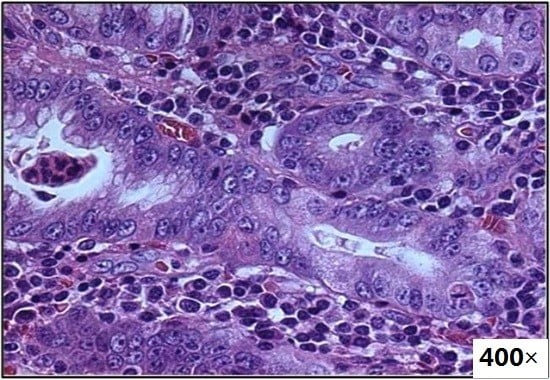
Morphological and Cellular Features of Innate Immune Reaction in Helicobacter pylori Gastritis: A Brief Review
Abstract
:1. Introduction
2. Innate Immune Cells in H. pylori Gastritis
| Cellular Actors | Role |
|---|---|
| Neutrophils | Marker of active disease |
| Mast cells | Starter of acute inflammatory reaction |
| Eosinophils | Producer of pro-fibrogenic/angiogenic factors |
| Macrophages | Scavenger of pathogens |
| Dendritic cells | Promoter of chronic infection |
3. Role of Neutrophils in H. pylori Gastritis
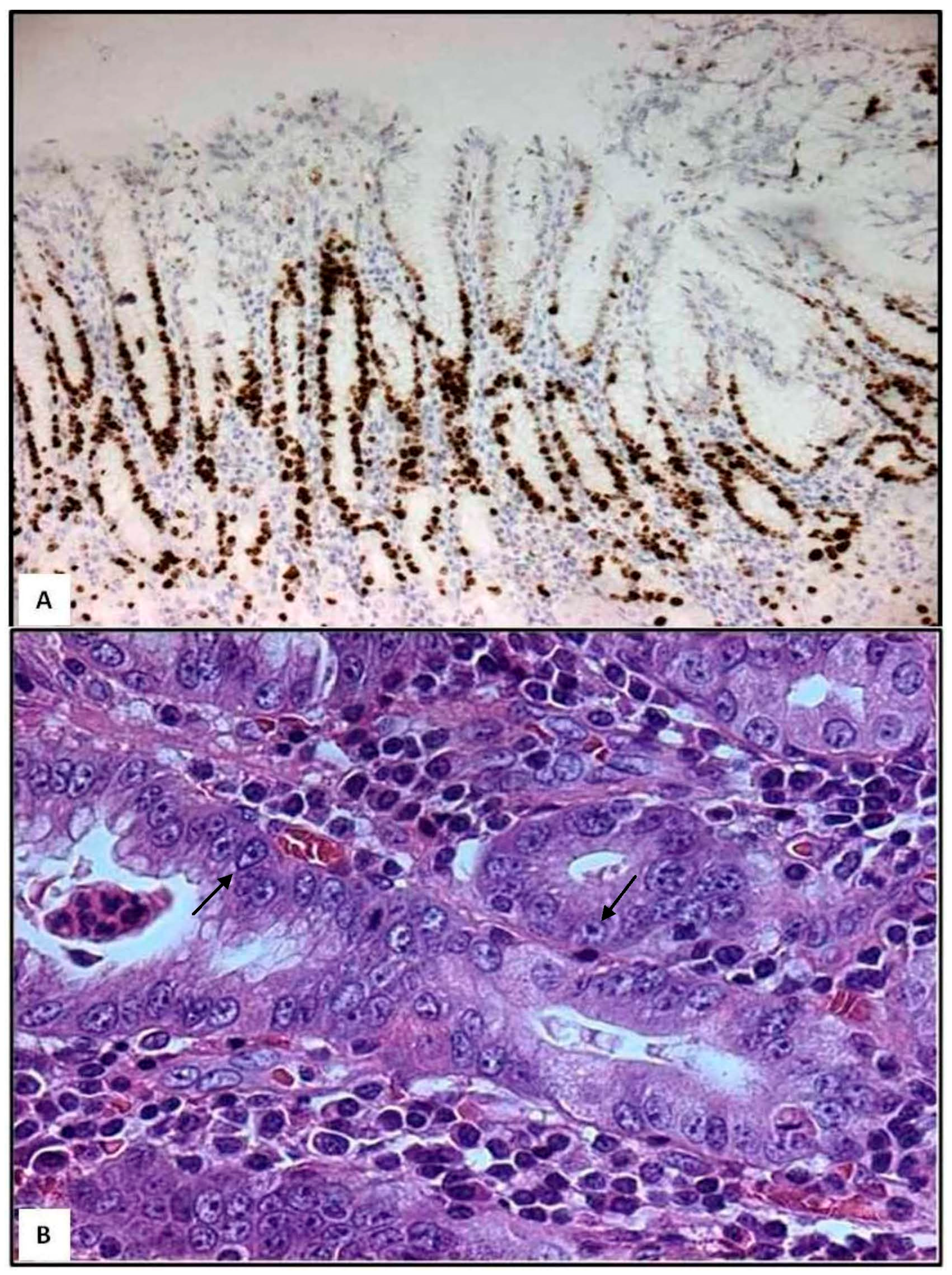
4. Role of Mast Cells in H. pylori Gastritis

5. Role of Eosinophils in H. pylori Gastritis
6. Role of Macrophages in H. pylori Gastritis
7. Role of Dendritic Cells in H. pylori Gastritis
8. Conclusions
- Histopathological and ultrastructural studies have revealed that not only neutrophils and eosinophils, but also mast cells and dendritic cells may directly infiltrate gastric foveolar epithelium during H. pylori infection. Therefore, these innate immune cells occupy strategic positions in order to evoke chronic active inflammation more easily.
- Although H. pylori was initially considered as a non-invasive pathogen, several studies have demonstrated that H. pylori is a facultative intracellular bacterium within epithelial cells, neutrophils, macrophages and dendritic cells.
- Highly virulent H. pylori strains arrest the normal phagosome maturation process in macrophages and dendritic cells, and generate large autophagosomes (also defined megasomes) where H. pylori can multiply, impairing immunological defense.
Author Contributions
Conflicts of Interest
References
- Bonilla, F.A.; Oettgen, H.C. Adaptive immunity. J. Allergy Clin. Immunol. 2010, 125, S33–S40. [Google Scholar] [CrossRef] [PubMed]
- Turvey, S.E.; Broide, D.H. Innate immunity. J. Allergy Clin. Immunol. 2010, 125, S24–S32. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A. Molecular pathways linking inflammation and cancer. Curr. Mol. Med. 2010, 10, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Dixon, M.F.; Genta, R.M.; Yardley, J.H.; Correa, P.; International Workshop on the Histopathology of Gastritis, Houston 1994. Classification and grading of gastritis: The updated sydney system. Am. J. Surg. Pathol. 1996, 20, 1161–1181. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014, 513, 202–209. [Google Scholar]
- Nakajima, S.; Bamba, N.; Hattori, T. Histological aspects and role of mast cells in Helicobacter pylori-infected gastritis. Aliment. Pharmacol. Ther. 2004, 20, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Caruso, R.A.; Parisi, A.; Crisafulli, C.; Bonanno, A.; Rigoli, L.; Branca, G.; Scardigno, M.; Fedele, F. Intraepithelial infiltration by mast cells in human Helicobacter pylori active gastritis. Ultrastruct. Pathol. 2011, 35, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Necchi, V.; Manca, R.; Ricci, V.; Solcia, E. Evidence for transepithelial dendritic cells in human H. pylori active gastritis. Helicobacter 2009, 14, 208–222. [Google Scholar] [CrossRef] [PubMed]
- Lee, I. Critical pathogenic steps to high risk Helicobacter pylori gastritis and gastric carcinogenesis. World J. Gastroenterol. 2014, 20, 6412–6419. [Google Scholar] [CrossRef] [PubMed]
- Caruso, R.A.; Fedele, F.; di Bella, C.; Mazzon, E.; Rigoli, L. Foveolar cells phagocytose apoptotic neutrophils in chronic active Helicobacter pylori gastritis. Virchows Arch. 2012, 461, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.J.; Borregaard, N.; Wynn, T.A. Phenotypic and functional plasticity of cells of innate immunity: Macrophages, mast cells and neutrophils. Nat. Immunol. 2011, 12, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Doener, F.; Michel, A.; Reuter, S.; Friedrich, P.; Böhm, L.; Relle, M.; Codarri, L.; Tenzer, S.; Klein, M.; Bopp, T.; et al. Mast cell-derived mediators promote murine neutrophil effector functions. Int. Immunol. 2013, 25, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Hofman, V.; Lassalle, S.; Selva, E.; Kalem, K.; Steff, A.; Hébuterne, X.; Sicard, D.; Auberger, P.; Hofman, P. Involvement of mast cells in gastritis caused by Helicobacter pylori: A potential role in epithelial cell apoptosis. J. Clin. Pathol. 2007, 60, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Prevete, N.; Rossi, F.W.; Rivellese, F.; Lamacchia, D.; Pelosi, C.; Lobasso, A.; Necchi, V.; Solcia, E.; Fiocca, R.; Ceppa, P.; et al. Helicobacter pylori HP(2-20) induces eosinophil activation and accumulation in superficial gastric mucosa and stimulates VEGF-α and TGF-β release by interacting with formyl-peptide receptors. Int. J. Immunopathol. Pharmacol. 2013, 26, 647–662. [Google Scholar] [PubMed]
- Piazuelo, M.B.; Camargo, M.C.; Mera, R.M.; Delgado, A.G.; Peek, R.M., Jr.; Correa, H.; Schneider, B.G.; Sicinschi, L.A.; Mora, Y.; Bravo, L.E.; et al. Eosinophils and mast cells in chronic gastritis: Possible implications in carcinogenesis. Hum. Pathol. 2008, 39, 1360–1369. [Google Scholar] [CrossRef] [PubMed]
- Dubois, A.; Borén, T. Helicobacter pylori is invasive and it may be a facultative intracellular organism. Cell. Microbiol. 2007, 9, 1108–1116. [Google Scholar] [CrossRef] [PubMed]
- Fehlings, M.; Drobbe, L.; Moos, V.; Renner Viveros, P.; Hagen, J.; Beigier-Bompadre, M.; Pang, E.; Belogolova, E.; Churin, Y.; Schneider, T.; et al. Comparative analysis of the interaction of Helicobacter pylori with human dendritic cells, macrophages, and monocytes. Infect. Immun. 2012, 80, 2724–2734. [Google Scholar] [CrossRef] [PubMed]
- Ginhoux, F.; Jung, S. Monocytes and macrophages: Developmental pathways and tissue homeostasis. Nat. Rev. Immunol. 2014, 14, 392–404. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.A.; Schlesinger, L.S.; Kang, B. Virulent strains of Helicobacter pylori demonstrate delayed phagocytosis and stimulate homotypic phagosome fusion in macrophages. J. Exp. Med. 2000, 191, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Hussey, S.; Travassos, L.H.; Jones, N.L. Autophagy as an emerging dimension to adaptive and innate immunity. Semin. Immunol. 2009, 21, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Wu, J.J.; Lei, H.Y. When Helicobacter pylori invades and replicates in the cells. Autophagy 2009, 5, 540–542. [Google Scholar] [CrossRef] [PubMed]
- Rittig, M.G.; Shaw, B.; Letley, D.P.; Thomas, R.J.; Argent, R.H.; Atherton, J.C. Helicobacter pylori-induced homotypic phagosome fusion in human monocytes is independent of the bacterial vacA and cag status. Cell. Microbiol. 2003, 5, 887–899. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.T.; Allen, L.A. Role of urease in megasome formation and Helicobacter pylori survival in macrophages. J. Leukoc. Biol. 2006, 79, 1214–1225. [Google Scholar] [CrossRef] [PubMed]
- Menaker, R.J.; Ceponis, P.J.; Jones, N.L. Helicobacter pylori induces apoptosis of macrophages in association with alterations in the mitochondrial pathway. Infect. Immun. 2004, 72, 2889–2898. [Google Scholar] [CrossRef] [PubMed]
- Mahnke, K.; Ring, S.; Johnson, T.S.; Schallenberg, S.; Schönfeld, K.; Storn, V.; Bedke, T.; Enk, A.H. Induction of immunosuppressive functions of dendritic cells in vivo by CD4+CD25+ regulatory T cells: Role of B7-H3 expression and antigen presentation. Eur. J. Immunol. 2007, 37, 2117–2126. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.; Germain, C.; Fiori, P.L.; Khamri, W.; Foster, G.R.; Ghosh, S.; Lechler, R.I.; Bamford, K.B.; Lombardi, G. Chronic exposure to Helicobacter pylori impairs dendritic cell function and inhibits Th1 development. Infect. Immun. 2007, 75, 810–819. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ieni, A.; Barresi, V.; Rigoli, L.; Fedele, F.; Tuccari, G.; Caruso, R.A. Morphological and Cellular Features of Innate Immune Reaction in Helicobacter pylori Gastritis: A Brief Review. Int. J. Mol. Sci. 2016, 17, 109. https://doi.org/10.3390/ijms17010109
Ieni A, Barresi V, Rigoli L, Fedele F, Tuccari G, Caruso RA. Morphological and Cellular Features of Innate Immune Reaction in Helicobacter pylori Gastritis: A Brief Review. International Journal of Molecular Sciences. 2016; 17(1):109. https://doi.org/10.3390/ijms17010109
Chicago/Turabian StyleIeni, Antonio, Valeria Barresi, Luciana Rigoli, Francesco Fedele, Giovanni Tuccari, and Rosario Alberto Caruso. 2016. "Morphological and Cellular Features of Innate Immune Reaction in Helicobacter pylori Gastritis: A Brief Review" International Journal of Molecular Sciences 17, no. 1: 109. https://doi.org/10.3390/ijms17010109