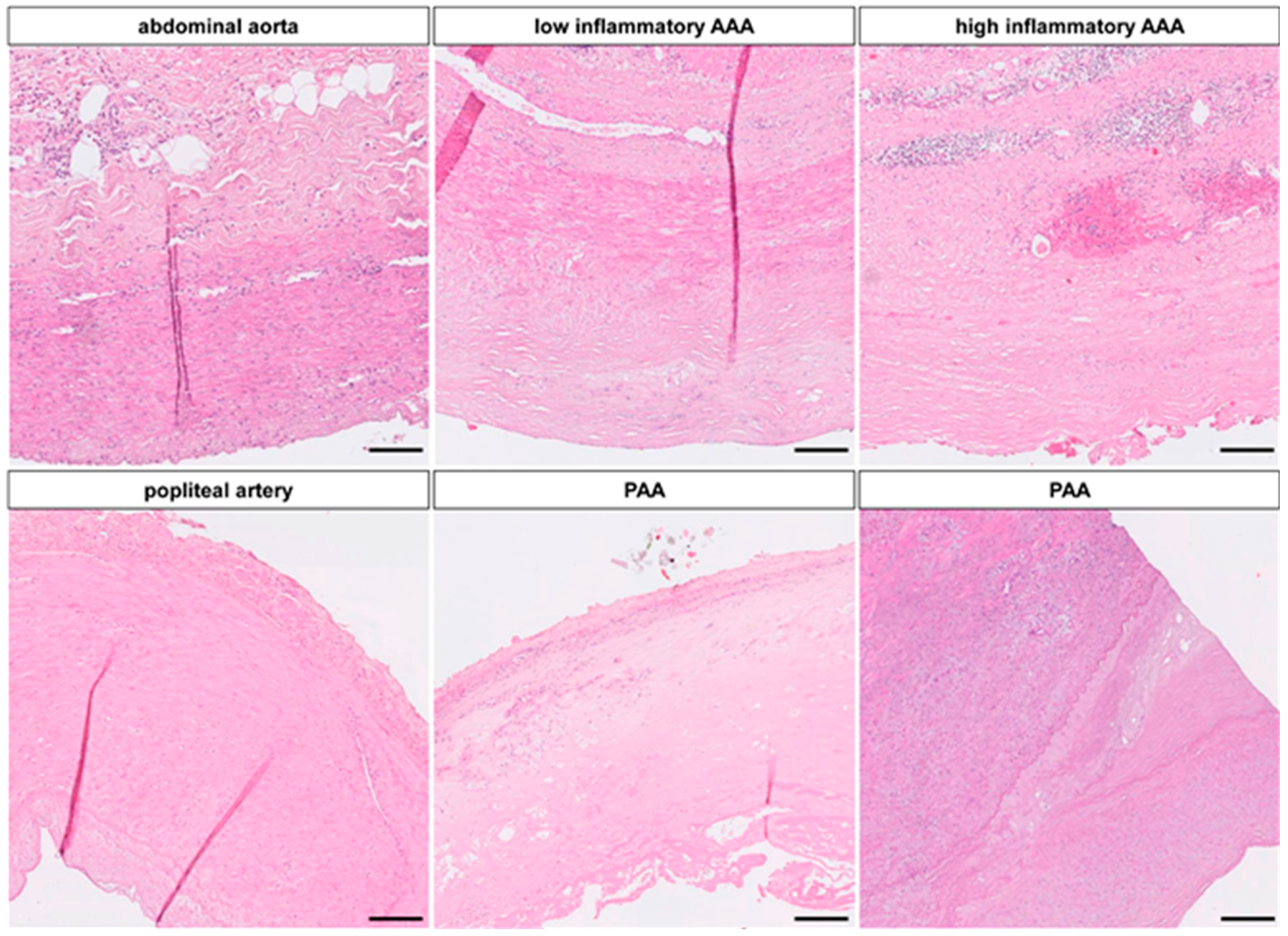
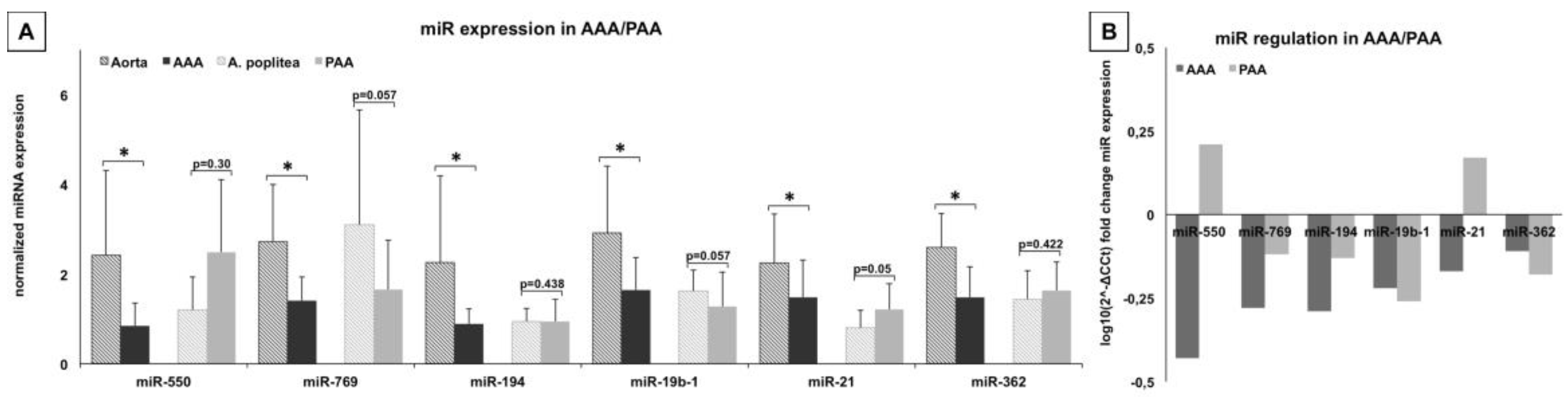

2.1.3. miR-Signature in Popliteal Artery Aneurysm (PAA) Differs Significantly from AAA
2.1.4. Signal Transduction, Tissue Remodeling and Cell Interaction Pathways Are Key in Aneurysm Pathogenesis
| Mode of Interaction | KEGG Pathway | miR Identified in This Study: miR-21, -19b, -194, -362, -769, -550 (in p-Value) | miR Identified in Other Studies: miR-29a/b/c, 155, -205, -516a (in p-Value) |
|---|---|---|---|
| Signal transduction | MAPK signaling (04010) | 22.08 (53) | 6.47 (39) |
| mTOR signaling (04150) | 15.97 (16) | 10.92 (14) | |
| Wnt signaling (04310) | 12.91 (31) | 4.06 (23) | |
| ErbB signaling (04012) | 12.9 (22) | 5.44 (17) | |
| TGF-ß signaling (04350) | 12.29 (22) | 7.55 (19) | |
| JAK-stat signaling (04630) | 7.04 (27) | 1.69 (2) | |
| Hedgehog signaling (04340) | 6.79 (13) | 0.1 (15) | |
| Calcium signaling (04020) | 3.26 (24) | 0.47 (17) | |
| Cytokine cytokine interaction (04060) | 0.44 (27) | 1.63 (31) | |
| p53 signaling (04115) | 0.05 (7) | 1.36 (10) | |
| Cell-Cell interaction | Adherence junction (04520) | 4.59 (14) | 5.71 (15) |
| Tight junction (04530) | 3.7 (21) | 8.42 (26) | |
| Gap junction (04540) | 3.66 (16) | 0.85 (12) | |
| Cell adhesion molecules (04514) | 0.11 (12) | 1.19 (16) | |
| Cell-ECM interaction | Focal adhesion (04510) | 14.61 (39) | 10.72 (36) |
| ECM receptor interaction (04512) | 3.42 (14) | 13.13 (21) | |
| Cell degradation | Ubiquitin mediated proteolysis (04120) | 13.46 (29) | 4.02 (21) |
| Apoptosis (04210) | 2.33 (13) | 1.57 (12) | |
| NKcell mediated cytotoxicity (04650) | 0.06 (12) | 0.08 (11) | |
| Cell growth | Insulin signaling (04910) | 9.08 (27) | 4.81 (23) |
| VEGF signaling (04370) | 7.66 (16) | 1.21 (10) | |
| Regulation actin cytoskeleton (04810) | 5.39 (32) | 2.68 (28) | |
| Inflammation | B-cell receptor (04662) | 5.59 (13) | 2.13 (10) |
| T-cell receptor (04660) | 3.24 (15) | 6.28 (18) | |
| Toll like receptor signaling (04620) | 2.89 (16) | 0.95 (13) | |
| Coagulation cascade (04610) | 0.94 (4) | 1.62 (3) | |
| Leukocyte migration (04670) | 0.6 (13) | 0.21 (12) |
2.2. Discussion
3. Experimental Section
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nordon, I.M.; Hinchliffe, R.J.; Loftus, I.M.; Thompson, M.M. Pathophysiology and epidemiology of abdominal aortic aneurysms. Nat. Rev. Cardiol. 2011, 8, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Lederle, F.A.; Wilson, S.E.; Johnson, G.R.; Reinke, D.B.; Littooy, F.N.; Acher, C.W.; Ballard, D.J.; Messina, L.M.; Gordon, I.L.; Chute, E.P.; et al. Immediate repair compared with surveillance of small abdominal aortic aneurysms. N. Engl. J. Med. 2002, 346, 1437–1444. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, K.E.; Torp-Pedersen, C.; Gislason, G.H.; Egfjord, M.; Rasmussen, H.B.; Hansen, P.R. Angiotensin-converting enzyme inhibitors and angiotensin ii receptor blockers in patients with abdominal aortic aneurysms: Nation-wide cohort study. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Collaborators, R.; Bown, M.J.; Sweeting, M.J.; Brown, L.C.; Powell, J.T.; Thompson, S.G. Surveillance intervals for small abdominal aortic aneurysms: A meta-analysis. JAMA 2013, 309, 806–813. [Google Scholar]
- Shimizu, K.; Mitchell, R.N.; Libby, P. Inflammation and cellular immune responses in abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Golledge, J.; Muller, J.; Daugherty, A.; Norman, P. Abdominal aortic aneurysm: Pathogenesis and implications for management. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2605–2613. [Google Scholar] [CrossRef] [PubMed]
- Ailawadi, G.; Moehle, C.W.; Pei, H.; Walton, S.P.; Yang, Z.; Kron, I.L.; Lau, C.L.; Owens, G.K. Smooth muscle phenotypic modulation is an early event in aortic aneurysms. J. Thorac. Cardiovasc. Surg. 2009, 138, 1392–1399. [Google Scholar] [CrossRef] [PubMed]
- Norman, P.E.; Curci, J.A. Understanding the effects of tobacco smoke on the pathogenesis of aortic aneurysm. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1473–1477. [Google Scholar] [CrossRef] [PubMed]
- Hinterseher, I.; Erdman, R.; Elmore, J.R.; Stahl, E.; Pahl, M.C.; Derr, K.; Golden, A.; Lillvis, J.H.; Cindric, M.C.; Jackson, K.; et al. Novel pathways in the pathobiology of human abdominal aortic aneurysms. Pathobiol. J. Immunopathol. Mol. Cell. Biol. 2013, 80, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Biros, E.; Moran, C.S.; Rush, C.M.; Gabel, G.; Schreurs, C.; Lindeman, J.H.; Walker, P.J.; Nataatmadja, M.; West, M.; Holdt, L.M.; et al. Differential gene expression in the proximal neck of human abdominal aortic aneurysm. Atherosclerosis 2014, 233, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Rijbroek, A.; Moll, F.L.; von Dijk, H.A.; Meijer, R.; Jansen, J.W. Inflammation of the abdominal aortic aneurysm wall. Eur. J. Vasc. Surg. 1994, 8, 41–46. [Google Scholar] [CrossRef]
- Van Rooij, E. The art of microRNA research. Circ. Res. 2011, 108, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Kloosterman, W.P.; Plasterk, R.H. The diverse functions of microRNAs in animal development and disease. Dev. Cell 2006, 11, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Huntzinger, E.; Izaurralde, E. Gene silencing by microRNAs: Contributions of translational repression and mRNA decay. Nat. Rev. Genet. 2011, 12, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Maegdefessel, L.; Spin, J.M.; Raaz, U.; Eken, S.M.; Toh, R.; Azuma, J.; Adam, M.; Nagakami, F.; Heymann, H.M.; Chernugobova, E.; et al. miR-24 limits aortic vascular inflammation and murine abdominal aneurysm development. Nat. Commun. 2014, 5, 5214. [Google Scholar] [CrossRef] [PubMed]
- Maegdefessel, L.; Azuma, J.; Toh, R.; Deng, A.; Merk, D.R.; Raiesdana, A.; Leeper, N.J.; Raaz, U.; Schoelmerich, A.M.; McConnell, M.V.; et al. MicroRNA-21 blocks abdominal aortic aneurysm development and nicotine-augmented expansion. Sci. Transl. Med. 2012, 4, 122ra122. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.W.; Kumar, S.; Son, D.J.; Jang, I.H.; Griendling, K.K.; Jo, H. Prevention of abdominal aortic aneurysm by anti-microRNA-712 or anti-microRNA-205 in angiotensin II-infused mice. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1412–1421. [Google Scholar] [CrossRef] [PubMed]
- Maegdefessel, L.; Azuma, J.; Toh, R.; Merk, D.R.; Deng, A.; Chin, J.T.; Raaz, U.; Schoelmerich, A.M.; Raiesdana, A.; Leeper, N.J.; et al. Inhibition of microRNA-29b reduces murine abdominal aortic aneurysm development. J. Clin. Investing. 2012, 122, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Cheuk, B.L.; Cheng, S.W. Identification and characterization of microRNAs in vascular smooth muscle cells from patients with abdominal aortic aneurysms. J. Vasc. Surg. 2014, 59, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Biros, E.; Moran, C.S.; Wang, Y.; Walker, P.J.; Cardinal, J.; Golledge, J. MicroRNA profiling in patients with abdominal aortic aneurysms: The significance of miR-155. Clin. Sci. 2014, 126, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Pahl, M.C.; Derr, K.; Gabel, G.; Hinterseher, I.; Elmore, J.R.; Schworer, C.M.; Peeler, T.C.; Franklin, D.P.; Gray, J.L.; Carey, D.J.; et al. MicroRNA expression signature in human abdominal aortic aneurysms. BMC Med. Genom. 2012, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Huang, Y.; Lu, X.; Lu, M.; Huang, X.; Li, W.; Jiang, M. Identification and characteristics of microRNAs with altered expression patterns in a rat model of abdominal aortic aneurysms. Tohoku J. Exp. Med. 2010, 222, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Ruddy, J.M.; Jones, J.A.; Ikonomidis, J.S. Pathophysiology of thoracic aortic aneurysm (TAA): Is it not one uniform aorta? Role of embryologic origin. Prog. Cardiovasc. Dis. 2013, 56, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Shi, J.Y.; Zhu, G.Q.; Shi, B. miR-17–92 cluster regulates cell proliferation and collagen synthesis by targeting tgfb pathway in mouse palatal mesenchymal cells. J. Cell. Biochem. 2012, 113, 1235–1244. [Google Scholar] [CrossRef] [PubMed]
- Adam, M.; Raaz, U.; Spin, J.M.; Tsao, P.S. MicroRNAs in abdominal aortic aneurysm. Curr. Vasc. Pharmacol. 2013, 13, 280–290. [Google Scholar] [CrossRef]
- Altuvia, Y.; Landgraf, P.; Lithwick, G.; Elefant, N.; Pfeffer, S.; Aravin, A.; Brownstein, M.J.; Tuschl, T.; Margalit, H. Clustering and conservation patterns of human microRNAs. Nucleic Acids Res. 2005, 33, 2697–2706. [Google Scholar] [CrossRef] [PubMed]
- Molina-Pinelo, S.; Pastor, M.D.; Suarez, R.; Romero-Romero, B.; Gonzalez de la Pena, M.; Salinas, A.; Garcia-Carbonero, R.; de Miguel, M.J.; Rodriguez-Panadero, F.; Carnero, A.; et al. MicroRNA clusters: Dysregulation in lung adenocarcinoma and copd. Eur. Respir. J. 2014, 43, 1740–1749. [Google Scholar] [CrossRef] [PubMed]
- Scherr, M.; Elder, A.; Battmer, K.; Barzan, D.; Bomken, S.; Ricke-Hoch, M.; Schroder, A.; Venturini, L.; Blair, H.J.; Vormoor, J.; et al. Differential expression of miR-17~92 identifies BCL2 as a therapeutic target in BCR-ABL-positive B-lineage acute lymphoblastic leukemia. Leukemia 2014, 28, 554–565. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Hussien, H.; Hanemaaijer, R.; Kleemann, R.; Verhaaren, B.F.; van Bockel, J.H.; Lindeman, J.H. The pathophysiology of abdominal aortic aneurysm growth: Corresponding and discordant inflammatory and proteolytic processes in abdominal aortic and popliteal artery aneurysms. J. Vasc. Surg. 2010, 51, 1479–1487. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Krishna, S.; Walker, P.J.; Norman, P.; Golledge, J. Transforming growth factor-β and abdominal aortic aneurysms. Cardiovasc. Pathol. 2013, 22, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Doyle, A.J.; Redmond, E.M.; Gillespie, D.L.; Knight, P.A.; Cullen, J.P.; Cahill, P.A.; Morrow, D.J. Differential expression of Hedgehog/Notch and transforming growth factor-β in human abdominal aortic aneurysms. J. Vasc. Surg. 2015, 62, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Deuse, T.; Stubbendorff, M.; Chernogubova, E.; Erben, R.G.; Eken, S.M.; Jin, H.; Li, Y.; Busch, A.; Heeger, C.H.; et al. Local microRNA modulation using a novel anti-miR-21-eluting stent effectively prevents experimental in-stent restenosis. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1945–1953. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.W.; Zhao, Z.; Li, F.N.; Zhang, J. The effect pathways analysis in the abdominal aortic aneurysms. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 1245–1251. [Google Scholar] [PubMed]
- Blann, A.D.; Devine, C.; Amiral, J.; McCollum, C.N. Soluble adhesion molecules, endothelial markers and atherosclerosis risk factors in abdominal aortic aneurysm: A comparison with claudicants and healthy controls. Blood Coagul. Fibrinolysis 1998, 9, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Lindholt, J.S.; Shi, G.P. Chronic inflammation, immune response, and infection in abdominal aortic aneurysms. Eur. J. Vasc. Endovasc. Surg. 2006, 31, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Vandesompele, J.; de Preter, K.; Pattyn, F.; Poppe, B.; van Roy, N.; de Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3. [Google Scholar] [CrossRef] [Green Version]
- Vlachos, I.S.; Kostoulas, N.; Vergoulis, T.; Georgakilas, G.; Reczko, M.; Maragkakis, M.; Paraskevopoulou, M.D.; Prionidis, K.; Dalamagas, T.; Hatzigeorgiou, A.G. Diana miRPath v.2.0: Investigating the combinatorial effect of microRNAs in pathways. Nucleic Acids Res. 2012, 40, W498–W504. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Busch, A.; Busch, M.; Scholz, C.-J.; Kellersmann, R.; Otto, C.; Chernogubova, E.; Maegdefessel, L.; Zernecke, A.; Lorenz, U. Aneurysm miRNA Signature Differs, Depending on Disease Localization and Morphology. Int. J. Mol. Sci. 2016, 17, 81. https://doi.org/10.3390/ijms17010081
Busch A, Busch M, Scholz C-J, Kellersmann R, Otto C, Chernogubova E, Maegdefessel L, Zernecke A, Lorenz U. Aneurysm miRNA Signature Differs, Depending on Disease Localization and Morphology. International Journal of Molecular Sciences. 2016; 17(1):81. https://doi.org/10.3390/ijms17010081
Chicago/Turabian StyleBusch, Albert, Martin Busch, Claus-Jürgen Scholz, Richard Kellersmann, Christoph Otto, Ekaterina Chernogubova, Lars Maegdefessel, Alma Zernecke, and Udo Lorenz. 2016. "Aneurysm miRNA Signature Differs, Depending on Disease Localization and Morphology" International Journal of Molecular Sciences 17, no. 1: 81. https://doi.org/10.3390/ijms17010081






