Selenium-Substituted Hydroxyapatite/Biodegradable Polymer/Pamidronate Combined Scaffold for the Therapy of Bone Tumour
Abstract
:1. Introduction
2. Results and Discussion
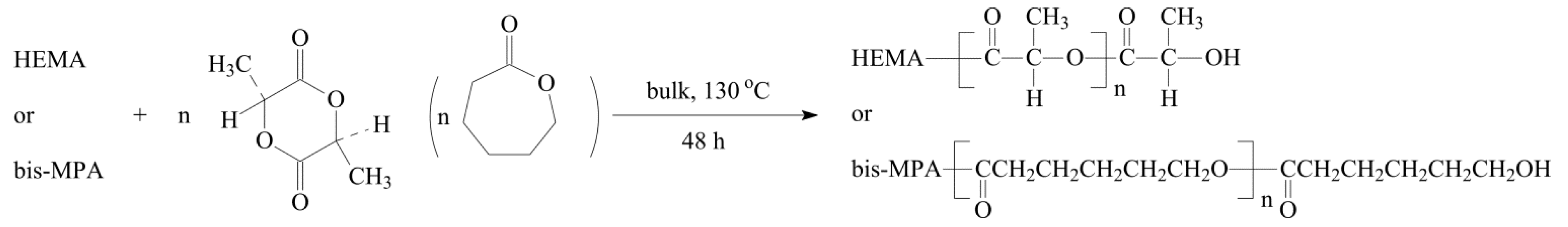
2.1. Polyester Structures from 2-Hydroxyethyl Methacrylate (HEMA) and Hyperbranched 2,2-Bis(hydroxymethyl)propionic Acid Polyester-16-Hydroxyl (Bis-MPA) Initiated Ring Opening Polymerization (ROP)
| Sample | I/M (mol/mol) | Yield (%) | DP c | DS c | Mn(NMR) c | Mn(GPC) d | MW/Mn d |
|---|---|---|---|---|---|---|---|
| HEMA-PCL50 | 1:50 | 75 | 72 | 1.3 | 10,800 | 12,300 | 1.28 |
| HEMA-PLA50 | 1:50 | 82 | 13 | 1.4 | 2800 | 3100 | 1.46 |
| HEMA-PCL100 | 1:100 | 64 | 101 | 1.1 | 12,800 | 14,200 | 1.12 |
| HEMA-PLA100 | 1:100 | 71 | 26 | 1.1 | 4300 | 4700 | 1.65 |
| HEMA-PCL250 | 1:250 | 68 | 115 | 1.2 | 15,900 | 17,500 | 1.32 |
| HEMA-PLA250 | 1:250 | 64 | 27 | 1.3 | 5200 | 5700 | 1.78 |
| bis-MPA-PCL200 | 1:200 | 89 | 26 | 14.0 | 43,300 | 45,300 | 1.44 |
| bis-MPA-PLA200 | 1:200 | 34 | 18 | 11.0 | 30,300 | 33,100 | 1.77 |
| Cytotoxicity Tests | Microtox® 15 min-PE a | Spirotox 24 h-PE a | ||||||
|---|---|---|---|---|---|---|---|---|
| Concentration (mg·mL−1) | 0.8 | 0.4 | 0.2 | 0.1 | 1.0 | 0.5 | 0.25 | 0.125 |
| HEMA-PCL100 | 22 ± 2 | 12 ± 1 | 3 ± 2 | 1 ± 1 | 0 | 0 | 0 | 0 |
| HEMA-PLA100 | 26 ± 5 | 14 ± 1 | 2 ± 2 | 3 ± 3 | 0 | 0 | 0 | 0 |
| Bis-MPA-PCL200 | 1 ± 1 | 8 ± 3 | 3 ± 3 | 2 ± 1 | 0 | 0 | 0 | 0 |
2.2. In Vitro Pamidronate (PAM) Release Characteristics of the Manufactured Scaffolds
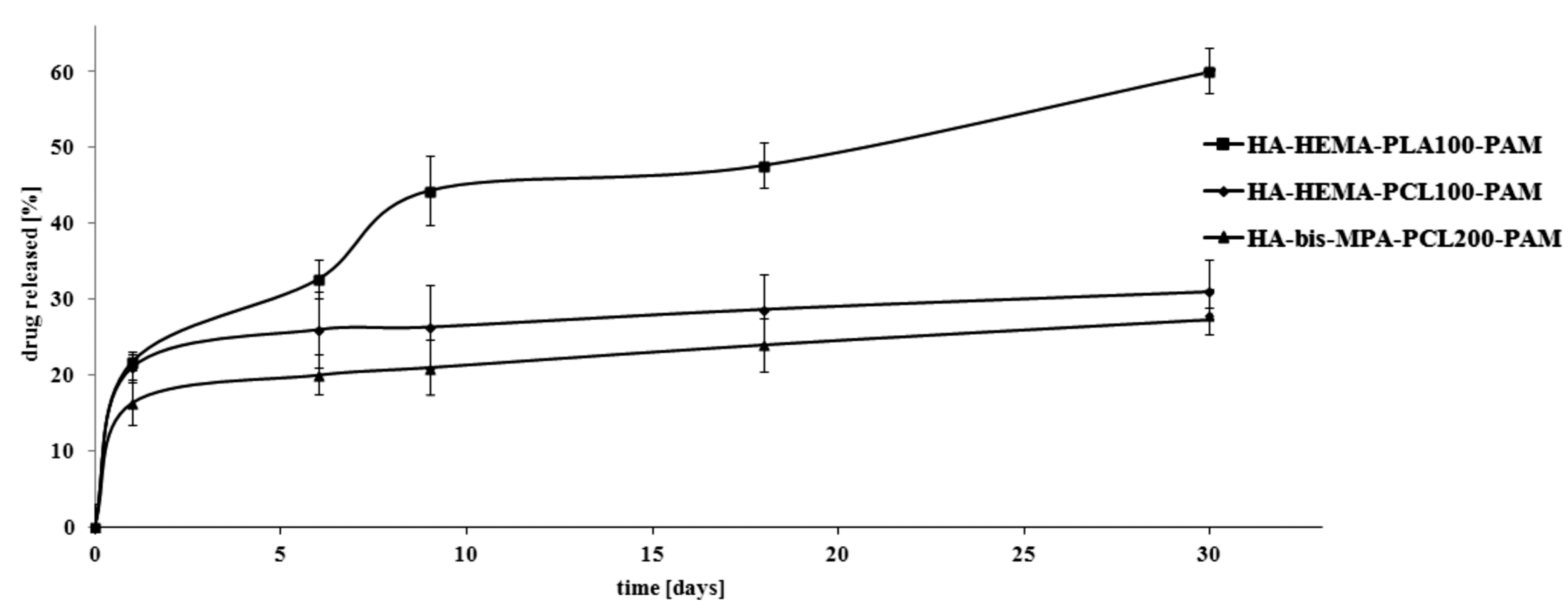

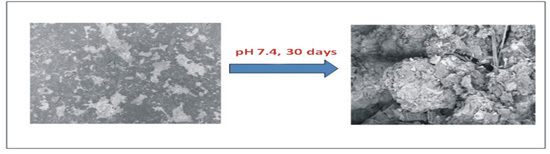
2.3. Hydrolytic Degradation Behavior of the Manufactured Scaffolds
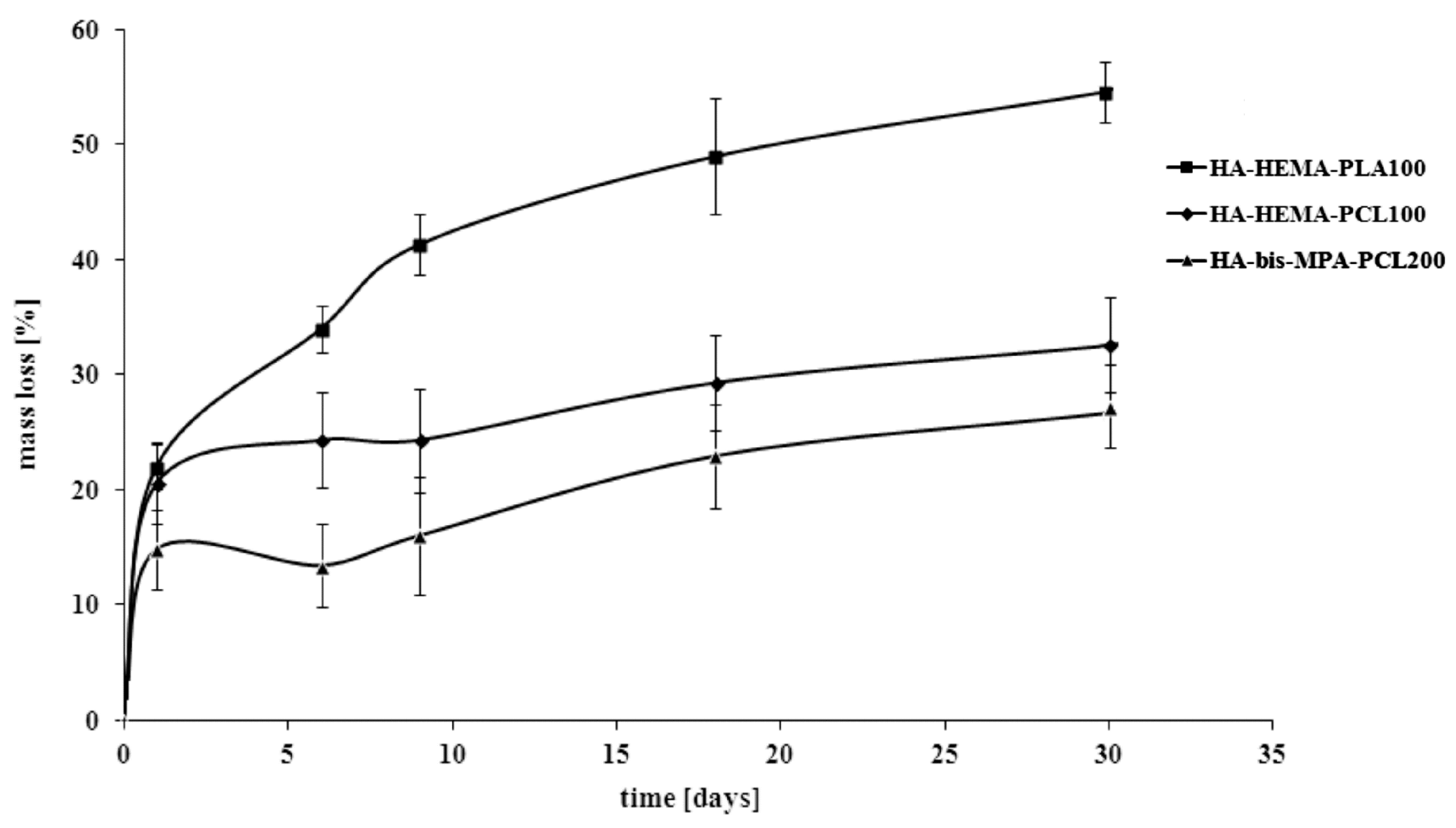
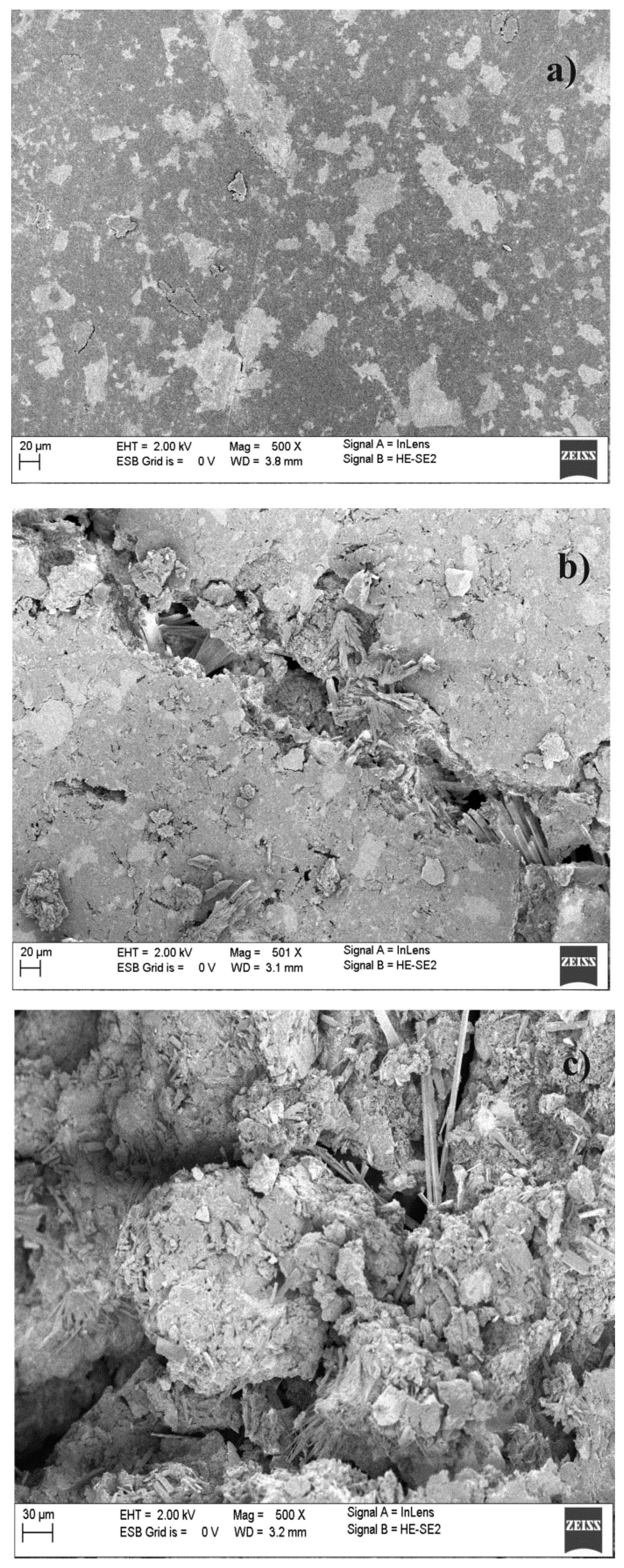
3. Experimental Section
3.1. Materials
3.2. Polymerization Procedure
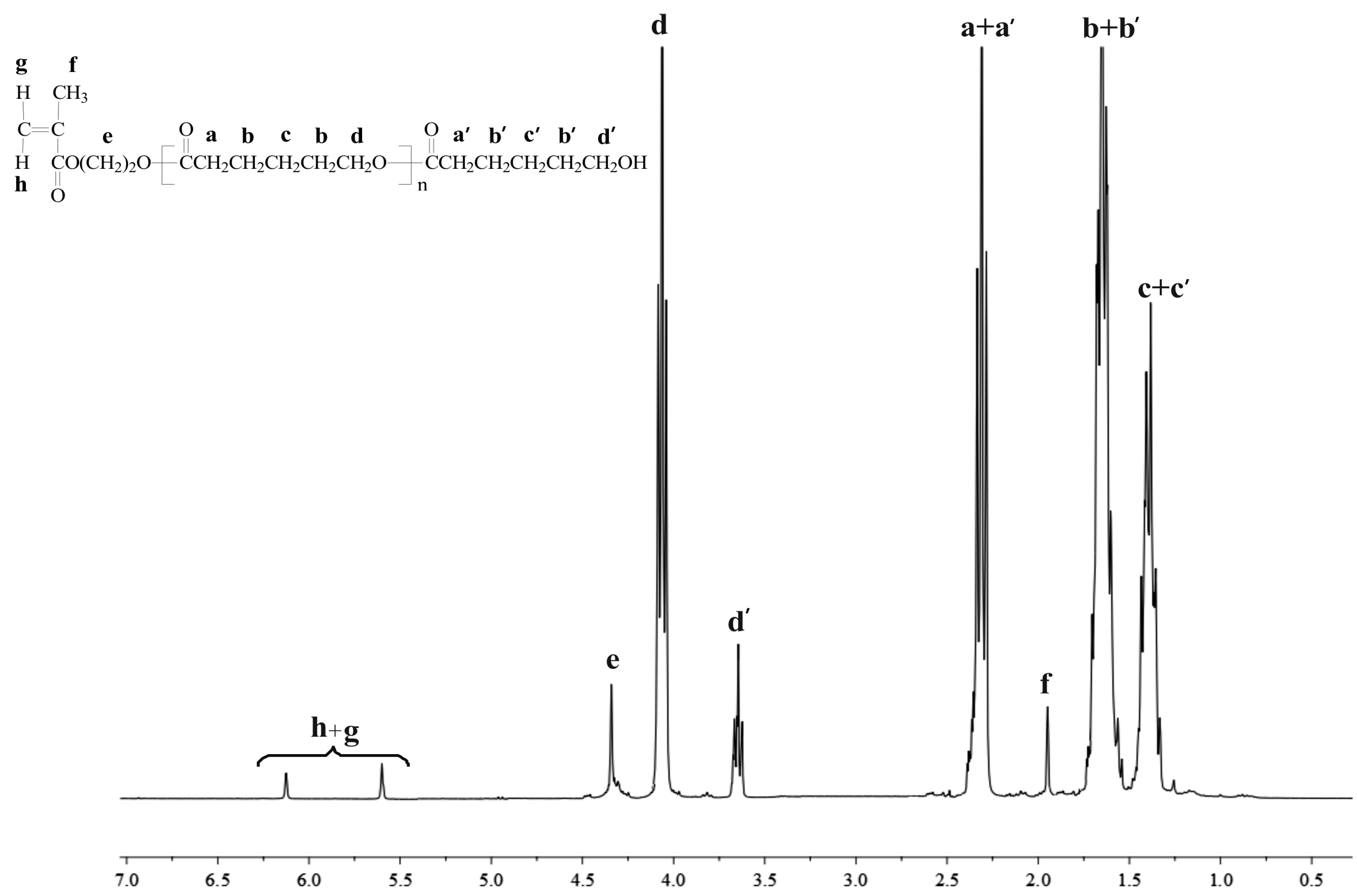
3.2.1. NMR Data for HEMA-PCL100
3.2.2. NMR Data for HEMA-PLA100
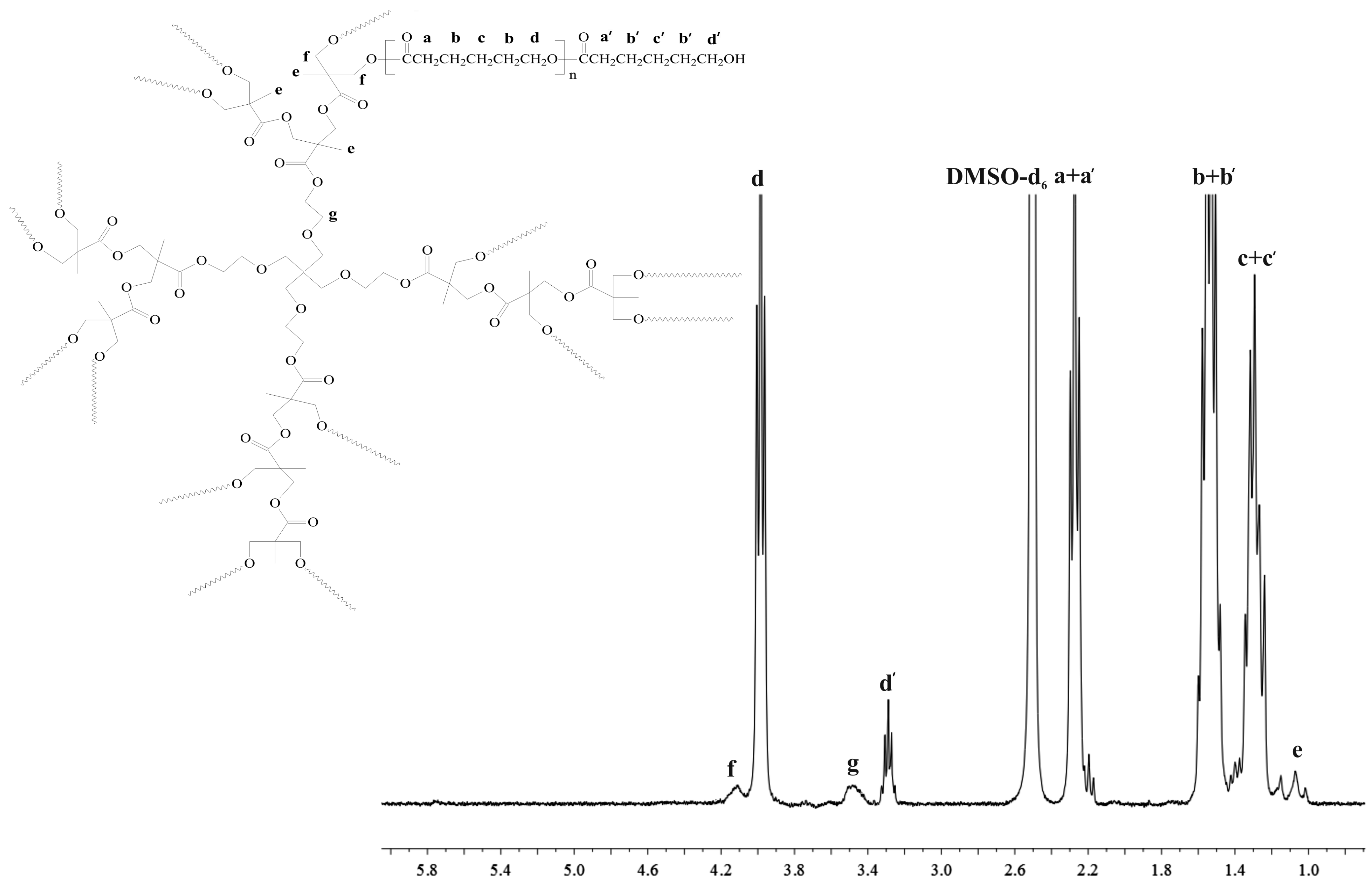
3.2.3. NMR Data for Bis-MPA-PCL200
3.2.4. NMR Data for Bis-MPA-PLA200
3.3. Toxicity Assays
3.4. Manufacturing of Combination Scaffolds for in Vitro and Hydrolytic Biodegradation Studies
3.5. In Vitro PAM Release Studies form the Manufactured Composites
3.6. Hydrolytic Degradation
3.7. Measurements
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mano, J.F.; Sousa, R.A.; Boesel, L.F.; Neves, N.M.; Reis, R.L. Bioinert, biodegradable and injectable polymeric matrix composites for hard tissue replacement: State of the art and recent developments. Compos. Sci. Technol. 2004, 64, 789–817. [Google Scholar]
- Chłopek, J. Composites in medicine. Composites 2001, 1, 50–54. [Google Scholar]
- Bonfield, W.; Grynpas, M.D.; Tully, A.E.; Bowman, J.; Abram, J. Hydroxyapatite reinforced polyethylene a mechanically compatible implant material for bone replacement. Biomaterials 1981, 2, 185–186. [Google Scholar] [CrossRef]
- Swain, R.E.; Wang, M.; Beale, B.; Bonfield, W. HAPEX™ for otologic applications. Biomed. Eng. Appl. Basis Commun. 1999, 11, 315–320. [Google Scholar]
- Broström, J.; Boss, A.; Chronakis, I.S. Biodegradable films of partly branched poly(l-lactide)-co-poly(epsilon-caprolactone) copolymer: Modulation of phase morphology, plasticization properties and thermal depolymerisation. Biomacromolecules 2004, 5, 1124–1134. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Bronich, T.K.; Kabanov, A.V.; Rauh, R.D.; Roovers, J. Synthesis and evaluation of a star amphiphilic block copolymer from poly(ε-caprolactone) and poly(ethylene glycol) as a potential drug delivery carrier. Bioconj. Chem. 2005, 16, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Montheard, J.P.; Chatzopoulos, M.; Chappard, D. 2-Hydroxyethylmethacrylate HEMA; Chemical properties and applications in biomedical fields. J. Macromol. Sci. Polym. Rev. 1992, 32, 1–34. [Google Scholar] [CrossRef]
- Dumitriu, S.; Dumitriu, D. Biocompatibility of Polymers in Polymeric Materials; Marcel Dekker: New York, NY, USA, 1994; pp. 99–158. [Google Scholar]
- Zeng, X.; Zhang, Y.; Nyström, A.M. Endocytic uptake and intracellular trafficking of bis-MPA-based hyperbranched copolymer micelles in breast cancer cells. Biomacromolecules 2012, 13, 3814–3822. [Google Scholar] [CrossRef] [PubMed]
- Almutairi, A.; Rossin, R.; Shokeen, M.; Hagooly, A.; Ananth, A.; Capoccia, B.; Guillaudeu, S.; Abendschein, D.; Anderson, C.J.; Welch, M.J.; et al. Biodegradable dendritic positron-emitting nanoprobes for the noninvasive imaging of angiogenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 685–690. [Google Scholar] [CrossRef]
- Padilla De Jesús, O.L.; Ihre, H.R.; Gagne, L.; Fréchet, J.M.; Szoka, F.C., Jr. Polyester dendritic systems for drug delivery applications: in vitro and in vivo evaluation. Bioconj. Chem. 2002, 13, 453–461. [Google Scholar] [CrossRef]
- Takwa, M.; Xiao, Y.; Simpson, N.; Malmström, E.; Hult, K.; Koning, C.E.; Heise, A.; Martinelle, M. Lipase catalyzed HEMA initiated ring-opening polymerization: In situ formation of mixed polyester methacrylates by transesterification. Biomacromolecules 2008, 9, 704–710. [Google Scholar] [CrossRef] [PubMed]
- Srivastavaa, R.K.; Kumarb, K.; Varmab, I.K.; Albertsson, A.-C. Chemo-enzymatic synthesis of comb polymers. Eur. Polym. J. 2007, 43, 808–817. [Google Scholar] [CrossRef]
- Lim, D.W.; Choi, S.C.; Park, T.G. A new class of biodegradable hydrogels stereocomplexed by enantiomeric oligo(lactide) side chains of poly(HEMA-g-OLA)s. Macromol. Rapid Commun. 2000, 21, 464–471. [Google Scholar] [CrossRef]
- Trollsås, M.; Claesson, H.; Atthoff, B.; Hedrick, J.L.; Pople, J.A.; Gast, A.P. Conformational and structural properties of high functionality dendrimer-like star polymers synthesized from living polymerization techniques. Macromol. Symp. 2000, 153, 87–108. [Google Scholar] [CrossRef]
- Atthoff, B.; Trollsås, M.; Claesson, H.; Hedrick, J.L. Poly(lactides) with controlled molecular architecture initiated from hydroxyl functional dendrimers and the effect on the hydrodynamic. Macromol. Rapid Commun. 1999, 200, 1333–1339. [Google Scholar] [CrossRef]
- Claesson, H.; Malmström, E.; Johansson, M.; Hult, A. Synthesis and characterisation of star branched polyesters with dendritic cores and the effect of structural variations on zero shear rate viscosity. Polymer 2002, 43, 3511–3518. [Google Scholar] [CrossRef]
- Velthoen, I.W.; Dijkstra, P.J.; Feijen, J. AB2 Functional polyesters via ring opening polymerization: Synthesis and characterization. Macromol. Rapid Commun. 2009, 210, 689–697. [Google Scholar] [CrossRef]
- Son, J.-S.; Kim, S.-G.; Oh, J.-S.; Appleford, M.; Oh, J.-S.; Ong, J.L.; Lee, K.-B. Hydroxyapatite/polylactide biphasic combination scaffold loaded with dexamethasone for bone regeneration. J. Biomed. Mat. Res. A 2011, 99, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Valencia, C.; López-Álvarez, M.; Cochόn-Cores, B.; Pereiro, I.; Serra, J.; Gonzàlez, P. Novel selenium-doped hydroxyapatite coatings for biomedical applications. J. Biomed. Mat. Res. A 2013, 101, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Kolmas, J.; Oledzka, E.; Sobczak, M.; Nalecz-Jawecki, G. Nanocrystalline hydroxyapatite doped with selenium oxyanions: A new material for potential biomedical applications. Mater. Sci. Eng. C 2014, 39, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Holben, D.H.; Smith, A.M. The diverse role of selenium within selenoproteins: A review. J. Am. Diet. Assoc. 1999, 99, 836–843. [Google Scholar] [CrossRef]
- Rayman, M.P. The importance of selenium to human health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef] [Green Version]
- Combs, G.F.; Gray, W.P. Chemopreventive agents: Selenium. Pharmacol. Ther. 1998, 79, 179–192. [Google Scholar] [CrossRef]
- Drake, E.N. Cancer chemoprevention: Selenium as a prooxidant, not an antioxidant. Med. Hypotheses 2006, 67, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Cao, J.J.; Combs, G.F. Selenium in bone health: Roles in antioxidant protection and cell proliferation. Nutrients 2013, 5, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Panzavolta, S.; Torricelli, P.; Bracci, B.; Fini, M.; Bigi, A. Alendronate and pamidronate calcium phosphate bone cements: Setting properties and in vitro response of osteoblast and osteoclast cells. J. Inorg. Biochem. 2009, 103, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Russell, R.G.G.; Watts, N.B.F.; Ebetino, F.H.; Rogers, M.J. Mechanisms of action of bisphosphonates: Similarities and differences and their potential influence on clinical efficacy. Osteoporos. Int. 2008, 19, 733–759. [Google Scholar] [CrossRef] [PubMed]
- Lipton, A. Implications of bone metastases and the benefits of bone-targeted therapy. Semin. Oncol. 2010, 37, S15–S29. [Google Scholar] [CrossRef] [PubMed]
- Body, J.J.; Mancini, I. Bisphosphonates for cancer patients: Why, how, and when? Support. Care Cancer 2002, 10, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Kolmas, J.; Sobczak, M.; Oledzka, E.; Nalecz-Jawecki, G.; Debek, C. Synthesis, characterization and in vitro evaluation of new composite bisphosphonate delivery systems. Int. J. Mol. Sci. 2014, 15, 16831–16847. [Google Scholar] [CrossRef] [PubMed]
- Sobczak, M.; Debek, C.; Oledzka, E.; Nalecz-Jawecki, G.; Kolodziejski, W.L.; Rajkiewicz, M. Segmented polyurethane elastomers derived from aliphatic polycarbonate and poly(ester-carbonate) soft segments for biomedical applications. J. Polym. Sci. A Polym. Chem. 2012, 50, 3904–3913. [Google Scholar] [CrossRef]
- Nalecz-Jawecki, G. Spirotox test—Spirostomum ambiguum acute toxicity test. In Small-Scale Freshwater Toxicity Investigations; Blaise, C., Férard, J.-F., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 299–322. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oledzka, E.; Sobczak, M.; Kolmas, J.; Nalecz-Jawecki, G. Selenium-Substituted Hydroxyapatite/Biodegradable Polymer/Pamidronate Combined Scaffold for the Therapy of Bone Tumour. Int. J. Mol. Sci. 2015, 16, 22205-22222. https://doi.org/10.3390/ijms160922205
Oledzka E, Sobczak M, Kolmas J, Nalecz-Jawecki G. Selenium-Substituted Hydroxyapatite/Biodegradable Polymer/Pamidronate Combined Scaffold for the Therapy of Bone Tumour. International Journal of Molecular Sciences. 2015; 16(9):22205-22222. https://doi.org/10.3390/ijms160922205
Chicago/Turabian StyleOledzka, Ewa, Marcin Sobczak, Joanna Kolmas, and Grzegorz Nalecz-Jawecki. 2015. "Selenium-Substituted Hydroxyapatite/Biodegradable Polymer/Pamidronate Combined Scaffold for the Therapy of Bone Tumour" International Journal of Molecular Sciences 16, no. 9: 22205-22222. https://doi.org/10.3390/ijms160922205