Enantioselective Pharmacokinetics of α-Lipoic Acid in Rats
Abstract
:1. Introduction
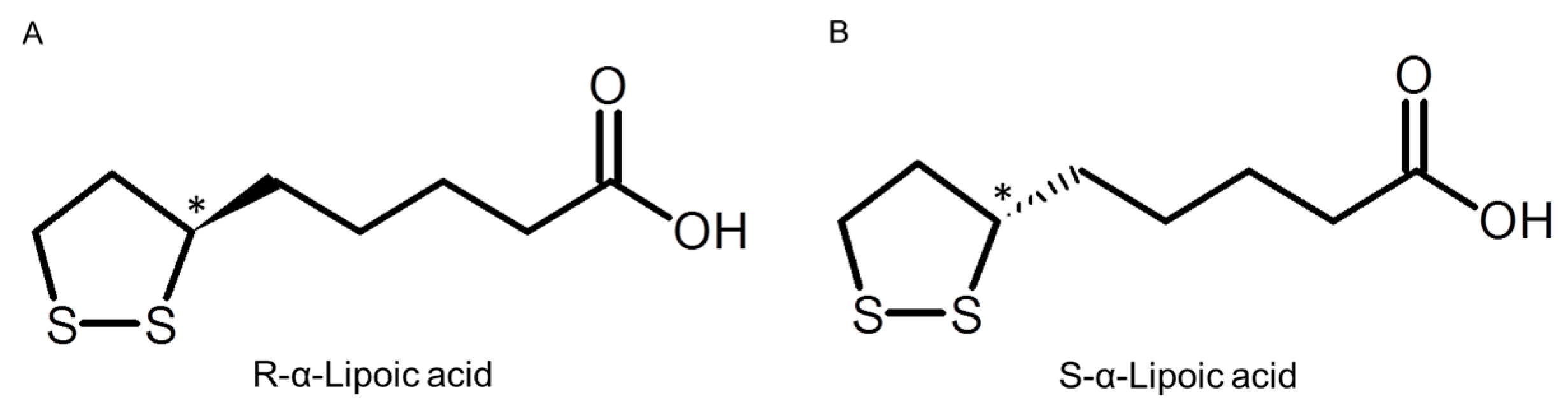
2. Results
2.1. Chiral Separation of LA (α-Lipoic Acid)

2.2. Stability in Different pH
| Time (min) | Residual Rate (%) | |||||
|---|---|---|---|---|---|---|
| pH 1.2 | pH 3.0 | pH 6.8 | ||||
| RLA | SLA | RLA | SLA | RLA | SLA | |
| 0 | 100 | 100 | 100 | 100 | 100 | 100 |
| 1 | 22.8 ± 3.7 | 22.6 ± 3.7 | 95.5 ± 1.0 | 95.4 ± 0.4 | 100.2 ± 2.1 | 100.0 ± 1.9 |
| 5 | 17.4 ± 2.9 | 17.4 ± 2.8 | 98.4 ± 2.0 | 97.8 ± 0.9 | 99.5 ± 0.4 | 99.4 ± 0.3 |
| 15 | 14.5 ± 3.5 | 14.3 ± 3.4 | 97.2 ± 0.7 | 98.1 ± 1.1 | 101.7 ± 1.8 | 101.4 ± 1.8 |
| 30 | 12.7 ± 1.2 | 12.7 ± 1.2 | 99.1 ± 3.2 | 99.2 ± 3.4 | 98.9 ± 1.3 | 99.2 ± 1.4 |
| 60 | 11.8 ± 0.7 | 11.7 ± 0.9 | 99.9 ± 1.4 | 99.7 ± 1.2 | 99.6 ± 1.1 | 99.8 ± 0.9 |
2.3. Pharmacokinetic Profiles
2.3.1. Oral and Intravenous Administration
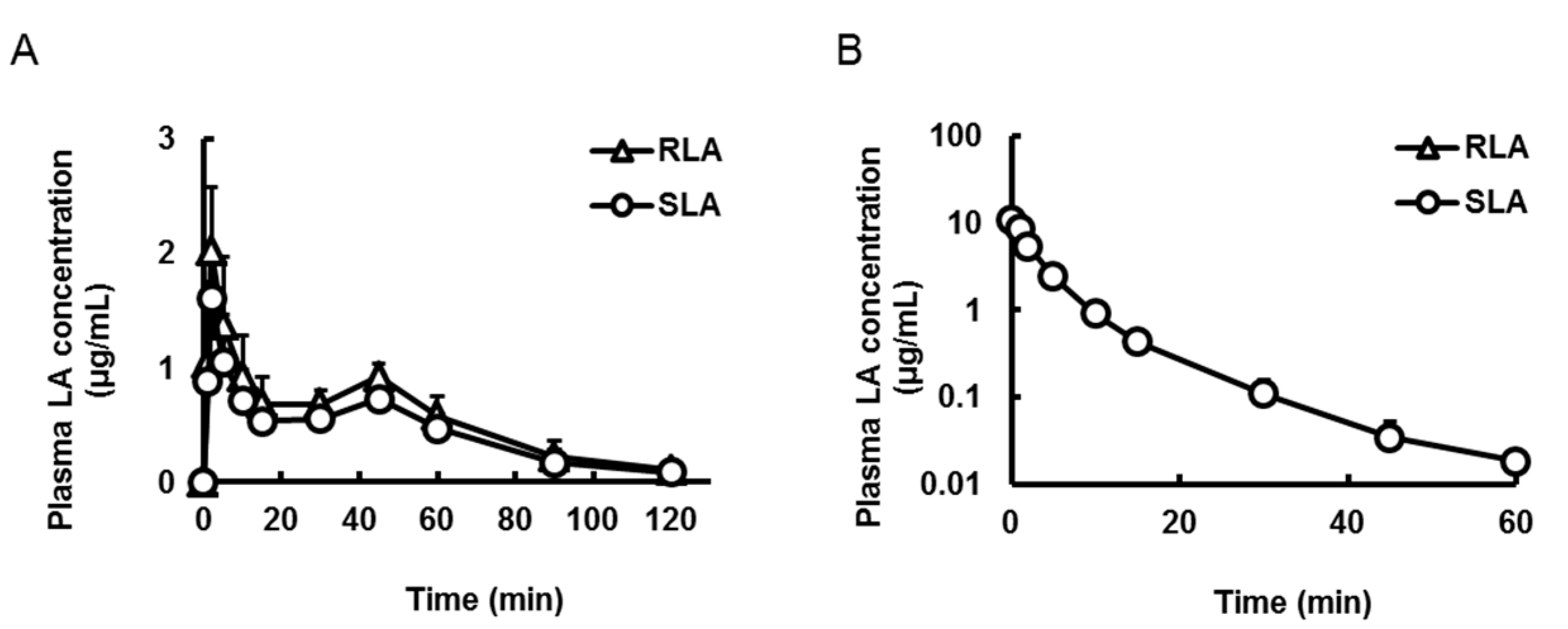
| Pharmacokinetic Parameters | Oral | Intravenous | ||
|---|---|---|---|---|
| RLA | SLA | RLA | SLA | |
| Cmax or C0 (µg/mL) | 2.0 ± 0.6 | 1.6 ± 0.4 * | 11.4 ± 3.6 | 10.9 ± 3.2 |
| Tmax (min) | 2.0 ± 0.0 | 2.0 ± 0.0 | not determined | not determined |
| T1/2 (min) | 26.7 ± 7.1 | 26.9 ± 7.1 | 10.9 ± 1.1 | 12.2 ± 2.3 |
| AUC (µg·min/mL) | 67.7 ± 6.8 | 53.8 ± 5.2 * | 48.2 ± 3.4 | 46.0 ± 2.3 |
2.3.2. Absorption from Stomach and Small Intestine
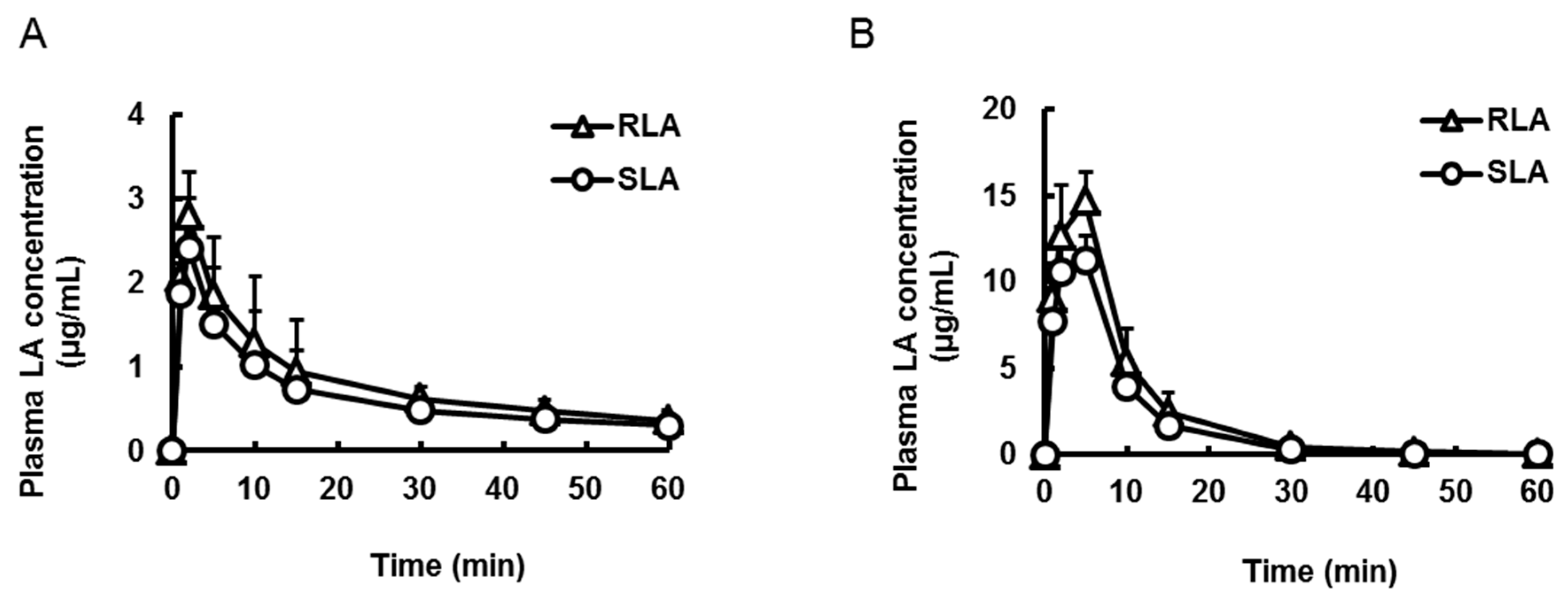
| Pharmacokinetic Parameters | Oral | Intraduodenal | ||
|---|---|---|---|---|
| RLA | SLA | RLA | SLA | |
| Cmax (µg/mL) | 2.8 ± 0.5 | 2.4 ± 0.6 * | 14.7 ± 1.7 | 11.5 ± 1.7 * |
| Tmax (min) | 2.0 ± 0.0 | 2.0 ± 0.0 | 5.0 ± 0.0 | 4.3 ± 1.5 |
| AUC (µg·min/mL) | 48.1 ± 15.6 | 38.8 ± 13.2 * | 154.2 ± 11.3 | 116.5 ± 4.4 * |
2.3.3. Hepatic Availability
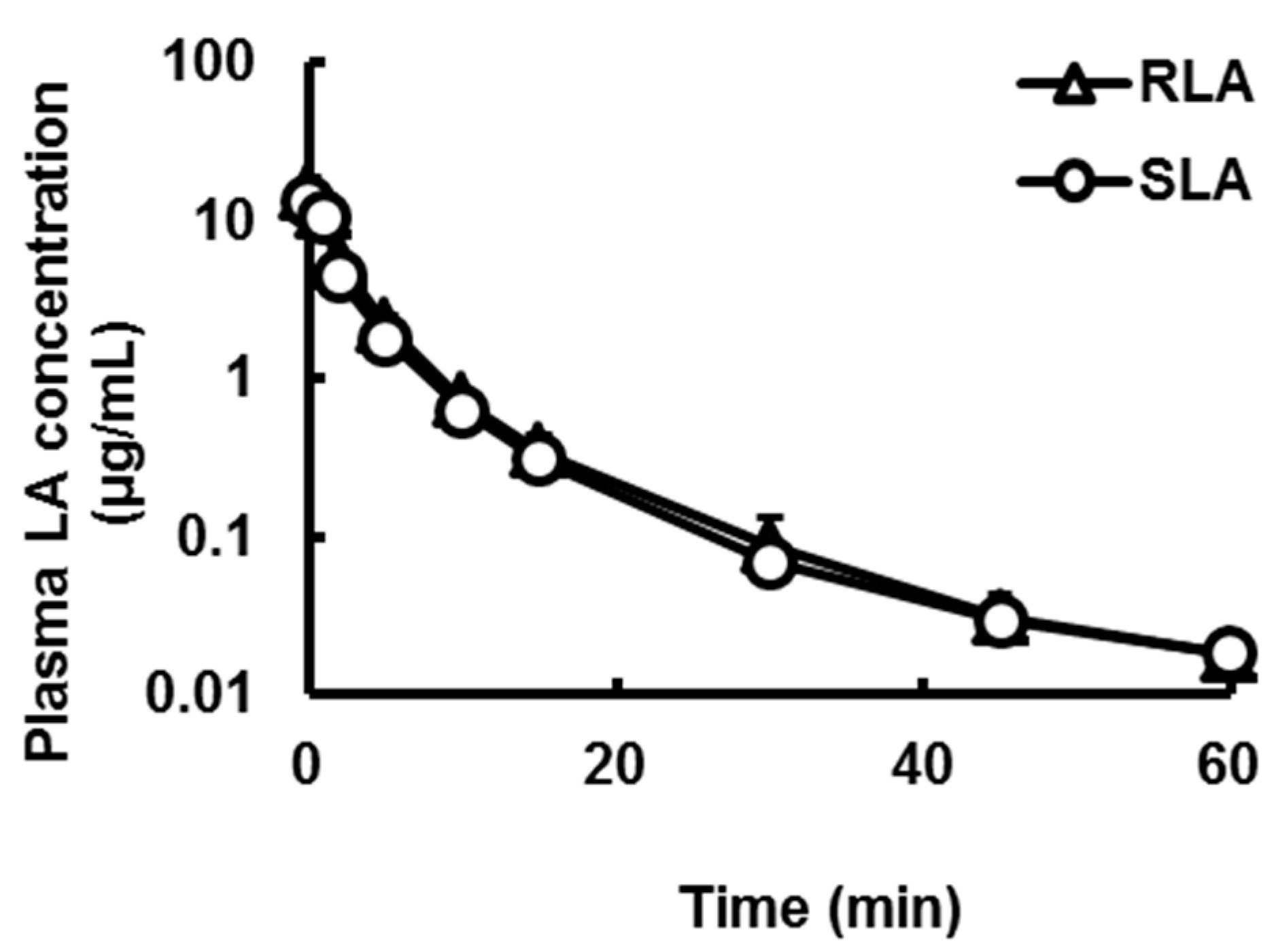
| Pharmacokinetic Parameters | RLA | SLA |
|---|---|---|
| C0 (µg/mL) | 14.7 ± 3.9 | 13.2 ± 2.7 |
| T1/2 (min) | 13.9 ± 0.6 | 15.7 ± 1.0 |
| AUC (µg·min/mL) | 47.5 ± 6.1 | 41.0 ± 5.1 * |
3. Discussion
4. Experimental Section
4.1. Chemical and Reagents
4.2. Animals
4.3. In Vitro Stability Test
4.4. Drug Administration
4.4.1. Oral and Intravenous Administration
4.4.2. Oral Administration under Pylorus Ligation
4.4.3. Intraduodenal Administration
4.4.4. Intraportal Administration
4.5. Blood Collection
4.6. Determination of LA Concentration by LC-MS/MS
4.7. Pharmacokinetics Analysis
4.8. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Snell, E.E.; Strong, F.M.; Peterson, W.H. Growth factors for bacteria: Fractionation and properties of an accessory factor for lactic acid bacteria. Biochem. J. 1937, 31, 1789–1799. [Google Scholar] [CrossRef] [PubMed]
- Reed, L.J.; De, B.B.; Gunsalus, I.C.; Hornberger, C.S. Crystalline α-lipoic acid; a catalytic agent associated with pyruvate dehydrogenase. Science 1951, 114, 93–94. [Google Scholar] [CrossRef] [PubMed]
- Bravo, M.; Orrego-Matte, H.; Walshe, J.M. Carbohydrate intermediates in hepatic cirrhosis: Role of lipoic acid. J. Lab. Clin. Med. 1961, 57, 213–222. [Google Scholar]
- Zulik, R.; Bako, F.; Budavari, J. Death-cap poisoning. Lancet 1972, 2. [Google Scholar] [CrossRef]
- Packer, L.; Witt, E.H.; Tritschler, H.J. α-Lipoic acid as a biological antioxidant. Free Radic. Biol. Med. 1995, 19, 227–250. [Google Scholar] [CrossRef]
- Biewenga, G.P.; Haenen, G.R.; Bast, A. The pharmacology of the antioxidant lipoic acid. Gen. Pharmacol. 1997, 29, 315–331. [Google Scholar] [CrossRef]
- Evans, J.L.; Goldfine, I.D. α-Lipoic acid: A multifunctional antioxidant that improves insulin sensitivity in patients with type 2 diabetes. Diabetes Technol. Ther. 2000, 2, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Parry, R.J. Biosynthesis of lipoic acid. 1. Incorporation of specifically tritiated octanoic acid into lipoic acid. J. Am. Chem. Soc. 1977, 99, 6464–6466. [Google Scholar] [CrossRef]
- Parry, R.J.; Trainor, D.A. Biosynthesis of lipoic acid. 2. Stereochemistry of sulfur introduction at C-6 of octanoic acid. J. Am. Chem. Soc. 1978, 100, 5243–5244. [Google Scholar] [CrossRef]
- Wada, H.; Shintani, D.; Ohlrogge, J. Why do mitochondria synthesize fatty acids? Evidence for involvement in lipoic acid production. Proc. Natl. Acad. Sci. USA 1997, 94, 1591–1596. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, T.; Yasuno, R.; Wada, H. Do mammalian cells synthesize lipoic acid?: Identification of a mouse cDNA encoding a lipoic acid synthase located in mitochondria. FEBS Lett. 2001, 498, 16–21. [Google Scholar] [CrossRef]
- Brookes, M.H.; Golding, B.T.; Howes, D.A.; Hudson, A.T. Proof that the absolute configuration of natural α-lipoic acid is R by the synthesis of its enantiomer ((S)-(−)-α-lipoic acid) from (S)-malic acid. J. Chem. Soc. Chem. Commun. 1983, 19, 1051–1053. [Google Scholar] [CrossRef]
- Reed, L.J. From lipoic acid to multi-enzyme complexes. Protein Sci. 1998, 7, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Khanna, S.; Roy, S.; Packer, L.; Sen, C.K. Cytokine-induced glucose uptake in skeletal muscle: Redox regulation and the role of α-lipoic acid. Am. J. Physiol 1999, 276, R1327–R1333. [Google Scholar] [PubMed]
- Bramanti, V.; Tomassoni, D.; Bronzi, D.; Grasso, S.; Curro, M.; Avitabile, M.; Volsi, G.L.; Renis, M.; Ientile, R.; Amenta, F.; et al. α-Lipoic acid modulates GFAP, vimentin, nestin, cyclin D1 and MAP-kinase expression in astroglial cell cultures. Neurochem. Res. 2010, 35, 2070–2077. [Google Scholar] [CrossRef] [PubMed]
- Krämer, K.; Packer, L. R-α-lipoic acid. In Nutraceuticals in Health and Disease Prevention; Krämer, K., Hoppe, P.P., Packer, L., Eds.; CRC Press: Boca Raton, FL, USA, 2001; pp. 129–164. [Google Scholar]
- Grasso, S.; Bramanti, V.; Tomassoni, D.; Bronzi, D.; Malfa, G.; Traini, E.; Napoli, M.; Renis, M.; Amenta, F.; Avola, R. Effect of lipoic acid and α-glyceryl-phosphoryl-choline on astroglial cell proliferation and differentiation in primary culture. J. Neurosci. Res. 2014, 92, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Streeper, R.S.; Henriksen, E.J.; Jacob, S.; Hokama, J.Y.; Fogt, D.L.; Tritschler, H.J. Differential effects of lipoic acid stereoisomers on glucose metabolism in insulin-resistant skeletal muscle. Am. J. Physiol. 1997, 273, E185–E191. [Google Scholar] [PubMed]
- Hagen, T.M.; Ingersoll, R.T.; Lykkesfeldt, J.; Liu, J.; Wehr, C.M.; Vinarsky, V.; Bartholomew, J.C.; Ames, A.B. (R)-α-Lipoic acid-supplemented old rats have improved mitochondrial function, decreased oxidative damage, and increased metabolic rate. FASEB J. 1999, 13, 411–418. [Google Scholar] [PubMed]
- Yang, Y.S.; Frey, P.A. 2-Ketoacid dehydrogenase complexes of Escherichia coli: Stereospecificities of the three components for (R)-lipoate. Arch. Biochem. Biophys. 1989, 268, 465–474. [Google Scholar] [CrossRef]
- Oehring, R.; Bisswanger, H. Incorporation of the enantiomers of lipoic acid into the pyruvate dehydrogenase complex from Escherichia coli in vivo. Biol. Chem. Hoppe Seyler 1992, 373, 333–335. [Google Scholar] [CrossRef] [PubMed]
- Gal, E.M. Reversal of selective toxicity of (−)-α-lipoic acid by thiamine in thiamine-deficient rats. Nature 1965, 207. [Google Scholar] [CrossRef]
- Wessel, K.; Borbe, H.; Ulrich, H.; Hettche, H.; Bisswanger, H.; Packer, L.; Klip, A. Use of R-(+)-α-lipoic acid, R-(−)-dihydrolipoic acid and metabolites in the form of the free acid or as salts or esters or amides for the preparation of drugs for the treatment of diabetes mellitus as well as of its sequelae. U.S. Patent 6284787 B1, 4 September 2001. [Google Scholar]
- Hermann, R.; Niebch, G.; Borbe, H.O.; Fieger-Büschges, H.; Ruus, P.; Nowak, H.; Riethmüller-Winzen, H.; Peukert, M.; Blume, H. Enantioselective pharmacokinetics and bioavailability of different racemic α-lipoic acid formulations in healthy volunteers. Eur. J. Pharm. Sci. 1996, 4, 167–174. [Google Scholar] [CrossRef]
- Niebch, G.; Buchele, B.; Blome, J.; Grieb, S.; Brandt, G.; Kampa, P.; Raffel, H.H.; Locher, M.; Borbe, H.O.; Nubert, I.; et al. Enantioselective high-performance liquid chromatography assay of (+)R- and (−)S-α-lipoic acid in human plasma. Chirality 1997, 9, 32–36. [Google Scholar] [CrossRef]
- Gleiter, C.H.; Schug, B.S.; Hermann, R.; Elze, M.; Blume, H.H.; Gundert-Remy, U. Influence of food intake on the bioavailability of thioctic acid enantiomers. Eur. J. Clin. Pharmacol. 1996, 50, 513–514. [Google Scholar] [CrossRef] [PubMed]
- Hermann, R.; Wildgrube, H.J.; Ruus, P.; Niebch, G.; Nowak, H.; Gleiter, C.H. Gastric emptying in patients with insulin dependent diabetes mellitus and bioavailability of thioctic acid-enantiomers. Eur. J. Pharm. Sci. 1998, 6, 27–37. [Google Scholar] [CrossRef]
- Breithaupt-Grogler, K.; Niebch, G.; Schneider, E.; Erb, K.; Hermann, R.; Blume, H.H.; Schug, B.S.; Belz, G.G. Dose-proportionality of oral thioctic acid--coincidence of assessments via pooled plasma and individual data. Eur J. Pharm. Sci. 1999, 8, 57–65. [Google Scholar] [CrossRef]
- Hermann, R.; Mungo, J.; Cnota, P.J.; Ziegler, D. Enantiomer-selective pharmacokinetics, oral bioavailability, and sex effects of various α-lipoic acid dosage forms. Clin. Pharmacol. 2014, 6, 195–204. [Google Scholar] [CrossRef] [PubMed]
- McConnell, E.L.; Basit, A.W.; Murdan, S. Measurements of rat and mouse gastrointestinal pH, fluid and lymphoid tissue, and implications for in vivo experiments. J. Pharm. Pharmacol. 2008, 60, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Ward, F.W.; Coates, M.E. Gastrointestinal pH measurement in rats: Influence of the microbial flora, diet and fasting. Lab. Anim. 1987, 21, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Lui, C.Y.; Amidon, G.L.; Berardi, R.R.; Fleisher, D.; Youngberg, C.; Dressman, J.B. Comparison of gastrointestinal pH in dogs and humans: Implications on the use of the beagle dog as a model for oral absorption in humans. J. Pharm. Sci. 1986, 75, 271–274. [Google Scholar] [CrossRef] [PubMed]
- FDA’s policy statement for the development of new stereoisomeric drugs. Chirality 1992, 4, 338–340.
- Maddux, B.A.; See, W.; Lawrence, J.C., Jr.; Goldfine, A.L.; Goldfine, I.D.; Evans, J.L. Protection against oxidative stress-induced insulin resistance in rat L6 muscle cells by mircomolar concentrations of alpha-lipoic acid. Diabetes 2001, 50, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Saito, K.; Iwasaki, Y.; Ito, R.; Nakazawa, H. Enantiomeric determination of α-lipoic acid in dietary supplements by liquid chromatography/mass spectrometry. Bunseki Kagaku 2012, 61, 109–114. [Google Scholar] [CrossRef]
- Schupke, H.; Hempel, R.; Peter, G.; Hermann, R.; Wessel, K.; Engel, J.; Kronbach, T. New metabolic pathways of α-lipoic acid. Drug Metab. Dispos. 2001, 29, 855–862. [Google Scholar] [PubMed]
- Boxenbaum, H. Interspecies scaling, allometry, physiological time, and the ground plan of pharmacokinetics. J. Pharmacokinet. Biopharm. 1982, 10, 201–227. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, C.; Garnett, M. Electrochemical behavior of the super antioxidant, α-lipoic acid. Int. J. Electrochem. Sci. 2011, 6, 3607–3630. [Google Scholar]
- Ikuta, N.; Sugiyama, H.; Shimosegawa, H.; Nakane, R.; Ishida, Y.; Uekaji, Y.; Nakata, D.; Pallauf, K.; Rimbach, G.; Terao, K.; et al. Analysis of the enhanced stability of R(+)-alpha lipoic acid by the complex formation with cyclodextrins. Int. J. Mol. Sci. 2013, 14, 3639–3655. [Google Scholar] [CrossRef] [PubMed]
- Suji, G.; Khedkar, S.A.; Singh, S.K.; Kishore, N.; Coutinho, E.C.; Bhor, V.M.; Sivakami, S. Binding of lipoic acid induces conformational change and appearance of a new binding site in methylglyoxal modified serum albumin. Protein J. 2008, 27, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Uchida, R.; Iwamoto, K.; Nagayama, S.; Miyajima, A.; Okamoto, H.; Ikuta, N.; Fukumi, H.; Terao, K.; Hirota, T. Effect of γ-cyclodextrin inclusion complex on the absorption of R-α-lipoic acid in rats. Int. J. Mol. Sci. 2015, 16, 10105–10120. [Google Scholar] [CrossRef] [PubMed]
- Takaishi, N.; Yoshida, K.; Satsu, H.; Shimizu, M. Transepithelial transport of α-lipoic acid across human intestinal Caco-2 cell monolayers. J. Agric. Food Chem. 2007, 55, 5253–5259. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, D.; Ametov, A.; Barinov, A.; Dyck, P.J.; Gurieva, I.; Low, P.A.; Munzel, U.; Yakhno, N.; Raz, I.; Novosadova, M.; et al. Oral treatment with α-lipoic acid improves symptomatic diabetic polyneuropathy. The sSYDNEY 2 trial. Diabetes Care 2006, 29, 2365–2370. [Google Scholar] [CrossRef] [PubMed]
- Tabata, K.; Yamaoka, K.; Kaibara, A.; Suzuki, S.; Terakawa, M.; Hata, T. Moment analysis program available on Microsoft Excel®. Drug Metab. Pharmacokinet. 1999, 14, 286–293. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uchida, R.; Okamoto, H.; Ikuta, N.; Terao, K.; Hirota, T. Enantioselective Pharmacokinetics of α-Lipoic Acid in Rats. Int. J. Mol. Sci. 2015, 16, 22781-22794. https://doi.org/10.3390/ijms160922781
Uchida R, Okamoto H, Ikuta N, Terao K, Hirota T. Enantioselective Pharmacokinetics of α-Lipoic Acid in Rats. International Journal of Molecular Sciences. 2015; 16(9):22781-22794. https://doi.org/10.3390/ijms160922781
Chicago/Turabian StyleUchida, Ryota, Hinako Okamoto, Naoko Ikuta, Keiji Terao, and Takashi Hirota. 2015. "Enantioselective Pharmacokinetics of α-Lipoic Acid in Rats" International Journal of Molecular Sciences 16, no. 9: 22781-22794. https://doi.org/10.3390/ijms160922781





