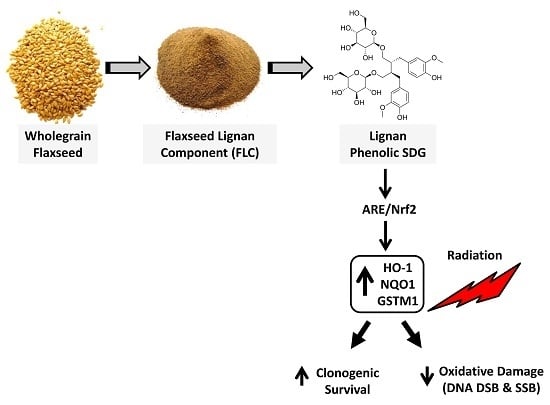
The Flaxseed-Derived Lignan Phenolic Secoisolariciresinol Diglucoside (SDG) Protects Non-Malignant Lung Cells from Radiation Damage
Abstract
:1. Introduction
2. Results
2.1. SDG Prevents Formation of DNA Single Strand Breaks in Irradiated Lung Cells

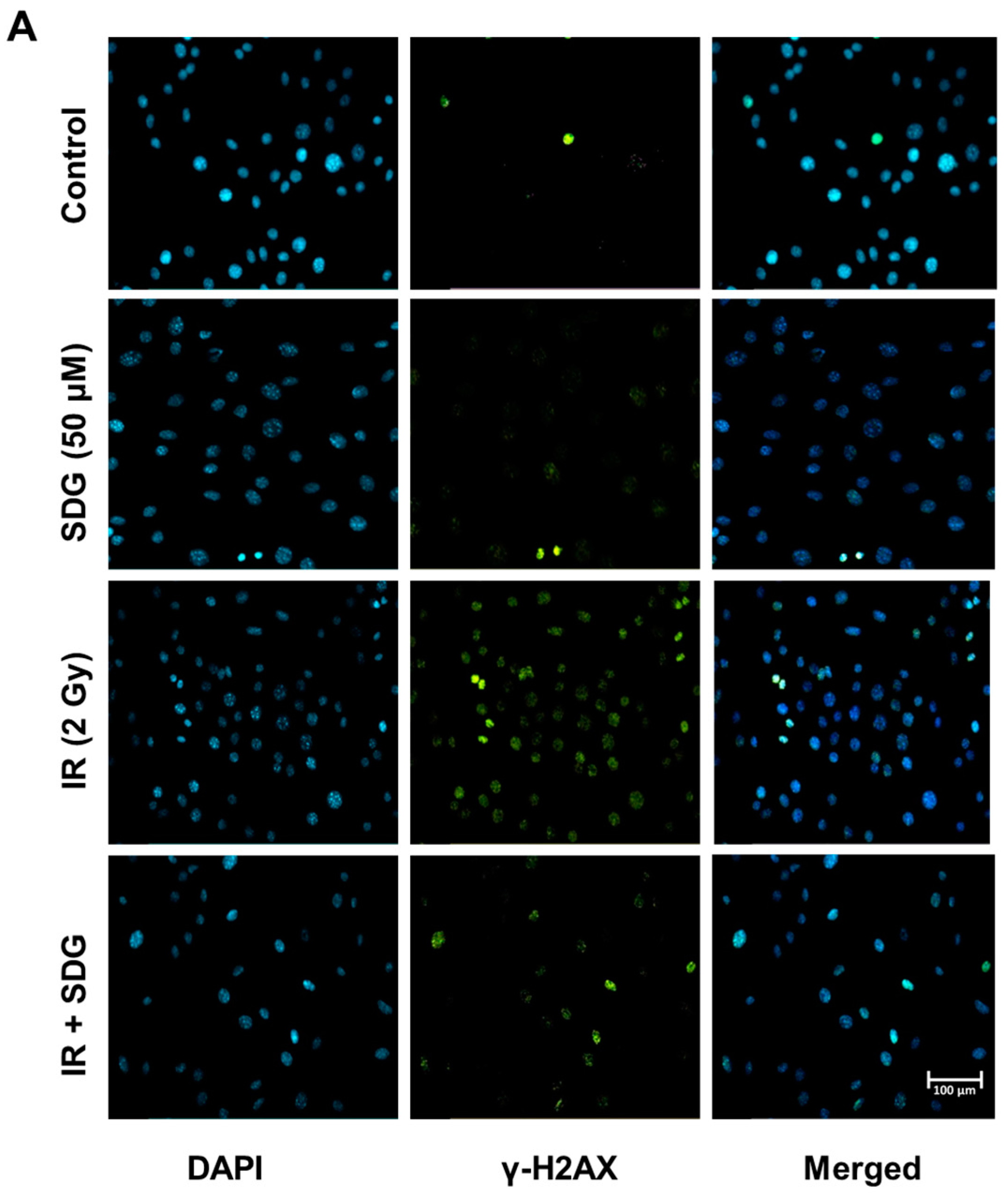
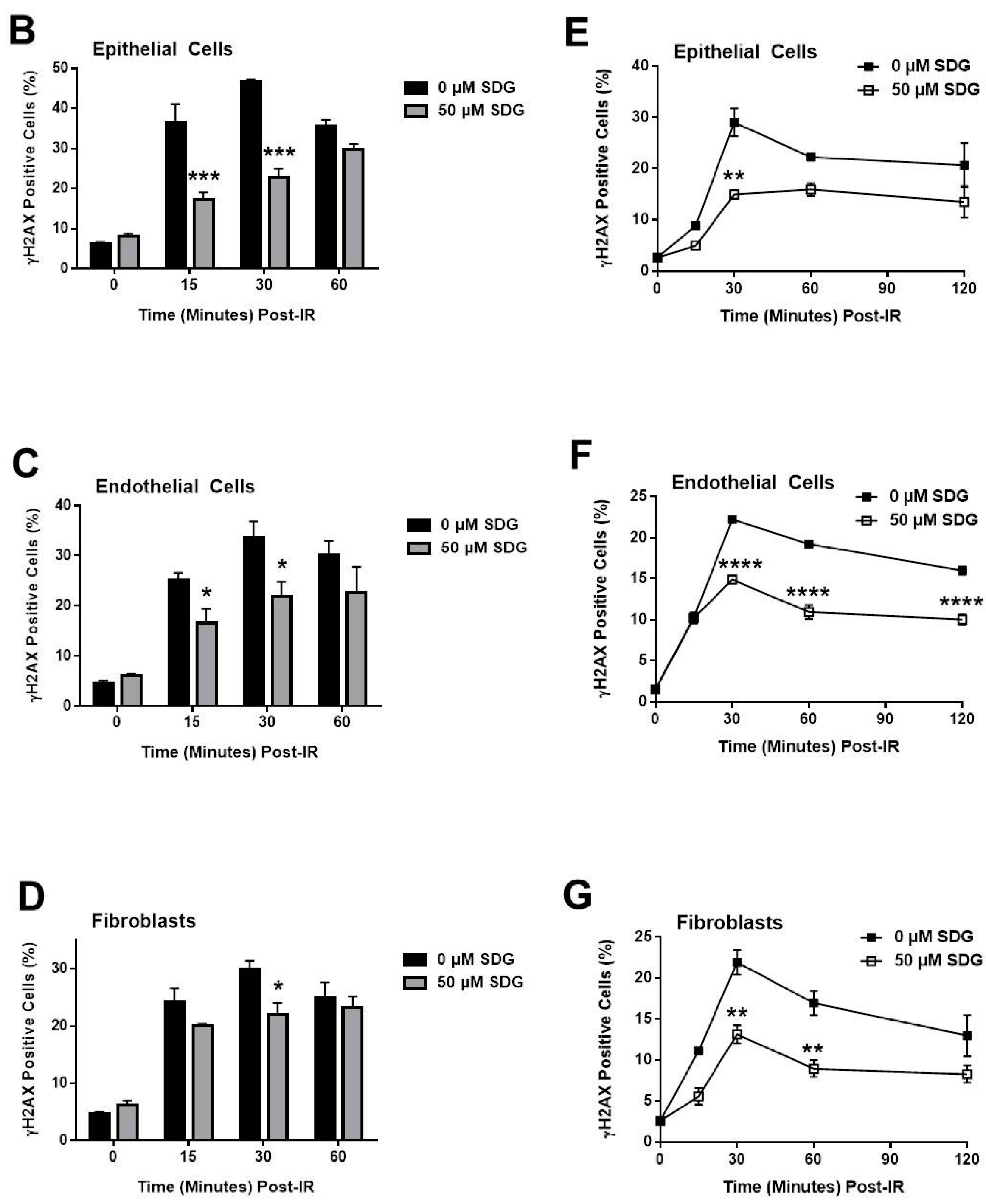
2.2. SDG Abrogates the Induction of γ-H2AX in Irradiated Murine Lung Cells
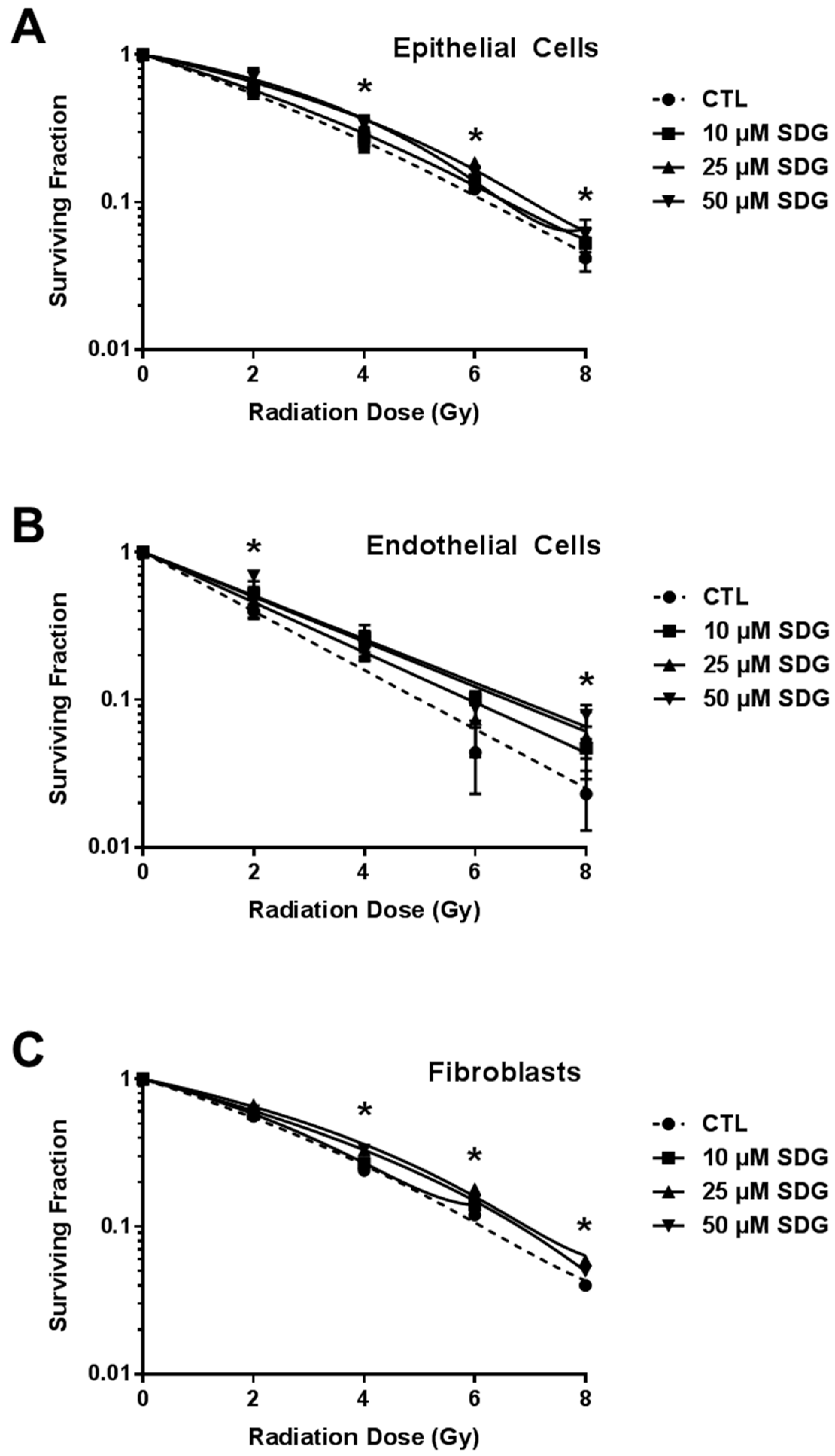
2.3. SDG Treatment Increases Colony Forming Ability of Irradiated Lung Cells
2.4. SDG Treatment Modifies the Gene and Protein Levels of Antioxidant Enzymes in Murine Lung Cells
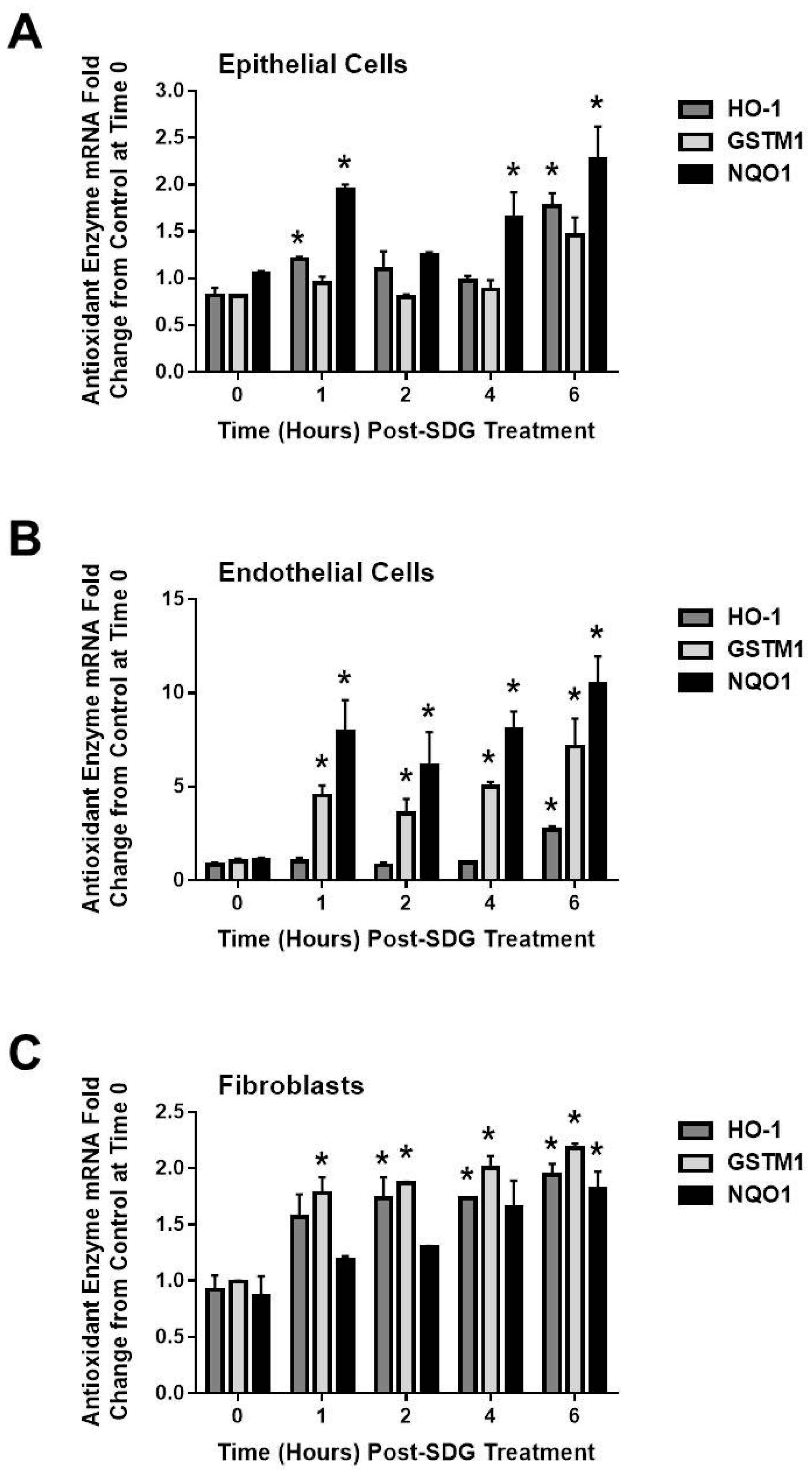
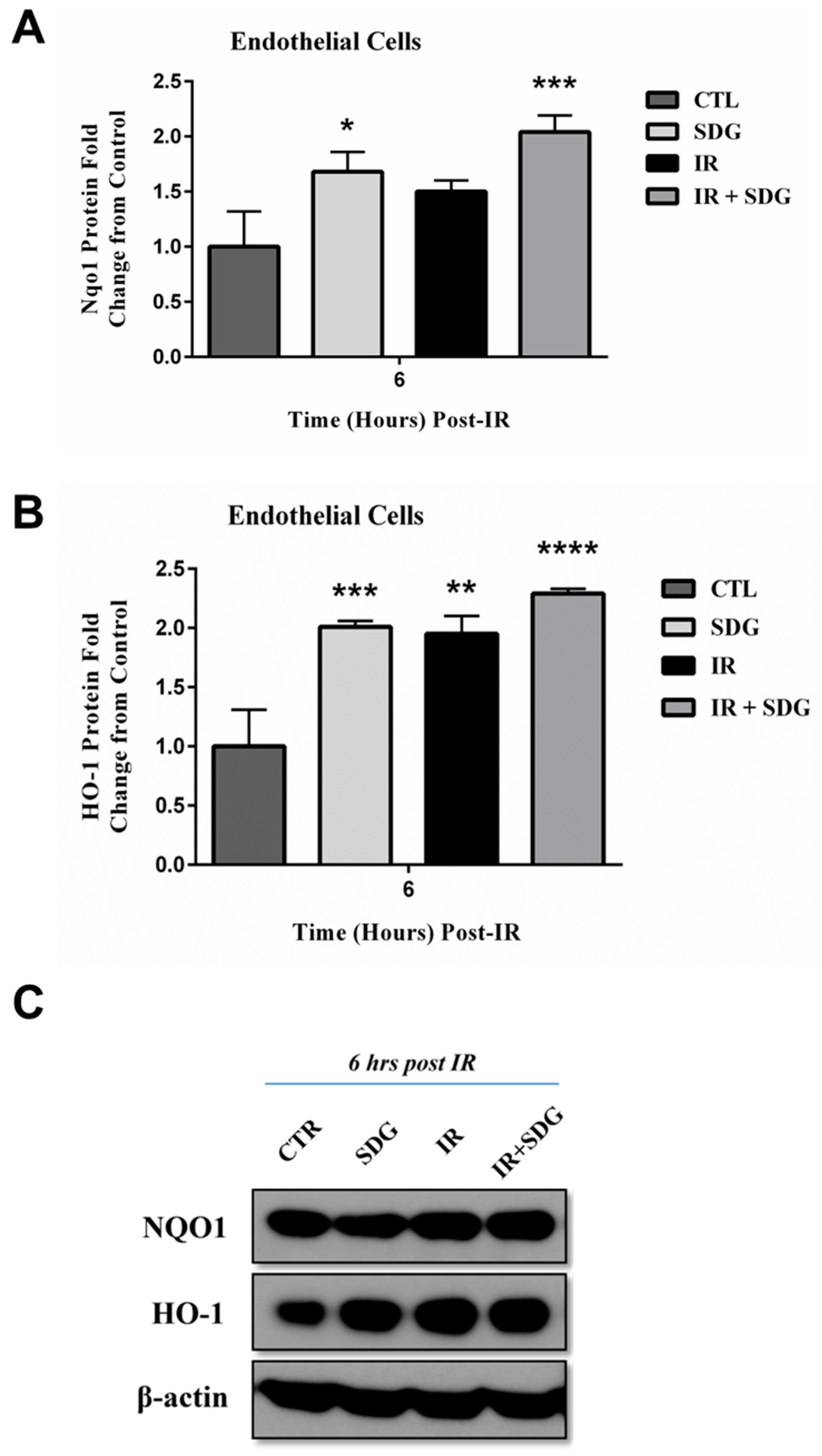
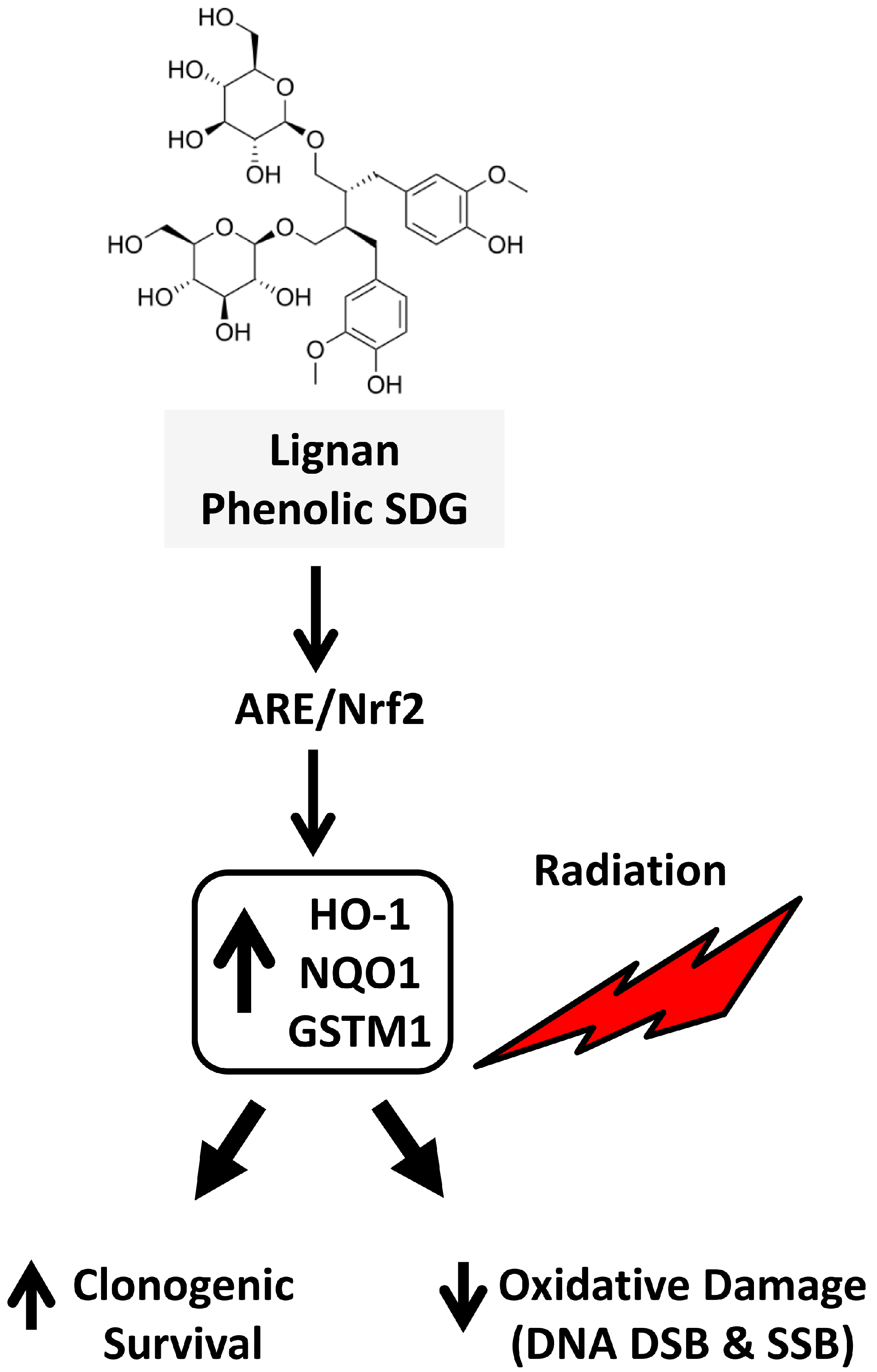
3. Discussion
4. Materials and Methods
4.1. Cell Lines
4.2. Reagents
4.3. COMET Analysis
4.4. Immunostaining
4.5. Flow Cytometry for γ-H2AX
4.6. Clonogenic Survival
4.7. Quantitative Real Time PCR (qPCR)
4.8. Western Blotting
4.9. Statistics
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| DSBs | Double strand breaks |
| SSBs | Single strand breaks |
| FS | flaxseed |
| GSTM1 | glutathione S-transferase mu 1 |
| HO-1 | heme oxygenase-1 |
| IR | irradiation |
| NQO-1 | NAD(P)H dehydrogenase, quinone 1 |
| qPCR | quantitative real time PCR |
| ROS | reactive oxygen species |
| SDG | secoisolariciresinol diglucoside |
| SEM | standard error of the mean |
References
- Sharma, R. Polyphenols in Health and Disease: Practice and Mechanisms of Benefits. In Polyphenols in Human Health and Disease; Elsevier Inc.: Atlanta, GA, USA, 2013; Volume 1, pp. 757–778. [Google Scholar]
- Klaunig, J.E.; Wang, Z.; Pu, X.; Zhou, S. Oxidative stress and oxidative damage in chemical carcinogenesis. Toxicol. Appl. Pharmacol. 2011, 254, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Hada, M.; Georgakilas, A.G. Formation of clustered DNA damage after high-LET irradiation: A review. J. Radiat. Res. 2008, 49, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Citrin, D.; Cotrim, A.P.; Hyodo, F.; Baum, B.J.; Krishna, M.C.; Mitchell, J.B. Radioprotectors and mitigators of radiation-induced normal tissue injury. Oncologist 2010, 15, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.H.; Okoye, C.C.; Patel, R.B.; Siva, S.; Biswas, T.; Ellis, R.J.; Yao, M.; Machtay, M.; Lo, S.S. Complications from Stereotactic Body Radiotherapy for Lung Cancer. Cancers 2015, 7, 981–1004. [Google Scholar] [CrossRef] [PubMed]
- Arora, R.; Gupta, D.; Chawla, R.; Sagar, R.; Sharma, A.; Kumar, R.; Prasad, J.; Singh, S.; Samanta, N.; Sharma, R.K. Radioprotection by plant products: Present status and future prospects. Phytother. Res. 2005, 19, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Hosseinimehr, S.J. Trends in the development of radioprotective agents. Drug Discov. Today 2007, 12, 794–805. [Google Scholar] [CrossRef] [PubMed]
- Maurya, D.K.; Devasagayam, T.P.; Nair, C.K. Some novel approaches for radioprotection and the beneficial effect of natural products. Indian J. Exp. Biol. 2006, 44, 93–114. [Google Scholar] [PubMed]
- Dziegielewski, J.; Goetz, W.; Baulch, J.E. Heavy ions, radioprotectors and genomic instability: Implications for human space exploration. Radiat. Environ. Biophys. 2010, 49, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Kinniry, P.; Amrani, Y.; Vachani, A.; Solomides, C.C.; Arguiri, E.; Workman, A.; Carter, J.; Christofidou-Solomidou, M. Dietary flaxseed supplementation ameliorates inflammation and oxidative tissue damage in experimental models of acute lung injury in mice. J. Nutr. 2006, 136, 1545–1551. [Google Scholar] [PubMed]
- Lee, J.C.; Bhora, F.; Sun, J.; Cheng, G.; Arguiri, E.; Solomides, C.C.; Chatterjee, S.; Christofidou-Solomidou, M. Dietary flaxseed enhances antioxidant defenses and is protective in a mouse model of lung ischemia-reperfusion injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 294, L255–L265. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Krochak, R.; Blouin, A.; Kanterakis, S.; Chatterjee, S.; Arguiri, E.; Vachani, A.; Solomides, C.C.; Cengel, K.A.; Christofidou-Solomidou, M. Dietary flaxseed prevents radiation-induced oxidative lung damage, inflammation and fibrosis in a mouse model of thoracic radiation injury. Cancer Biol. Ther. 2009, 8, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Christofidou-Solomidou, M.; Tyagi, S.; Tan, K.S.; Hagan, S.; Pietrofesa, R.; Dukes, F.; Arguiri, E.; Heitjan, D.F.; Solomides, C.C.; Cengel, K.A. Dietary flaxseed administered post thoracic radiation treatment improves survival and mitigates radiation-induced pneumonopathy in mice. BMC Cancer 2011, 11, 269. [Google Scholar] [CrossRef] [PubMed]
- Kitts, D.D.; Yuan, Y.V.; Wijewickreme, A.N.; Thompson, L.U. Antioxidant activity of the flaxseed lignan secoisolariciresinol diglycoside and its mammalian lignan metabolites enterodiol and enterolactone. Mol. Cell. Biochem. 1999, 202, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K. Secoisolariciresinol diglucoside from flaxseed delays the development of type 2 diabetes in Zucker rat. J. Lab. Clin. Med. 2001, 138, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Christofidou-Solomidou, M.; Tyagi, S.; Pietrofesa, R.; Dukes, F.; Arguiri, E.; Turowski, J.; Grieshaber, P.A.; Solomides, C.C.; Cengel, K.A. Radioprotective role in lung of the flaxseed lignan complex enriched in the phenolic secoisolariciresinol diglucoside (SDG). Radiat. Res. 2012, 178, 568–580. [Google Scholar] [CrossRef] [PubMed]
- Pietrofesa, R.; Turowski, J.; Tyagi, S.; Dukes, F.; Arguiri, E.; Busch, T.M.; Gallagher-Colombo, S.M.; Solomides, C.C.; Cengel, K.A.; Christofidou-Solomidou, M. Radiation mitigating properties of the lignan component in flaxseed. BMC Cancer 2013, 13, 179. [Google Scholar] [CrossRef] [PubMed]
- Mishra, O.P.; Simmons, N.; Tyagi, S.; Pietrofesa, R.; Shuvaev, V.V.; Valiulin, R.A.; Heretsch, P.; Nicolaou, K.C.; Christofidou-Solomidou, M. Synthesis and antioxidant evaluation of (S,S)- and (R,R)-secoisolariciresinol diglucosides (SDGs). Bioorg. Med. Chem. Lett. 2013, 23, 5325–5328. [Google Scholar] [CrossRef] [PubMed]
- Mishra, O.P.; Pietrofesa, R.; Christofidou-Solomidou, M. Novel synthetic (S,S) and (R,R)-secoisolariciresinol diglucosides (SDGs) protect naked plasmid and genomic DNA From gamma radiation damage. Radiat. Res. 2014, 182, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Mah, L.J.; El-Osta, A.; Karagiannis, T.C. γh2AX: A sensitive molecular marker of DNA damage and repair. Leukemia 2010, 24, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Yuan, Y.V.; Kitts, D.D. Antioxidant activities of the flaxseed lignan secoisolariciresinol diglucoside, its aglycone secoisolariciresinol and the mammalian lignans enterodiol and enterolactone in vitro. Food Chem. Toxicol. 2007, 45, 2219–2227. [Google Scholar] [CrossRef] [PubMed]
- Dukes, F.; Kanterakis, S.; Lee, J.; Pietrofesa, R.; Andersen, E.S.; Arguiri, E.; Tyagi, S.; Showe, L.; Amrani, Y.; Christofidou-Solomidou, M. Gene expression profiling of flaxseed in mouse lung tissues-modulation of toxicologically relevant genes. BMC Complement. Altern. Med. 2012, 12, 47. [Google Scholar] [CrossRef] [PubMed]
- Aprotosoaie, A.C.; Trifan, A.; Gille, E.; Petreus, T.; Bordeianu, G.; Miron, A. Can phytochemicals be a bridge to develop new radioprotective agents? Phytochem. Rev. 2015, 14, 555–566. [Google Scholar] [CrossRef]
- Chowdhury, D.; Keogh, M.C.; Ishii, H.; Peterson, C.L.; Buratowski, S.; Lieberman, J. Gamma-H2AX dephosphorylation by protein phosphatase 2A facilitates DNA double-strand break repair. Mol. Cell 2005, 20, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Benkovic, V.; Kopjar, N.; Horvat Knezevic, A.; Dikic, D.; Basic, I.; Ramic, S.; Viculin, T.; Knezevic, F.; Orolic, N. Evaluation of radioprotective effects of propolis and quercetin on human white blood cells in vitro. Biol. Pharm. Bull. 2008, 31, 1778–1785. [Google Scholar] [CrossRef] [PubMed]
- Nair, C.K.; Salvi, V.P. Protection of DNA from gamma-radiation induced strand breaks by Epicatechin. Mutat. Res. 2008, 650, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Sebastià, N.; Almonacid, M.; Villaescusa, J.I.; Cervera, J.; Such, E.; Silla, M.A.; Soriano, J.M.; Montoro, A. Radioprotective activity and cytogenetic effect of resveratrol in human lymphocytes: An in vitro evaluation. Food Chem. Toxicol. 2013, 51, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Richi, B.; Kale, R.K.; Tiku, A.B. Radio-modulatory effects of green tea catechin EGCG on pBR322 plasmid DNA and murine splenocytes against gamma-radiation induced damage. Mutat. Res. 2012, 747, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Begum, N.; Prasad, N.R. Apigenin, a dietary antioxidant, modulates gamma radiation-induced oxidative damages in human peripheral blood lymphocytes. Biomed. Prev. Nutr. 2012, 2, 16–24. [Google Scholar] [CrossRef]
- Pradeep, K.; Park, S.H.; Ko, K.C. Hesperidin a flavanoglycone protects against γ-irradiation induced hepatocellular damage and oxidative stress in Sprague-Dawley rats. Eur. J. Pharmacol. 2008, 587, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, P.; Kumar, A.; Ali, M.; Mishra, K.P. Radioprotection of plasmid and cellular DNA and Swiss mice by silibinin. Mutat. Res. 2010, 695, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Moon, C.; Kim, S.H.; Kim, J.C.; Jin, W.H.; Nam, H.L.; Jae, W.P.; Shin, T. Protective effect of phlorotannin components phloroglucinol and eckol on radiation-induced intestinal injury in mice. Phytother. Res. 2008, 22, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Maier, P.; Wenz, F.; Herskind, C. Radioprotection of normal tissue cells. Strahlenther. Onkol. 2014, 190, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Heredia, A.; Davis, C.; Redfield, R. Synergistic inhibition of HIV-1 in activated and resting peripheral blood mononuclear cells, monocyte-derived macrophages, and selected drug-resistant isolates with nucleoside analogues combined with a natural product, resveratrol. J Acquir Immune Defic. Syndr. 2000, 25, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.P.; Bodiwala, H.S. Recent advances in anti-HIV natural products. Nat. Prod. Rep. 2010, 27, 1781–1800. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.R.; Scott, E.; Brown, V.A.; Gescher, A.J.; Steward, W.P.; Brown, K. Clinical trials of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Cornish, S.M.; Chilibeck, P.D.; Paus-Jennsen, L.; Biem, H.J.; Khozani, T.; Senanayake, V.; Vatanparast, H.; Little, J.P.; Whiting, S.J.; Pahwa, P. A randomized controlled trial of the effects of flaxseed lignan complex on metabolic syndrome composite score and bone mineral in older adults. Appl. Physiol. Nutr. Metab. 2009, 34, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Hallund, J.; Tetens, I.; Bugel, S.; Tholstrup, T.; Bruun, J.M. The effect of a lignan complex isolated from flaxseed on inflammation markers in healthy postmenopausal women. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Eickelberg, O.; Kohler, E.; Reichenberger, F.; Bertschin, S.; Woodtli, T.; Erne, P.; Perruchoud, A.P.; Roth, M. Extracellular matrix deposition by primary human lung fibroblasts in response to TGF-beta1 and TGF-beta3. Am. J. Physiol. 1999, 276, L814–L824. [Google Scholar] [PubMed]
- Lee, J.C.; Kinniry, P.A.; Arguiri, E.; Serota, M.; Kanterakis, S.; Chatterjee, S.; Solomides, C.C.; Javvadi, P.; Koumenis, C.; Cengel, K.A.; et al. Dietary curcumin increases antioxidant defenses in lung, ameliorates radiation-induced pulmonary fibrosis, and improves survival in mice. Radiat. Res. 2010, 173, 590–601. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.J.; Le Mesurier, S.M.; de Montfort, M.L.; Lykke, A.W. Development and characterization of type 2 pneumocyte-related cell lines from normal adult mouse lung. Pathology 1984, 16, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Johnsson, P.; Kamal-Eldin, A.; Lundgren, L.N.; Aman, P. HPLC method for analysis of secoisolariciresinol diglucoside in flaxseeds. J. Agric. Food Chem. 2000, 48, 5216–5219. [Google Scholar] [CrossRef] [PubMed]
- Eliasson, C.; Kamal-Eldin, A.; Andersson, R.; Aman, P. High-performance liquid chromatographic analysis of secoisolariciresinol diglucoside and hydroxycinnamic acid glucosides in flaxseed by alkaline extraction. J. Chromatogr. A 2003, 1012, 151–159. [Google Scholar] [CrossRef]
- Muir, A.D.; Westcott, N.D. Quantitation of the lignan secoisolariciresinol diglucoside in baked goods containing flax seed or flax meal. J. Agric. Food Chem. 2000, 48, 4048–4052. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velalopoulou, A.; Tyagi, S.; Pietrofesa, R.A.; Arguiri, E.; Christofidou-Solomidou, M. The Flaxseed-Derived Lignan Phenolic Secoisolariciresinol Diglucoside (SDG) Protects Non-Malignant Lung Cells from Radiation Damage. Int. J. Mol. Sci. 2016, 17, 7. https://doi.org/10.3390/ijms17010007
Velalopoulou A, Tyagi S, Pietrofesa RA, Arguiri E, Christofidou-Solomidou M. The Flaxseed-Derived Lignan Phenolic Secoisolariciresinol Diglucoside (SDG) Protects Non-Malignant Lung Cells from Radiation Damage. International Journal of Molecular Sciences. 2016; 17(1):7. https://doi.org/10.3390/ijms17010007
Chicago/Turabian StyleVelalopoulou, Anastasia, Sonia Tyagi, Ralph A. Pietrofesa, Evguenia Arguiri, and Melpo Christofidou-Solomidou. 2016. "The Flaxseed-Derived Lignan Phenolic Secoisolariciresinol Diglucoside (SDG) Protects Non-Malignant Lung Cells from Radiation Damage" International Journal of Molecular Sciences 17, no. 1: 7. https://doi.org/10.3390/ijms17010007