Transcription Factor Sp1 Promotes the Expression of Porcine ROCK1 Gene
Abstract
:1. Introduction
2. Results
2.1. Identification of the Promoter Region and Regulatory Elements of Porcine ROCK1 Gene.
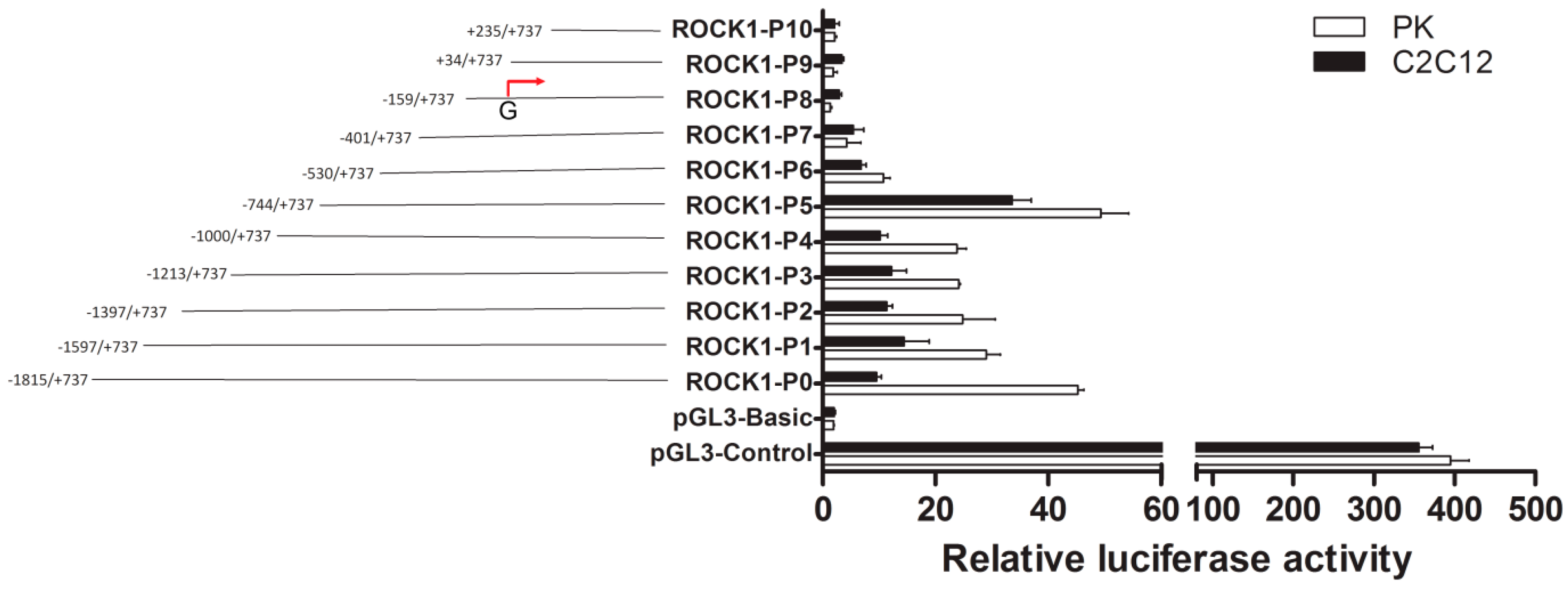
2.2. The Importance of Sp1 Binding Sites in Porcine ROCK1 Promoter

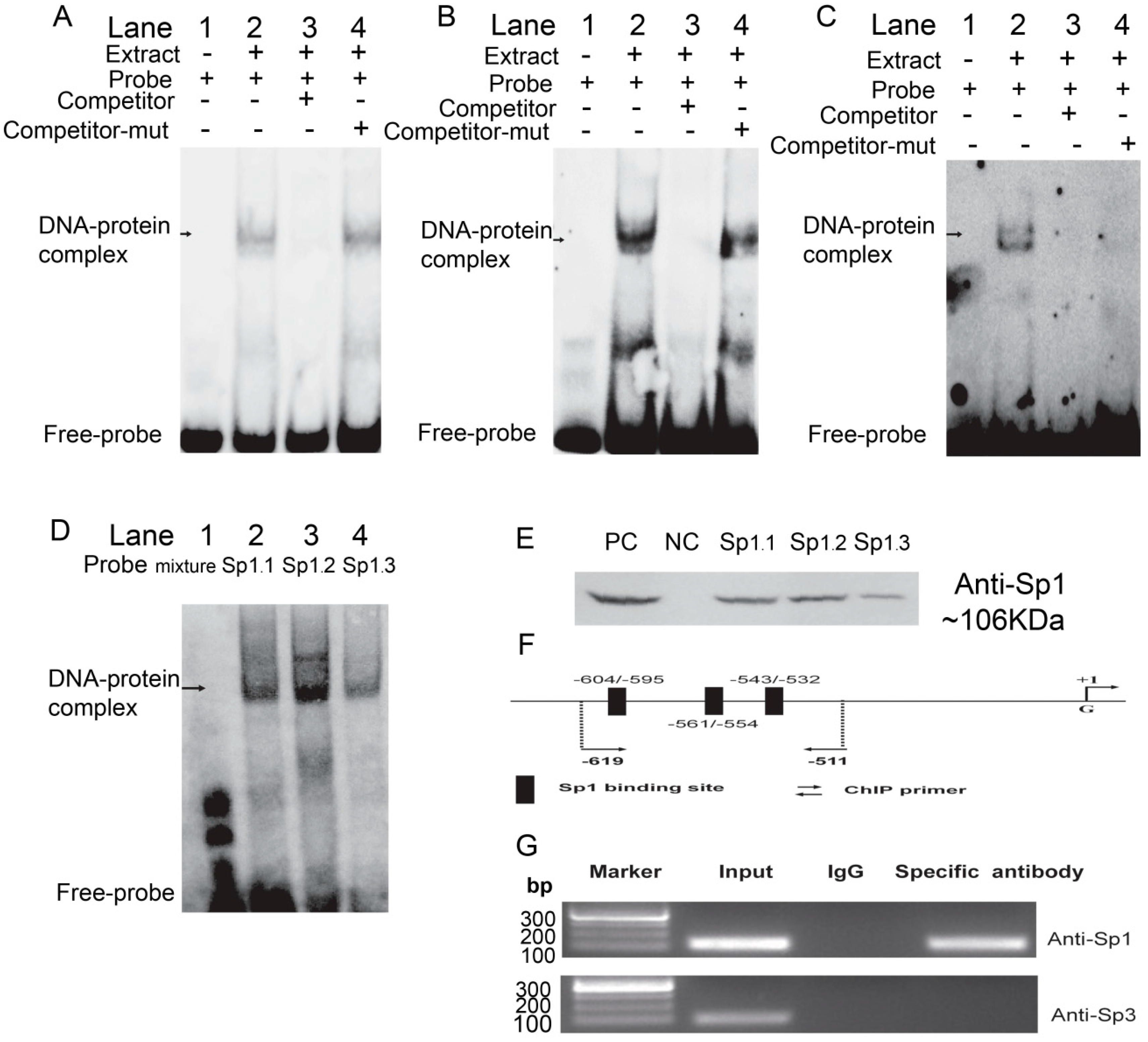
2.3. Sp1 Binds to the Porcine ROCK1 Promoter in Vitro and in Vivo
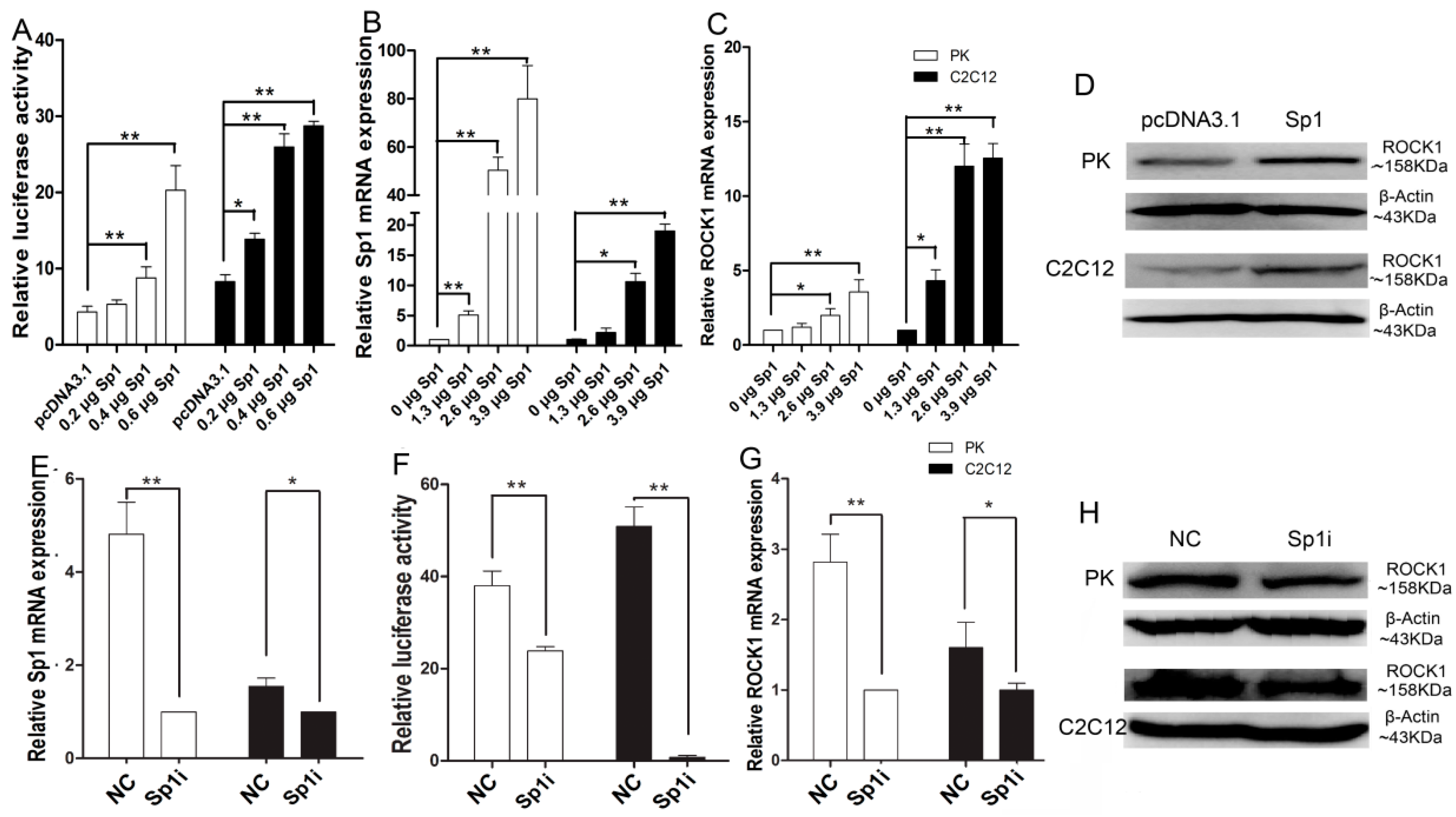
2.4. Sp1 Stimulates ROCK1 Gene Expression
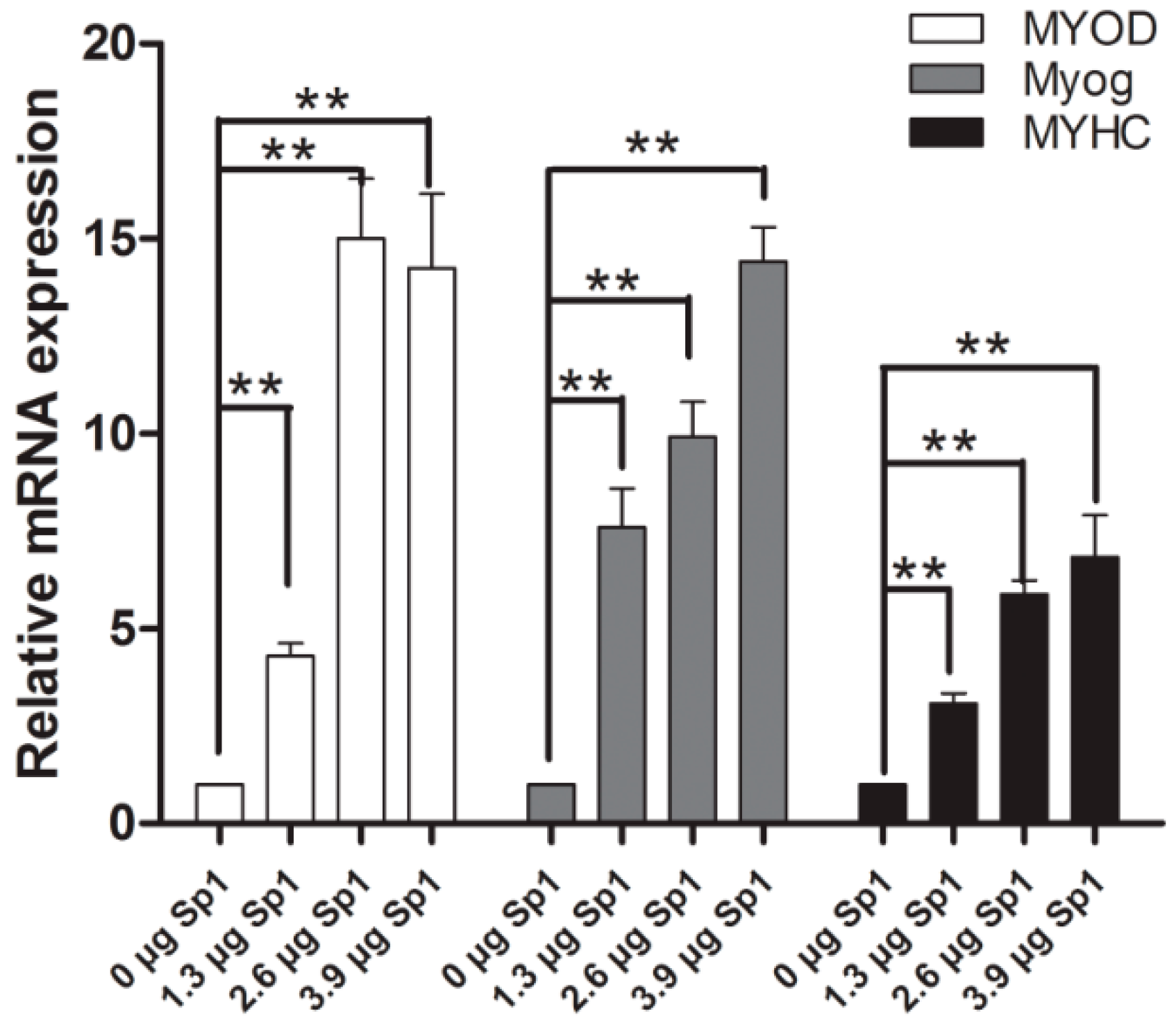
2.5. Sp1 Stimulates the Process of Myogenesis
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. In Silico Sequence Analysis
4.3. Rapid Amplification of 5’cDNA Ends (5’-RACE)
4.4. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.5. Plasmids’ Construction, Cell Culture, Transfection and Analysis
4.6. Electrophoretic Mobility Shift Assay (EMSA)
4.7. Chromatin Immunoprecipitation (ChIP) Assay
4.8. DNA Pull down Assay
4.9. RNA Interference
4.10. Western Blotting
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sun, J.; Zhang, D.H.; Zheng, Y.; Zhao, Q.; Zheng, M.H.; Kovacevic, Z.; Richardson, D.R. Targeting the Metastasis Suppressor, NDRG1, using novel iron chelators: Regulation of stress fiber-mediated tumor cell migration via modulation of the ROCK1/pMLC2 signaling pathway. Mol. Pharmacol. 2013, 83, 454–469. [Google Scholar] [CrossRef] [PubMed]
- Riento, K.; Ridley, A.J. Rocks: Multifunctional kinases in cell behavior. Nat. Rev. Mol. Cell Biol. 2003, 4, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Dong, H.S.; Oqani, R.K.; Lin, T.; Kang, J.W.; Jin, D.I. Distinct roles of ROCK1 and ROCK2 during development of porcine preimplantation embryos. Reproduction 2014, 148, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Ye, W.; Wu, J.; Liu, L.; Yang, L.; Gao, L.; Chen, B.; Zhang, F.; Yang, H.; Li, Y. Sp1-CD147 positive feedback loop promotes the invasion ability of ovarian cancer. Oncol. Rep. 2015, 34, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Huang, H.; Choi, K.; Lee, D.H.; Shi, J.; Liu, T.; Chun, K.H.; Seo, J.A.; Lima, I.S.; Zabolotny, J.M.; et al. ROCK1 isoform-specific deletion reveals a role for diet-induced insulin resistance. Am. J. Physiol. Endocrinol. Metab. 2014, 306, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, W.Z.; Chen, C.Y.; Pei, Y.G.; Long, X.Y. MiR-335 acts as a potential tumor suppressor miRNA via downregulating ROCK1 expression in hepatocellular carcinoma. Tumor Biol. 2015, 36, 6313–6319. [Google Scholar] [CrossRef] [PubMed]
- Kentrup, D.; Reuter, S.; Schnockel, U.; Grabner, A.; Edemir, B.; Pavenstadt, H.; Schober, O.; Schafers, M.; Schlatter, E.; Bussemaker, E. Hydroxyfasudil-Mediated Inhibition of ROCK1 and ROCK2 Improves Kidney Function in Rat Renal Acute Ischemia-Reperfusion Injury. PLoS ONE 2011, 6, e26419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castellani, L.; Salvati, E.; Alema, S.; Falcone, G. Fine regulation of RhoA and rock is required for skeletal muscle differentiation. J. Biol. Chem. 2006, 281, 15249–15257. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ying, Z.Z.; Tang, Z.L.; Long, L.Q.; Li, K. MicroRNA-148a Promotes Myogenic Differentiation by Targeting the ROCK1 Gene. J. Biol. Chem. 2012, 287, 21093–21101. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Chen, X.Y.; Xiong, R.P.; Ning, Y.L.; Li, P.; Peng, Y.; Liu, P.; Zhao, Y.; Yang, N.; Zhou, Y.G. Dexamethasone inhibits U937 cell adhesion via the down-regulation of ROCK1 activity. Acta Biochim. Pol. 2012, 59, 557–560. [Google Scholar] [PubMed]
- Vignozzi, L.; Morelli, A.; Filippi, S.; Ambrosini, S.; Mancina, R.; Luconi, M.; Mungai, S.; Vannelli, G.B.; Zhang, X.H.; Forti, G.; et al. Testosterone regulates RhoA/Rho-Kinase signaling in two distinct animal models of chemical diabetes. J. Sex. Med. 2007, 4, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.; Dobashi, K.; Iizuka, K.; Horie, T.; Suzuki, K.; Tukagoshi, H.; Nakazawa, T.; Nakazato, Y.; Mori, M. Contribution of small GTPase Rho and its target protein rock in a murine model of lung fibrosis. Am. J. Respir. Crit. Care Med. 2001, 163, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Chun, K.H.; Araki, K.; Jee, Y.; Lee, D.H.; Oh, B.C.; Huang, H.; Park, K.S.; Lee, S.W.; Zabolotny, J.M.; Kim, Y.B. Regulation of glucose transport by ROCK1 differs from that of ROCK2 and is controlled by actin polymerization. Endocrinology 2012, 153, 1649–1662. [Google Scholar] [CrossRef] [PubMed]
- Hirose, T.; Horvitz, H.R. An Sp1 transcription factor coordinates caspase-dependent and -independent apoptotic pathways. Nature 2013, 500, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Ramirez, R.; Martinez-Hernandez, E.; Sandoval, A.; Felix, R. Transcription Factor Sp1 Regulates T-Type Ca2+ Channel Ca(V)3.1 Gene Expression. J. Cell. Physiol. 2014, 229, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rubio, S.; Lopez-Sanchez, L.; Munoz-Castaneda, J.; Linares, C.I.; Aguilar-Melero, P.; Rodriguez-Peralvarez, M.; Sanchez-Sanchez, R.; Fernandez-Alvarez, A.; Casado, M.; Montero-Alvarez, J.L.; et al. GCDCA down-regulates gene expression by increasing Sp1 binding to the NOS-3 promoter in an oxidative stress dependent manner. Biochem. Pharmacol. 2015, 96, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Gazzoli, I.; Kolodner, R.D. Regulation of the human MSH6 gene by the Sp1 transcription factor and alteration of promoter activity and expression by polymorphisms. Mol. Cell. Biol. 2003, 23, 7992–8007. [Google Scholar] [CrossRef] [PubMed]
- Beishline, K.; Azizkhan-Clifford, J. Sp1 and the ‘hallmarks of cancer’. FEBS J. 2015, 282, 224–258. [Google Scholar] [CrossRef] [PubMed]
- Yanuaryska, R.D.; Miyoshi, K.; Adiningrat, A.; Horiguchi, T.; Tanimura, A.; Hagita, H.; Noma, T. Sp6 regulation of Rock1 promoter activity in dental epithelial cells. J. Med. Investig. 2014, 61, 306–317. [Google Scholar] [CrossRef]
- Aso, T.; Conaway, J.W.; Conaway, R.C. Role of core promoter structure in assembly of the RNA polymerase II preinitiation complex. A common pathway for formation of preinitiation intermediates at many TATA and TATA-less promoters. J. Biol. Chem. 1994, 269, 26575–26583. [Google Scholar] [PubMed]
- Gidoni, D.; Dynan, W.S.; Tjian, R. Multiple specific contacts between a mammalian transcription factor and its cognate promoters. Nature 1984, 312, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.A.; Jung, Y.S.; Kim, J.Y.; Kim, H.M.; Lim, I.K. Inhibition of breast cancer invasion by TIS21-Akt1-Sp1-Nox4 pathway targeting actin nucleators, mDia genes. Oncogene 2015. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Yoon, G.; Cho, J.J.; Cho, J.H.; Cho, Y.S.; Chae, J.I.; Shim, J.H. Licochalcone A induces apoptosis in malignant pleural mesothelioma through downregulation of Sp1 and subsequent activation of mitochondria-related apoptotic pathway. Int. J. Oncol. 2015, 46, 1385–1392. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, Q.; Qiang, Q.; Shan, H.; Shi, M.; Chen, B.; Zhao, S.; Yuan, L. Sp1-mediated transcriptional regulation of MALAT1 plays a critical role in tumor. J. Cancer Res. Clin. Oncol. 2015, 141, 1909–1920. [Google Scholar] [CrossRef] [PubMed]
- Narayan, V.A.; Kriwacki, R.W.; Caradonna, J.P. Structures of zinc finger domains from transcription factor Sp1. Insights into sequence-specific protein-DNA recognition. J. Biol. Chem. 1997, 272, 7801–7809. [Google Scholar] [CrossRef] [PubMed]
- Kadonaga, J.T.; Carner, K.R.; Masiarz, F.R.; Tjian, R. Isolation of cDNA encoding transcription factor Sp1 and functional analysis of the DNA binding domain. Cell 1987, 51, 1079–1090. [Google Scholar] [CrossRef]
- Gidoni, D.; Kadonaga, J.T.; Barrera-Saldana, H.; Takahashi, K.; Chambon, P.; Tjian, R. Bidirectional SV40 transcription mediated by tandem Sp1 binding interactions. Science 1985, 230, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Gartel, A.L.; Goufman, E.; Najmabadi, F.; Tyner, A.L. Sp1 and Sp3 activate p21 (WAF1/CIP1) gene transcription in the Caco-2 colon adenocarcinoma cell line. Oncogene 2000, 19, 5182–5188. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.S.; Kim, M.S.; Edenberg, H.J.; Hur, M.W. Sp3 and Sp4 can repress transcription by competing with Sp1 for the core cis-elements on the human ADH5/FDH minimal promoter. J. Biol. Chem. 1999, 274, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Sordella, R.; Jiang, W.; Chen, G.C.; Curto, M.; Settleman, J. Modulation of Rho GTPase signaling regulates a switch between adipogenesis and myogenesis. Cell 2003, 113, 147–158. [Google Scholar] [CrossRef]
- Goetsch, K.P.; Kallmeyer, K.; Niesler, C.U. Decorin modulates collagen I-stimulated, but not fibronectin-stimulated, migration of C2C12 myoblasts. Matrix Biol. 2011, 30, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, T.; Kii, I.; Kudo, A. Inactivation of Rho/ROCK signaling is crucial for the nuclear accumulation of FKHR and myoblast fusion. J. Biol. Chem. 2004, 279, 47311–47319. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, T.K.; Weintraub, H. Differences and similarities in DNA-binding preferences of MyoD and E2A protein complexes revealed by binding site selection. Science 1990, 250, 1104–1110. [Google Scholar] [CrossRef] [PubMed]
- Zhong, T.; Jin, P.F.; Dong, E.N.; Li, L.; Wang, L.J.; Zhang, H.P. Caprine sex affects skeletal muscle profile and MRFs expression during postnatal development. Anim. Sci. J. 2013, 84, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Pas, M.F.W.I.; Everts, M.E.; Haagsman, H.P. Muscle Development of Livestock Animals: Physiology, Genetics, and Meat Quality; CABI Pub.: Wallingford, Oxfordshire, UK; Cambridge, MA, USA, 2004; Volume 411, p. 42. [Google Scholar]
- Tang, Q.; Zhao, S.; Wu, J.; Zheng, F.; Yang, L.; Hu, J.; Hann, S.S. Inhibition of integrin-linked kinase expression by emodin through crosstalk of AMPKalpha and ERK1/2 signaling and reciprocal interplay of Sp1 and c-Jun. Cell Signal. 2015, 27, 1469–1477. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Wu, J.; Zheng, F.; Tang, Q.; Yang, L.; Li, L.; Wu, W.; Hann, S.S. β-elemene inhibited expression of DNA methyltransferase 1 through activation of ERK1/2 and AMPKalpha signalling pathways in human lung cancer cells: the role of Sp1. J. Cell. Mol. Med. 2015, 19, 630–641. [Google Scholar] [CrossRef] [PubMed]
- Hata, T.; Goto, C.; Soga, J.; Hidaka, T.; Fujii, Y.; Idei, N.; Fujimura, N.; Maruhashi, T.; Mikami, S.; Kihara, Y.; et al. Measurement of Rho-associated kinase (ROCK) activity in humans: Validity of leukocyte p-MBS/t-MBS in comparison with vascular response to fasudil. Atherosclerosis 2011, 214, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Xiong, Y.; Qian, H.; Lei, M.; Xu, D.; Ren, Z. Selection of reference genes for gene expression studies in porcine skeletal muscle using SYBR green qPCR. J. Biotechnol. 2010, 150, 288–293. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Feng, X.; Zhan, M.; Huang, C.; Chen, K.; Tang, X.; Kang, T.; Xiong, Y.; Lei, M. Transcription Factor Sp1 Promotes the Expression of Porcine ROCK1 Gene. Int. J. Mol. Sci. 2016, 17, 112. https://doi.org/10.3390/ijms17010112
Zhang R, Feng X, Zhan M, Huang C, Chen K, Tang X, Kang T, Xiong Y, Lei M. Transcription Factor Sp1 Promotes the Expression of Porcine ROCK1 Gene. International Journal of Molecular Sciences. 2016; 17(1):112. https://doi.org/10.3390/ijms17010112
Chicago/Turabian StyleZhang, Ruirui, Xiaoting Feng, Mengsi Zhan, Cong Huang, Kun Chen, Xiaoyin Tang, Tingting Kang, Yuanzhu Xiong, and Minggang Lei. 2016. "Transcription Factor Sp1 Promotes the Expression of Porcine ROCK1 Gene" International Journal of Molecular Sciences 17, no. 1: 112. https://doi.org/10.3390/ijms17010112




