Enzymatic Kinetic Properties of the Lactate Dehydrogenase Isoenzyme C4 of the Plateau Pika (Ochotona curzoniae)
Abstract
:1. Introduction
2. Results
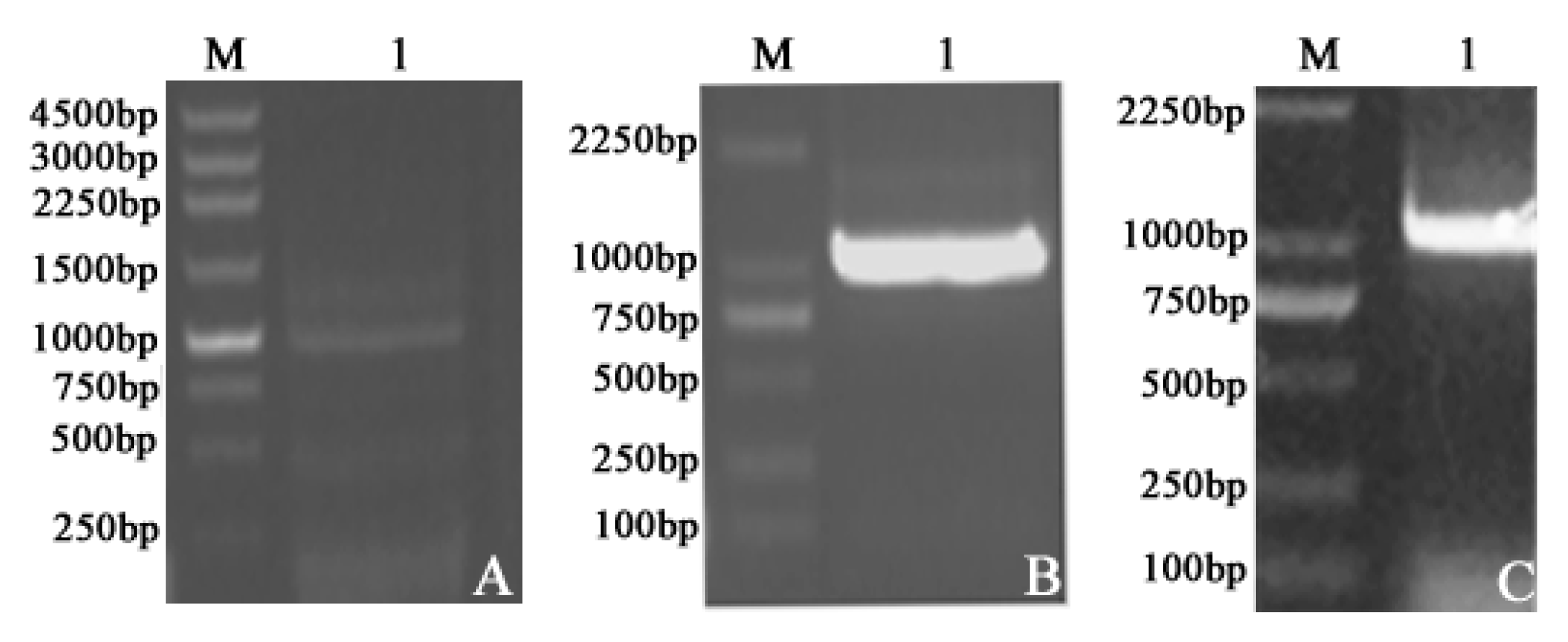
2.1. Plasmid Construction and Recombinant Protein Expression
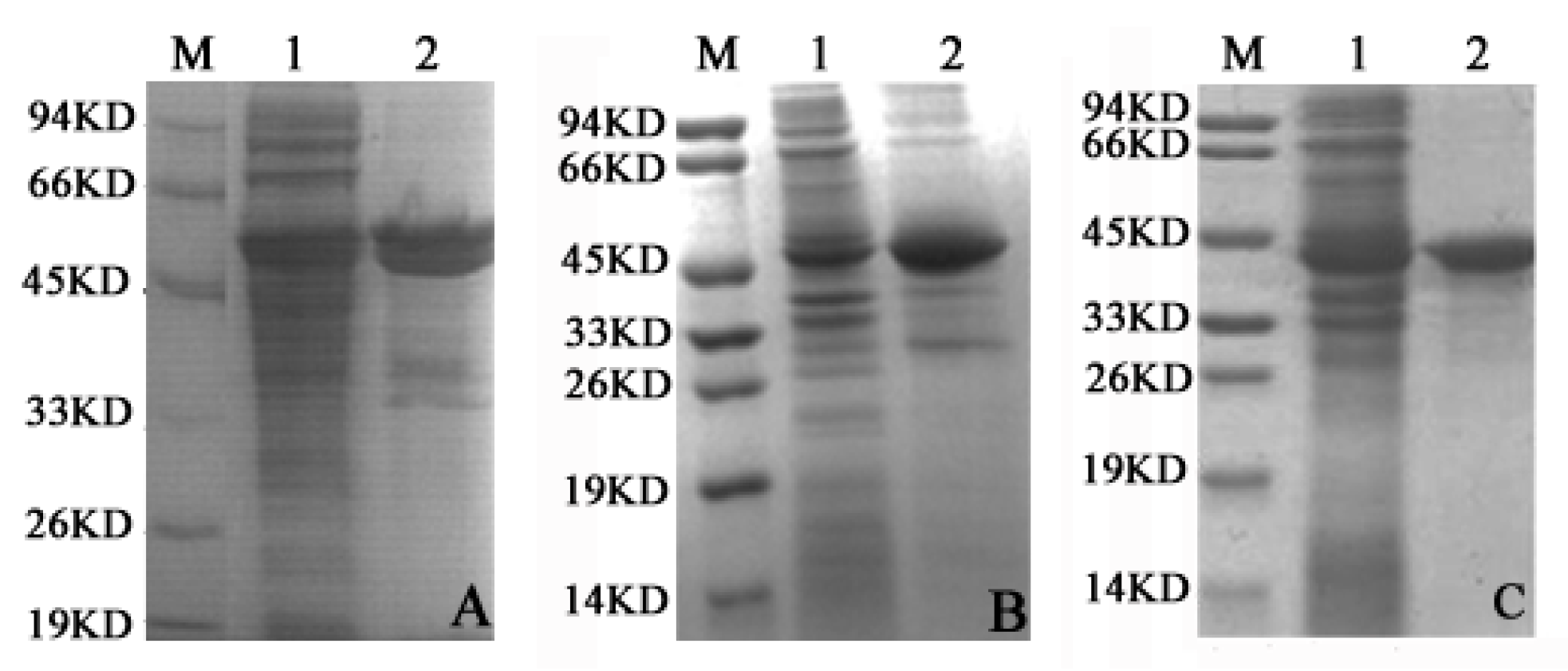
2.2. LDH-A4, LDH-B4 and LDH-C4 Purification and Activity Measurement
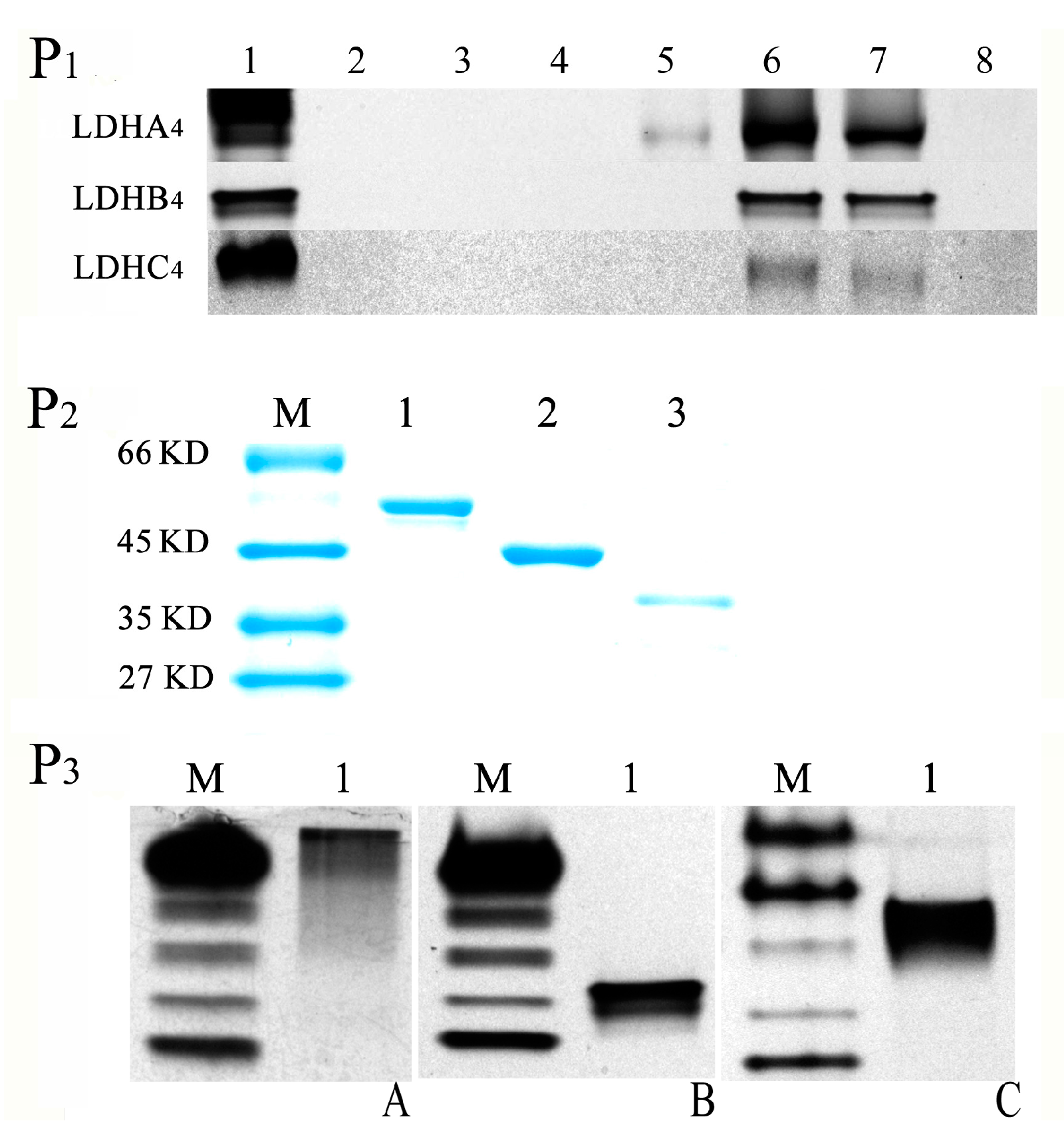
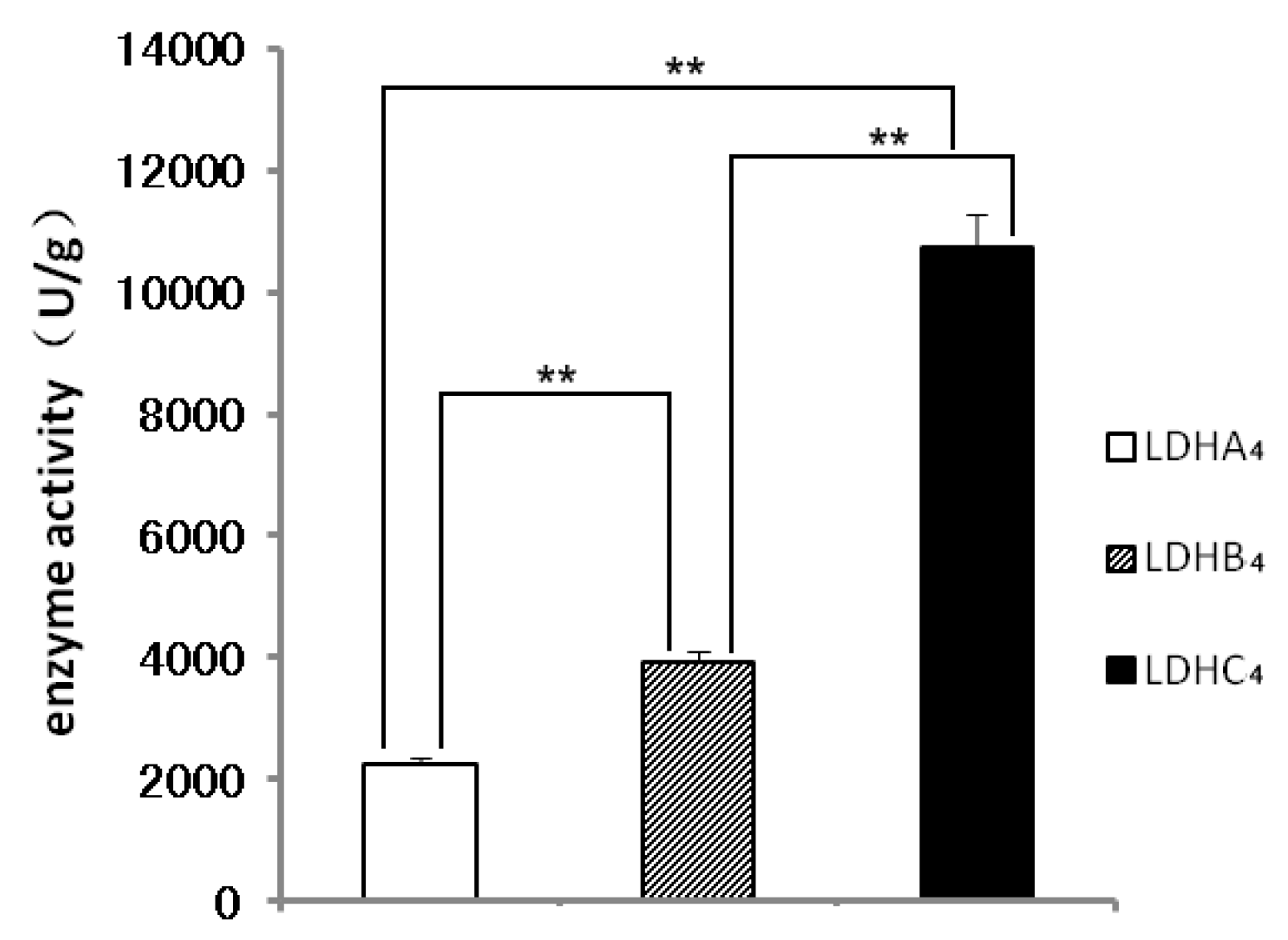
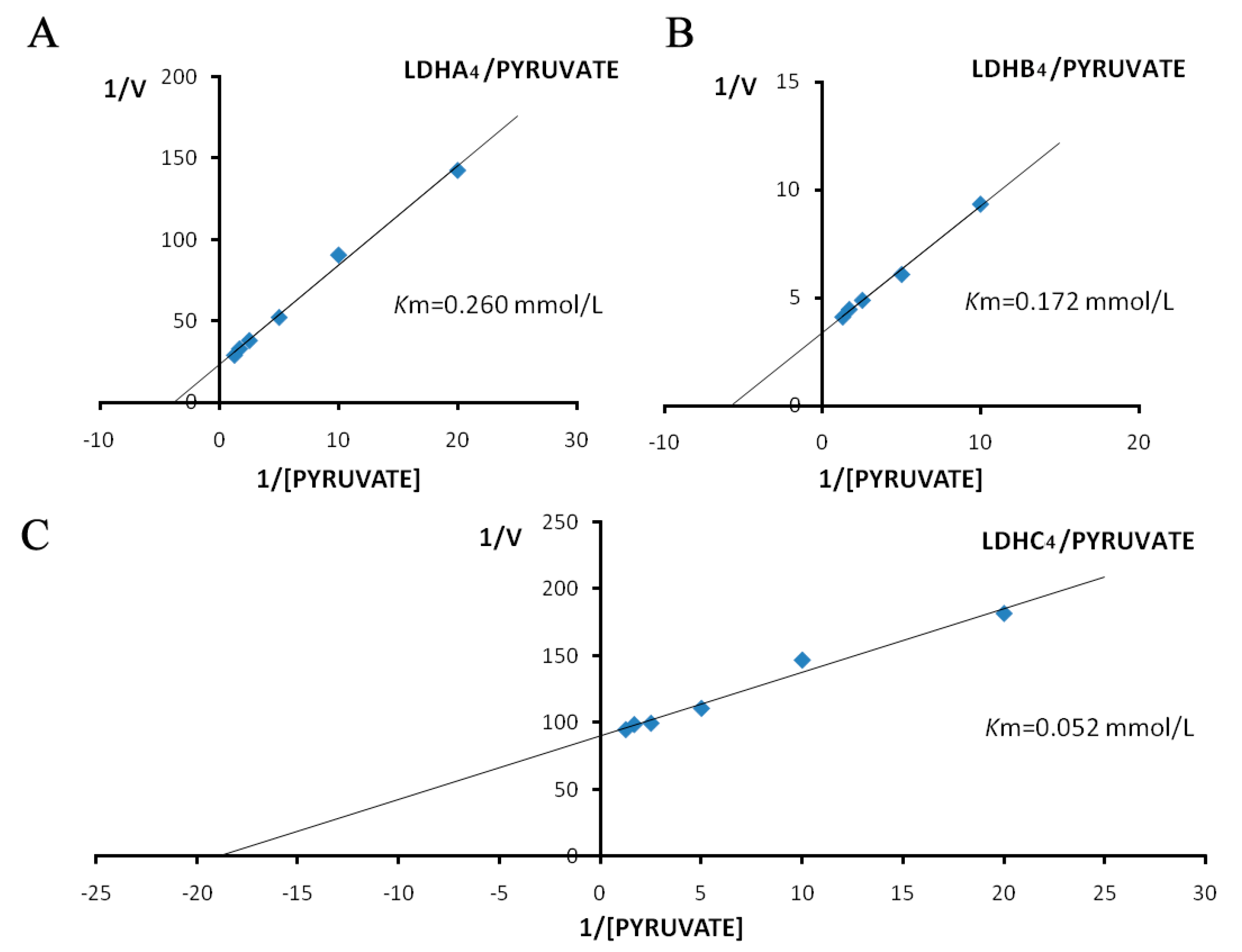
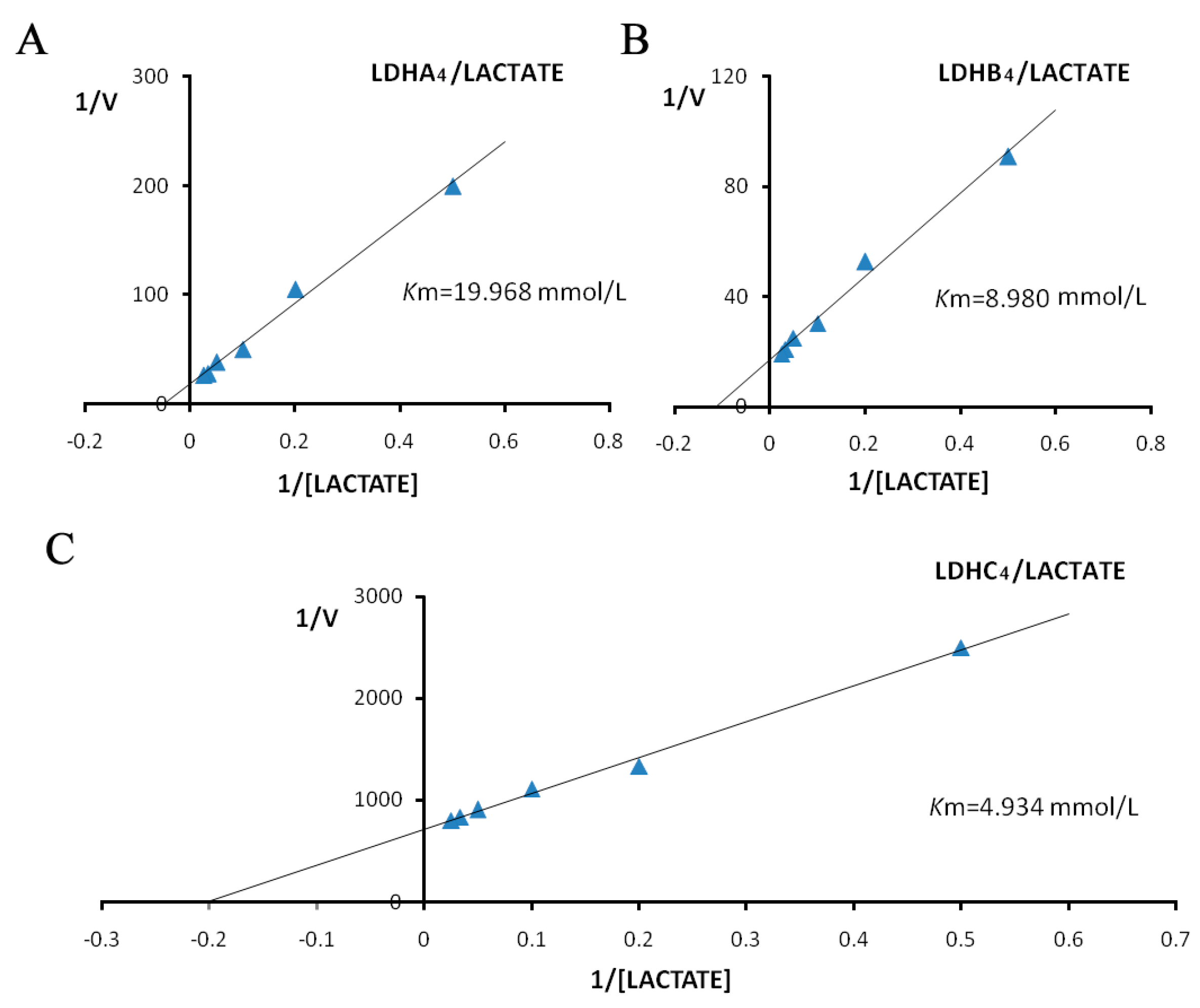
2.3. Enzyme Kinetics Properties of LDH-A4, LDH-B4, and LDH-C4
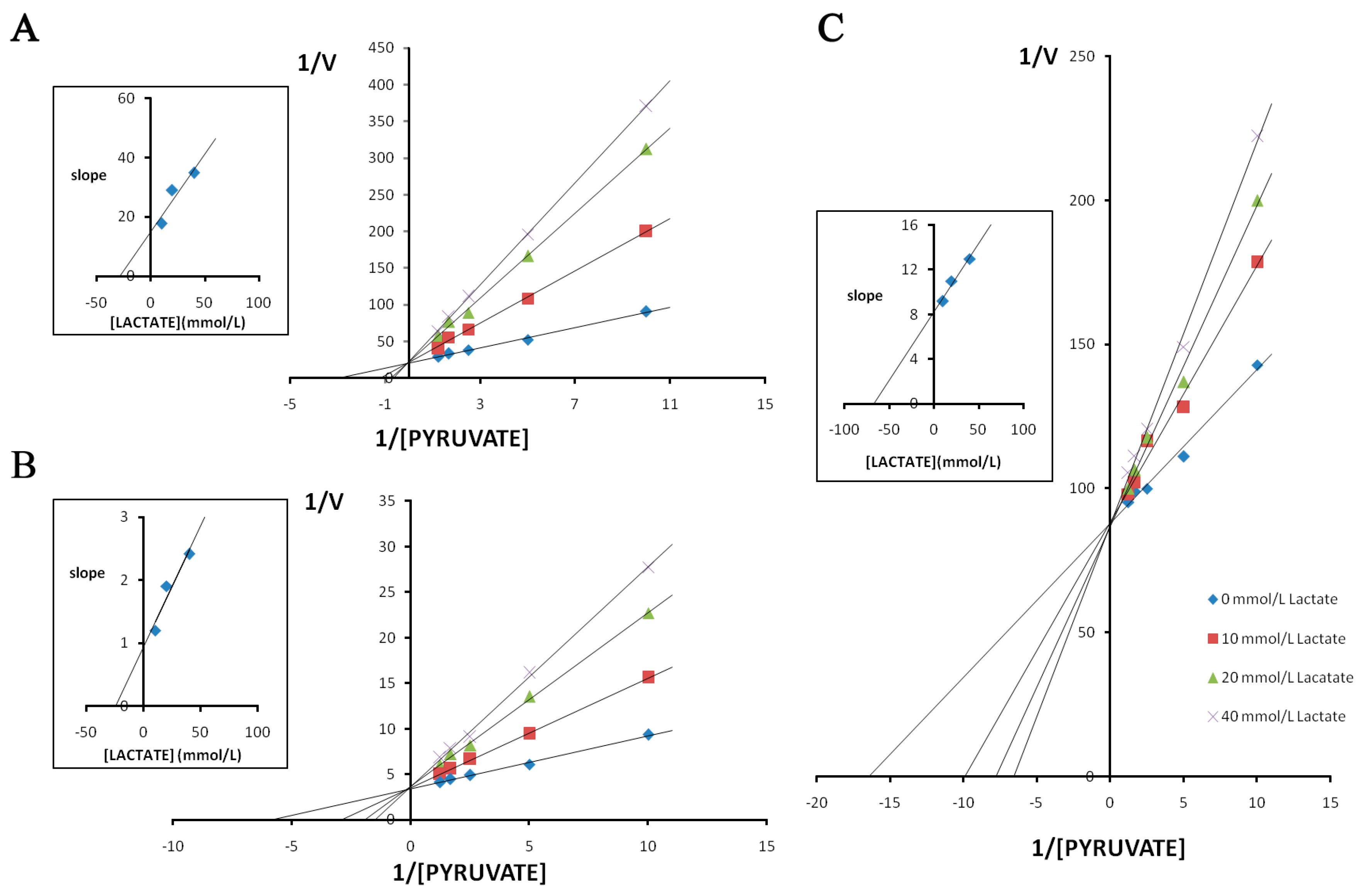
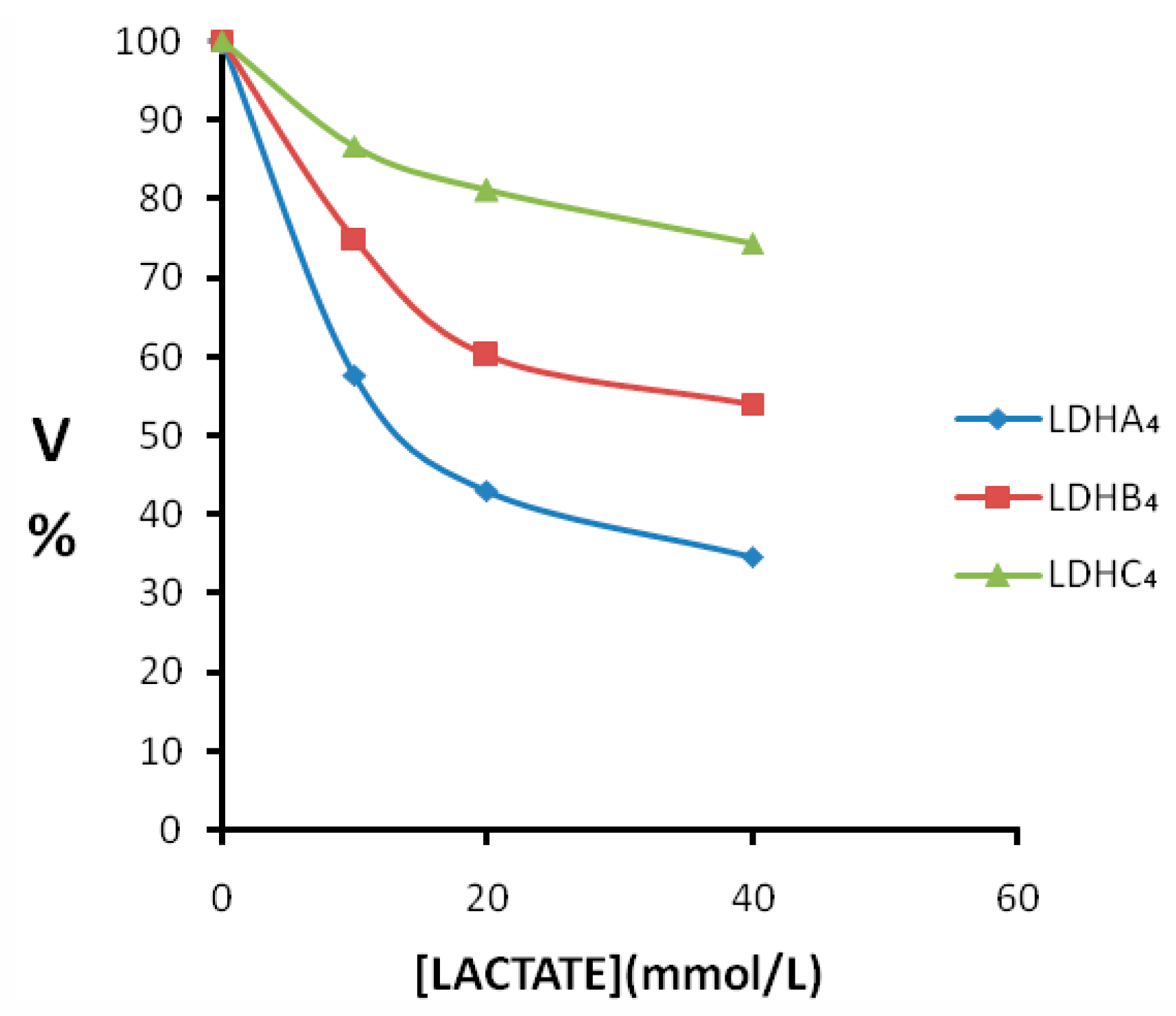
3. Discussion
4. Materials and Methods
4.1. Reagents and Animal Procedures
4.2. Expression Plasmids Construction
4.3. Protein Expression, Purification, Analysis and Enzyme Activity Measurement
4.4. LDH-A4, LDH-B4 and LDH-C4 Kinetics Properties
4.5. Statistics
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Smith, A.T.; Foggin, J.M. The plateau pika (Ochotona curzoniae) is a keystone species for biodiversity on the Tibetan plateau. Anim. Conserv. 1999, 2, 235–240. [Google Scholar] [CrossRef]
- Lai, C.H.; Smith, A.T. Keystone status of plateau pikas (Ochotona curzoniae): Effect of control on biodiversity of native birds. Biodivers. Conserv. 2003, 12, 1901–1912. [Google Scholar] [CrossRef]
- Yang, Y.Z.; Cao, Y.; Jin, G.E.; Bai, Z.Z.; Yun, M.L.; Ge, R.L. Molecular cloning and characterization of hemoglobin α and β chains from plateau pika (Ochotona curzoniae) living at high altitude. Gene 2007, 403, 118–124. [Google Scholar]
- Wang, X.J.; Wei, D.B.; Wei, L.; Qi, X.Z.; Zhu, S.H. Characteristics of pulmonary acinus structure in the plateau zokor (Myospalax baileyi) and plateau pika (Ochotona curzoniae). Acta Zool. Sin. 2008, 54, 531–539. [Google Scholar]
- Ge, R.L.; Kubo, K.; Kobayashi, T.; Sekiguchi, M.; Honda, T. Blunted hypoxic pulmonary vasoconstrictive response in the rodent Ochotona curzoniae (pika) at high altitude. Am. J. Physiol. Heart Circ. Physiol. 1998, 274, H1792–H1799. [Google Scholar]
- Wang, X.J.; Wei, D.B.; Wei, L.; Zhang, J.M.; Yu, H.Y. Physiological character of erythrocyte adapting to hypoxia in plateau zokor and plateau pika. Sichuan J. Zool. 2008, 6, 038. (In Chinese) [Google Scholar]
- Ye, R.R.; Cao, Y.F.; Bai, Q.H. Blood indices of plateau pika and relationship with hypoxia adaptation. Acta Anim. Sci. Sin. 1994, 2, 114–119. [Google Scholar]
- He, J.; Xu, C.; Meng, X.; Li, H.; Wang, Y. Comparative analysis in transport and intake of oxygen between pikas (Ochotona curzoniae) and rats. J. Prev. Med. Chin. People Lib. 1994, 12, 431–435. (In Chinese) [Google Scholar]
- Qi, X.Z.; Wang, X.J.; Zhu, S.H.; Rao, X.F.; Wei, L.; Wei, D.B. Hypoxic adaptation of the hearts of plateau zokor (Myospalax baileyi) and plateau pika (Ochotona curzoniae). Acta Physiol. Sin. 2008, 60, 348–354. (In Chinese) [Google Scholar]
- Wei, D.B.; Wei, L.; Zhang, J.M.; Yu, H.Y. Blood-gas properties of plateau zokor (Myospalax baileyi). Comp. Biochem. Phys. A 2006, 145, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.H.; Qi, X.Z.; Wang, X.J.; Rao, X.F.; Wei, L.; Wei, D.B. Difference in oxygen uptake in skeletal muscles between plateau zokor (Myospalax baileyi) and plateau pika (Ochotona curzoniac). Acta Physiol. Sin. 2009, 61, 373–378. (In Chinese) [Google Scholar]
- Sun, S.Z.; Wei, L.; Wei, D.B.; Wang, D.W.; Ma, B.Y. Differences of glycolysis in skeletal muscle and lactate metabolism in liver between plateau zokor (Myospalax baileyi) and plateau pika (Ochotona curzoniae). Acta Physiol. Sin. 2013, 65, 276–284. [Google Scholar]
- Li, H.G.; Guo, S.C.; Ren, Y.M.; Wang, D.P.; Yu, H.H.; Li, W.J.; Zhao, X.Q.; Chang, Z.J. VEGF189 expression is highly related to adaptation of the plateau pika (Ochotona curzoniae) inhabiting high altitudes. HighAlt. Med. Biol. 2013, 14, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.N.; Zhu, R.J.; Wang, D.W.; Wei, L.; Wei, D.B. Gene coding and mRNA expression of vascular endothelial growth factor as well as microvessel density in brain of plateau zokor: Comparison with other rodents. Acta Physiol. Sin. 2011, 63, 155–163. (In Chinese) [Google Scholar]
- Li, H.G.; Ren, Y.M.; Guo, S.C.; Cheng, L.; Wang, D.P.; Yang, J.; Chang, Z.J.; Zhao, X.Q. The protein level of hypoxia-inducible factor-1α is increased in the plateau pika (Ochotona curzoniae) inhabiting high altitudes. J. Exp. Zool. A Ecol. Genet. Physiol. 2009, 311, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.B.; Ning, H.X.; Zhu, S.S.; Sun, P.; Xu, S.X.; Chang, Z.J.; Zhao, X.Q. Cloning of hypoxia-inducible factor-1α cDNA from a high hypoxia tolerant mammal—plateau pika (Ochotona curzoniae). Biochem. Biophys. Res. Commun. 2004, 316, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.W.; Wei, L.; Wei, D.B.; Rao, X.F.; Qi, X.Z.; Wang, X.J.; Ma, B.Y. Testis-specific lactate dehydrogenase is expressed in somatic tissues of plateau pikas. FEBS Open Biol. 2013, 3, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Pichon, A.; Zhenzhong, B.; Favret, F.; Jin, G.; Shufeng, H.; Marchant, D.; Richalet, J.P.; Ge, R.L. Long-term ventilatory adaptation and ventilatory response to hypoxia in plateau pika (Ochotona curzoniae): Role of nNOS and dopamine. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, R978–R987. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, Z.L.; Zhao, X.Q.; Xu, B.H.; Ren, Y.H.; Tian, H.F. Natural selection and adaptive evolution of leptin in the ochotona family driven by the cold environmental stress. PLoS ONE 2008, 3, e1472. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhao, X.Q.; Guo, S.C.; Li, H.G.; Qi, D.L.; Wang, D.P.; Cao, J.H. Leptin cDNA cloning and its mRNA expression in plateau pikas (Ochotona curzoniae) from different altitudes on Qinghai-Tibet Plateau. Biochem. Biophys. Res. Commun. 2006, 345, 1405–1413. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.J.; Gao, W.X.; Gao, Y.Q.; Tang, S.; Huang, Q.Y.; Tan, X.L.; Chen, J.; Huang, T.S. Mitochondrial genome analysis of Ochotona curzoniae and implication of cytochrome c oxidase in hypoxic adaptation. Mitochondrion 2008, 8, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Everse, J.; Kaplan, N.O. Lactate dehydrogenases: Structure and function. Adv. Enzymol. Relat. Areas Mol. Biol. 1973, 37, 61–133. [Google Scholar] [PubMed]
- Li, S. Lactate dehydrogenase isoenzymes A (muscle), B (heart) and C (testis) of mammals and the genes coding for these enzymes. Biochem. Soc. Trans. 1989, 17, 304. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; O’brien, D.; Hou, E.; Versola, J.; Rockett, D.; Eddy, E. Differential activity and synthesis of lactate dehydrogenase isozymes A (muscle), B (heart), and C (testis) in mouse spermatogenic cells. Biol. Reprod. 1989, 40, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Cahn, R.D.; Zwilling, E.; Kaplan, N.O.; Levine, L. Nature and development of lactic dehydrogenases. Science 1962, 136, 962–969. [Google Scholar] [CrossRef] [PubMed]
- Fine, I.; Kaplan, N.; Kuftinec, D. Developmental changes of mammalian lactic dehydrogenases. Biochemistry 1963, 2, 116–121. [Google Scholar] [CrossRef]
- Goldberg, E. Reproductive implications of LDH-C4 and other testis-specific isozymes. Exp. Clin. Immunogenet. 1984, 2, 120–124. [Google Scholar]
- Coonrod, S.; Vitale, A.; Duan, C.; Bristol-Gould, S.; Herr, J.; Goldberg, E. Testis-Specific lactate dehydrogenase (LDH-C4; Ldh3) in murine oocytes and preimplantation embryos. J. Androl. 2006, 27, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, E. Lactate dehydrogenase-X from mouse testes and spermatozoa. Methods Enzymol. 1975, 41, 318. [Google Scholar] [PubMed]
- Goldberg, E. Lactate dehydrogenases and malate dehydrogenases in sperm: Studied by polyacrylamide gel electrophoresis. Ann. N. Y. Acad. Sci. 1964, 121, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Coronel, C.E.; Burgos, C.; de Gerez Burgos, N.M.; Rovai, L.E.; Blanco, A. Catalytic properties of the sperm-specific lactate dehydrogenase (LDH X or C4) from different species. J. Exp. Zool. 1983, 225, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.; Yañez, R.; Brown, D.M.; Dickey, A.; Parks, M.E.; McKee, R.W. Isolation and properties of lactate dehydrogenase isozyme X from Swiss mice. Biochim. Biophys. Acta 1971, 146, 454–460. [Google Scholar] [CrossRef]
- Allen, J. Multiple forms of lactic dehydrogenase in tissues of the mouse: Their specificity, cellular localization, and response to altered physiological conditions. Ann. N. Y. Acad. Sci. 1961, 94, 937–951. [Google Scholar] [CrossRef] [PubMed]
- Zinkham, W.H.; Blanco, A.; Clowry, L.J. An unusual isozyme of lactate dehydrogenase in mature testes: Localization, ontogeny, and kinetic properties. Ann. N. Y. Acad. Sci. 1964, 121, 571–588. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, J.; Withycombe, W.A. Organ specificity and lactate-dehydrogenase activity. Some properties of human spermatozoal lactate dehydrogenase. Biochem. J. 1965, 97, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Blanco, A.; Zinkham, W.H.; Kupchyk, L. Genetic control and ontogeny of lactate dehydrogenase in pigeon testes. J. Exp. Zool. 1964, 156, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Battellino, L.J.; Jaime, F.R.; Blanco, A. Kinetic properties of rabbit testicular lactate dehydrogenase isozyme. J. Biol. Chem. 1968, 243, 5185–5192. [Google Scholar] [PubMed]
- Schatz, L.; Segal, H.L. Reduction of α-ketoglutarate by homogeneous lactic dehydrogenase X of testicular tissue. J. Biol. Chem. 1969, 244, 4393–4397. [Google Scholar] [PubMed]
- Battellino, L.J.; Blanco, A. Catalytic properties of the lactate dehydrogenase isozyme “X” from mouse testis. J. Exp. Zool. 1970, 174, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Hawtrey, C.O.; Goldberg, E. Some kinetic aspects of sperm specific lactate dehydrogenase in mice. J. Exp. Zool. 1970, 174, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Fleisher, G.A.; Kolb, E.; Larner, J. Isolation and characterization of bovine lactate dehydrogenase X. Biochemistry 1970, 9, 4372–4380. [Google Scholar] [CrossRef]
- Rodríguez-Páez, L.; Chena-Taboada, M.A.; Cabrera-Hernández, A.; Cordero-Martínez, J.; Wong, C. Oxamic acid analogues as LDH-C4-specific competitive inhibitors. J. Enzym. Inhib. Med. Chem. 2011, 26, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Blanco, A.; Zinkham, W.H. Lactate dehydrogenases in human testes. Science 1963, 139, 601–602. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, E. Lactic and malic dehydrogenases in human spermatozoa. Science 1963, 139, 602–603. [Google Scholar] [CrossRef] [PubMed]
- Quistorff, B.; Grunnet, N. The isoenzyme pattern of LDH does not play a physiological role; except perhaps during fast transitions in energy metabolism. Aging 2011, 3, 457–460. [Google Scholar] [PubMed]
- Putz, M.V. On the Reducible Character of Haldane-Radić Enzyme Kinetics to Conventional and Logistic Michaelis-Menten Models. Molecules 2011, 16, 3128–3145. [Google Scholar] [CrossRef] [PubMed]
- Putz, M.V.; Ana-Maria, L.; Vasile, O. Full analytic progress curves of enzymic reactions in vitro. Int. J. Mol. Sci. 2006, 7, 469–484. [Google Scholar] [CrossRef]
- Clausen, J.; Ovlisen, B. Lactate dehydrogenase isoenzymes of human semen. Biochem. J. 1965, 97, 513–517. [Google Scholar] [CrossRef] [PubMed]
- LeVan, K.M.; Goldberg, E. Properties of human testis-specific lactate dehydrogenase expressed from Escherichia coli. Biochem. J. 1991, 273, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.; Rodrı́guez-Páez, L.; Nogueda, B.; Pérez, A.; Baeza, I. Selective inhibition of the sperm-specific lactate dehydrogenase isozyme-C4 by N-isopropyl oxamate. Biochim. Biophys. Acta 1997, 1343, 16–22. [Google Scholar] [CrossRef]
- Hereng, T.; Elgstøen, K.; Cederkvist, F.; Eide, L.; Jahnsen, T.; Skålhegg, B.; Rosendal, K. Exogenous pyruvate accelerates glycolysis and promotes capacitation in human spermatozoa. Hum. Reprod. 2011. [Google Scholar] [CrossRef] [PubMed]
- Harwood. Basic DNA and RNA Protocols. In Methods in Molecular Biology, 1st ed.; NJ Humana Press: Totowa, NJ, USA, 1994; Volume 58, pp. 491–510. [Google Scholar]
- Niu, X.; Guiltinan, M.J. DNA binding specificity of the wheat bZIP protein EmBP-1. Nucleic Acids Res. 1994, 22, 4969–4978. [Google Scholar] [CrossRef] [PubMed]
- Tang, W. The cause of deviation made in determining the molecular weight of His-tag fusion proteins by SDS-PAGE. Acta Phytophysiol. Sin. 2000, 26, 64–68. (In Chinese) [Google Scholar]
- Berta, M.A.; Mazure, N.; Hattab, M.; Pouysségur, J.; Brahimi-Horn, M.C. SUMOylation of hypoxia-inducible factor-1α reduces its transcriptional activity. Biochem. Biophys. Res. Commun. 2007, 360, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Odet, F.; Gabel, S.A.; Williams, J.; London, R.E.; Goldberg, E.; Eddy, E.M. Lactate dehydrogenase C and energy metabolism in mouse sperm. Biol. Reprod. 2011, 85, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Odet, F.; Duan, C.; Willis, W.D.; Goulding, E.H.; Kung, A.; Eddy, E.M.; Goldberg, E. Expression of the gene for mouse lactate dehydrogenase C (Ldhc) is required for male fertility. Biol. Reprod. 2008, 79, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Mukai, C.; Okuno, M. Glycolysis plays a major role for adenosine triphosphate supplementation in mouse sperm flagellar movement. Biol. Reprod. 2004, 71, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.C.; Ford, W.C.L. The role of glucose in supporting motility and capacitation in human spermatozoa. J. Androl. 2001, 22, 680–695. [Google Scholar] [PubMed]
- Zhang, J.Y.; Jin, Z.; Sun, T.; Jiang, Y.; Han, Q.Q.; Song, Y.Z.; Chen, Q.; Xia, X.S. Prokaryotic expression, purification, and polyclonal antibody production of a truncated recombinant rabies virus L protein. Iran J. Biotechnol. 2015, 13, e1022. [Google Scholar]
- Bergmeyer, H.U. Methods of Enzymatic Analysis; Academic Press: New York, NY, USA, 2012; Volume 3, pp. 1196–1201. [Google Scholar]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wei, L.; Wei, D.; Li, X.; Xu, L.; Wei, L. Enzymatic Kinetic Properties of the Lactate Dehydrogenase Isoenzyme C4 of the Plateau Pika (Ochotona curzoniae). Int. J. Mol. Sci. 2016, 17, 39. https://doi.org/10.3390/ijms17010039
Wang Y, Wei L, Wei D, Li X, Xu L, Wei L. Enzymatic Kinetic Properties of the Lactate Dehydrogenase Isoenzyme C4 of the Plateau Pika (Ochotona curzoniae). International Journal of Molecular Sciences. 2016; 17(1):39. https://doi.org/10.3390/ijms17010039
Chicago/Turabian StyleWang, Yang, Lian Wei, Dengbang Wei, Xiao Li, Lina Xu, and Linna Wei. 2016. "Enzymatic Kinetic Properties of the Lactate Dehydrogenase Isoenzyme C4 of the Plateau Pika (Ochotona curzoniae)" International Journal of Molecular Sciences 17, no. 1: 39. https://doi.org/10.3390/ijms17010039