A New HPLC-MS Method for Measuring Maslinic Acid and Oleanolic Acid in HT29 and HepG2 Human Cancer Cells
Abstract
:1. Introduction
2. Results and Discussion
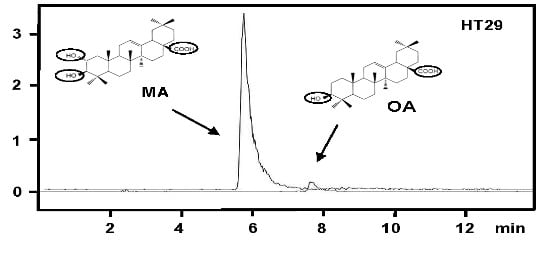
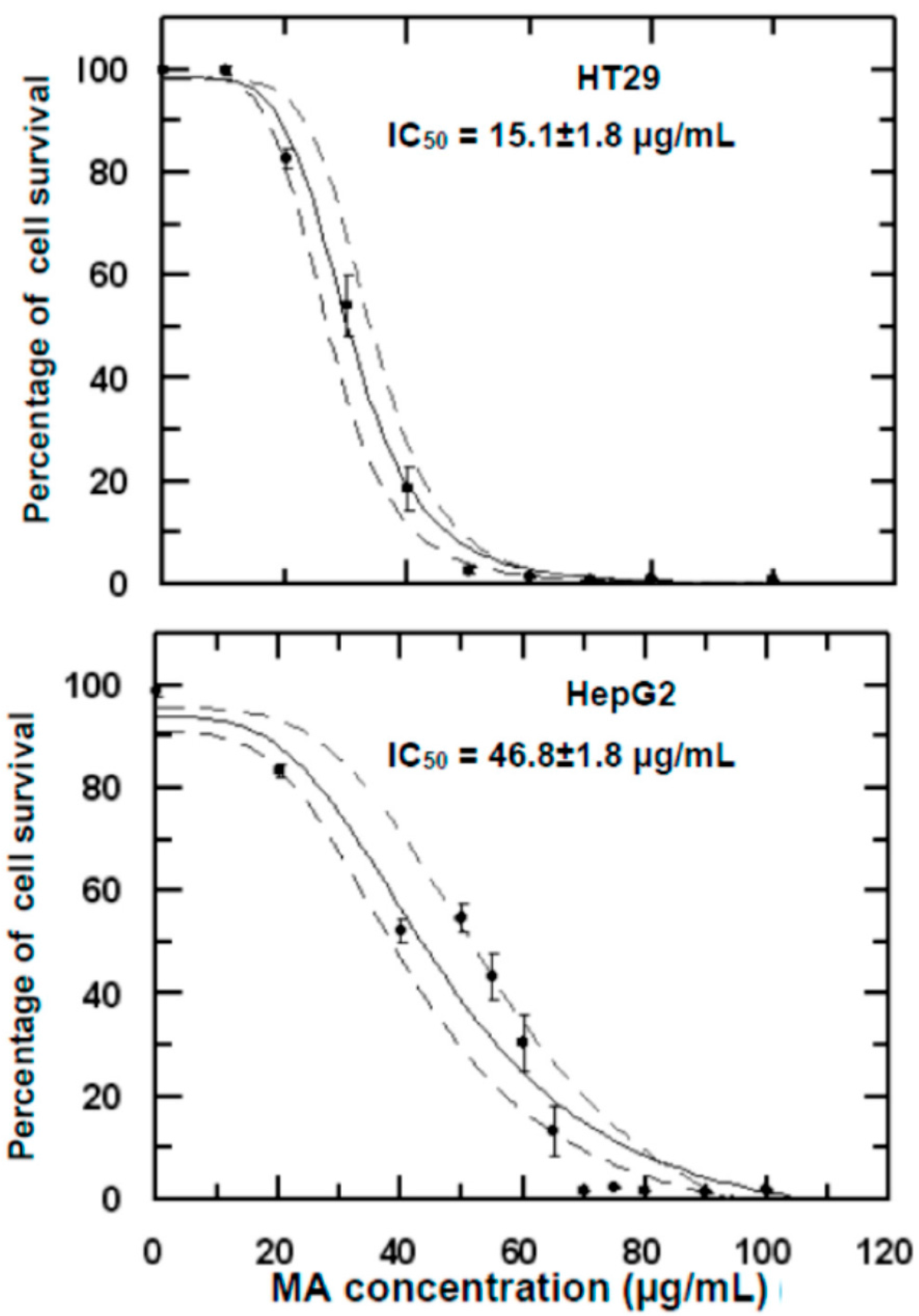
2.1. Growth Inhibitory Effects of Maslinic/Oleanolic Acid on HT29 and HepG2 Cancer Cells
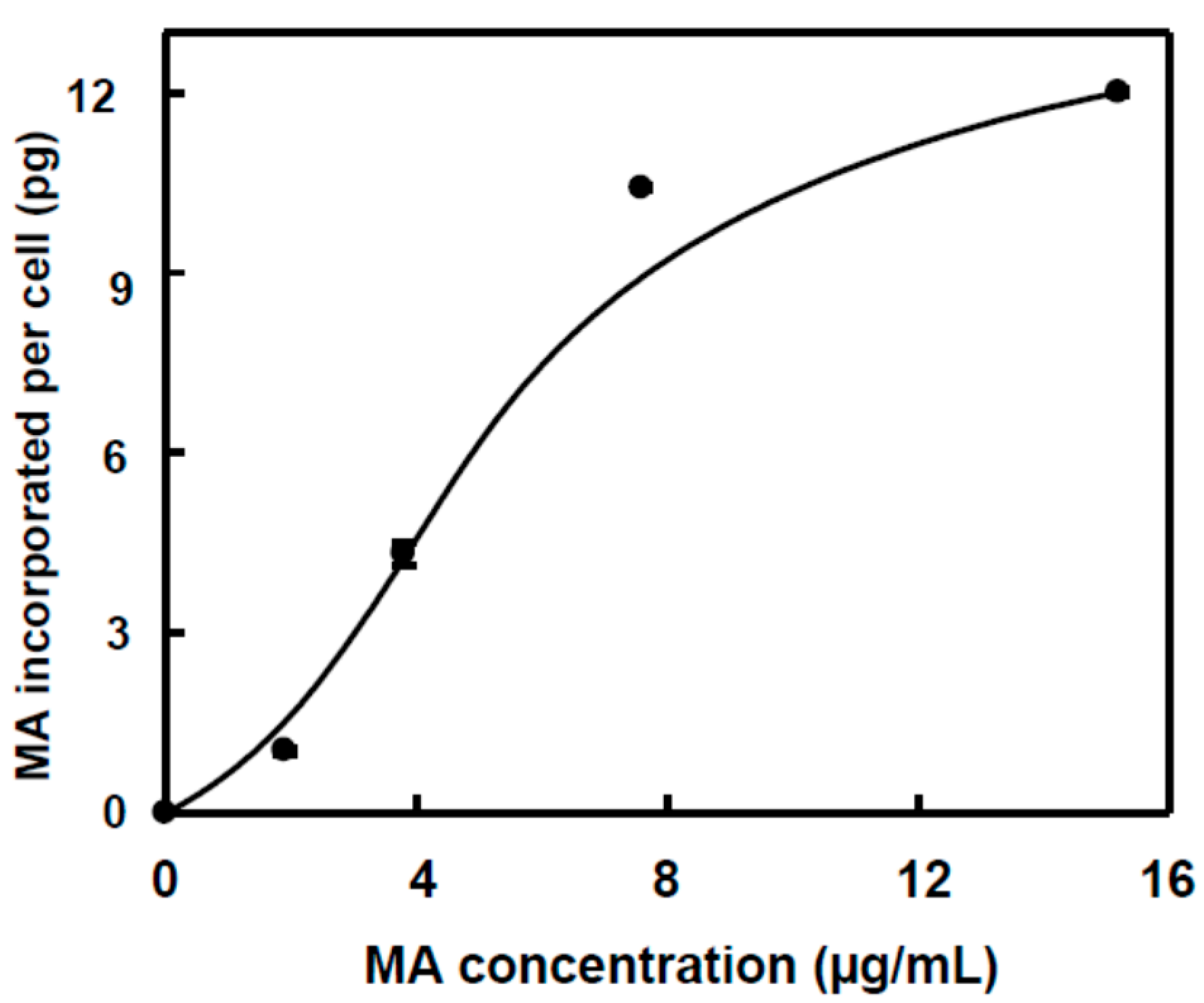
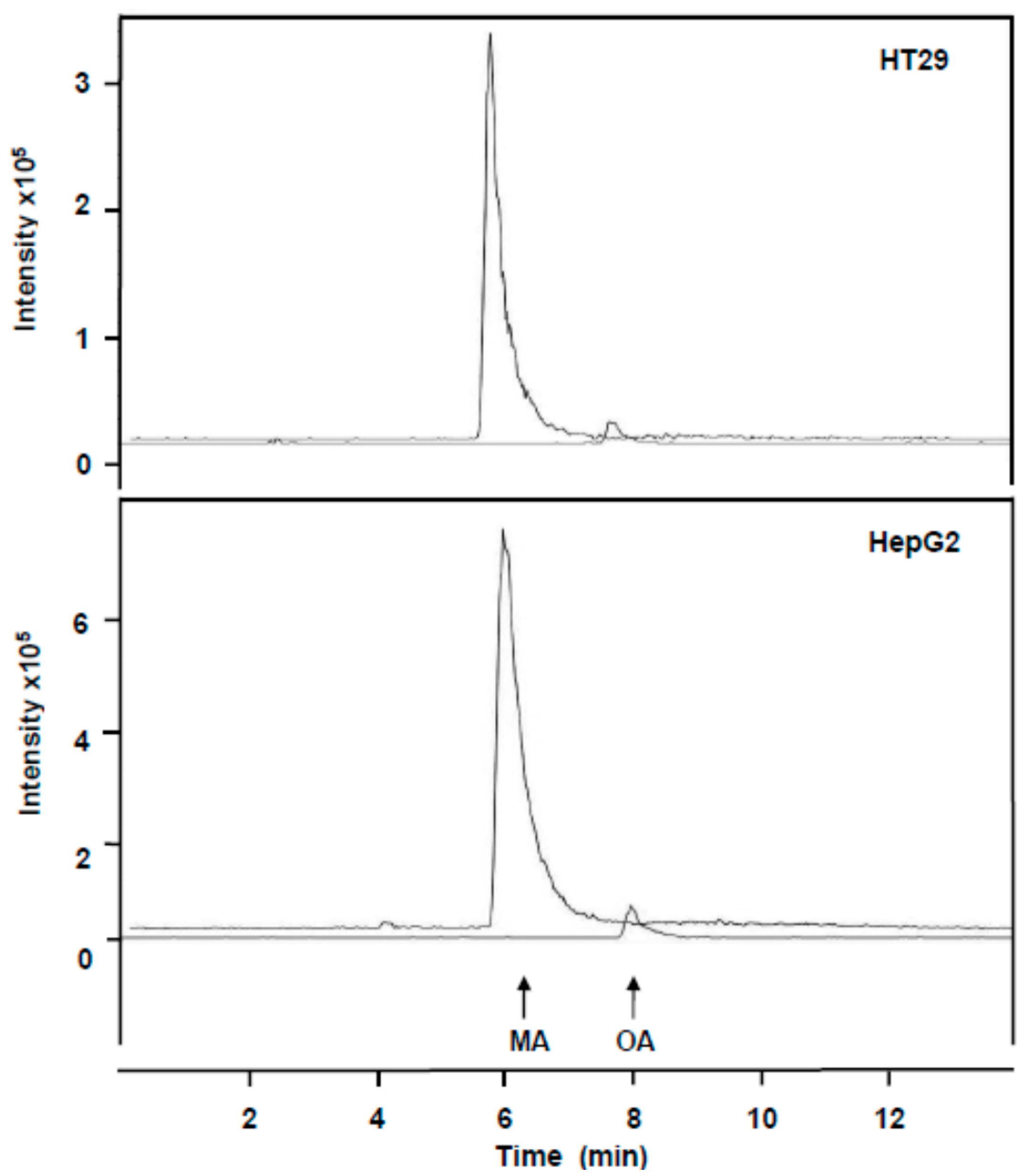
2.2. Incorporation of MA and OA to the Inner of HT29 and HepG2 Cancer Cells
| C+ | C- | IC50/8 | IC50/4 | IC50/2 | IC50 | |
|---|---|---|---|---|---|---|
| HT29 | ||||||
| µg MA added | 0 | 0 | 22.65 | 45.3 | 90.6 | 181.2 |
| µg MA in the medium | 0 | 0 | 17.5 ± 0.7 a | 29.8 ± 0.9 b | 36.6 ± 1.8 c | 117.0 ± 3.5 d |
| µg MA inside cells | 0 | 0 | 1.6 ± 0.1 a | 6.5 ± 0.3 b | 15.6 ± 0.8 c | 18.0 ± 0.9 c |
| HepG2 | ||||||
| µg MA added | 0 | 0 | 75.9 | 151.8 | 303.6 | 607.2 |
| µg MA in the medium | 0 | 0 | 56.5 ± 2.3 a | 129.0 ± 5.2 b | 179.3 ± 7.2 c | 407.5 ± 20.4 d |
| µg MA inside cells | 0 | 0 | 28.5 ± 1.4 a | 50.4 ± 2.5 b | 68.7 ± 3.4 c | 149.6 ± 7.5 d |

| C+ | C- | IC50/8 | IC50/4 | IC50/2 | IC50 | |
|---|---|---|---|---|---|---|
| HT29 | ||||||
| µg OA added | 0 | 0 | 0.47 | 0.93 | 1.85 | 3.70 |
| µg OA in the medium | 0 | 0 | 0 | 0 | 0 | 0 |
| µg OA inside cells | 0 | 0 | 0.01 ± 0.01 a | 0.50 ± 0.02 b | 1.00 ± 0.05 c | 2.17 ± 0.11 d |
| HepG2 | ||||||
| µg OA added | 0 | 0 | 1.52 | 3.04 | 6.07 | 12.14 |
| µg OA in the medium | 0 | 0 | 0 | 0 | 0 | 0 |
| µg OA inside cells | 0 | 0 | 1.16 ± 0.06 a | 4.57 ± 0.23 b | 7.19 ± 0.35 c | 7.88 ± 0.39 c |
3. Experimental Section
3.1. Materials and Chemicals
3.2. Cell Culture
3.3. Determination of the Effect on HT29 and HepG2 Cell Viability by MTT Assay
3.4. Extraction of Maslinic and Oleanolic Acid from HT29 and HepG2 Culture Cells and Medium
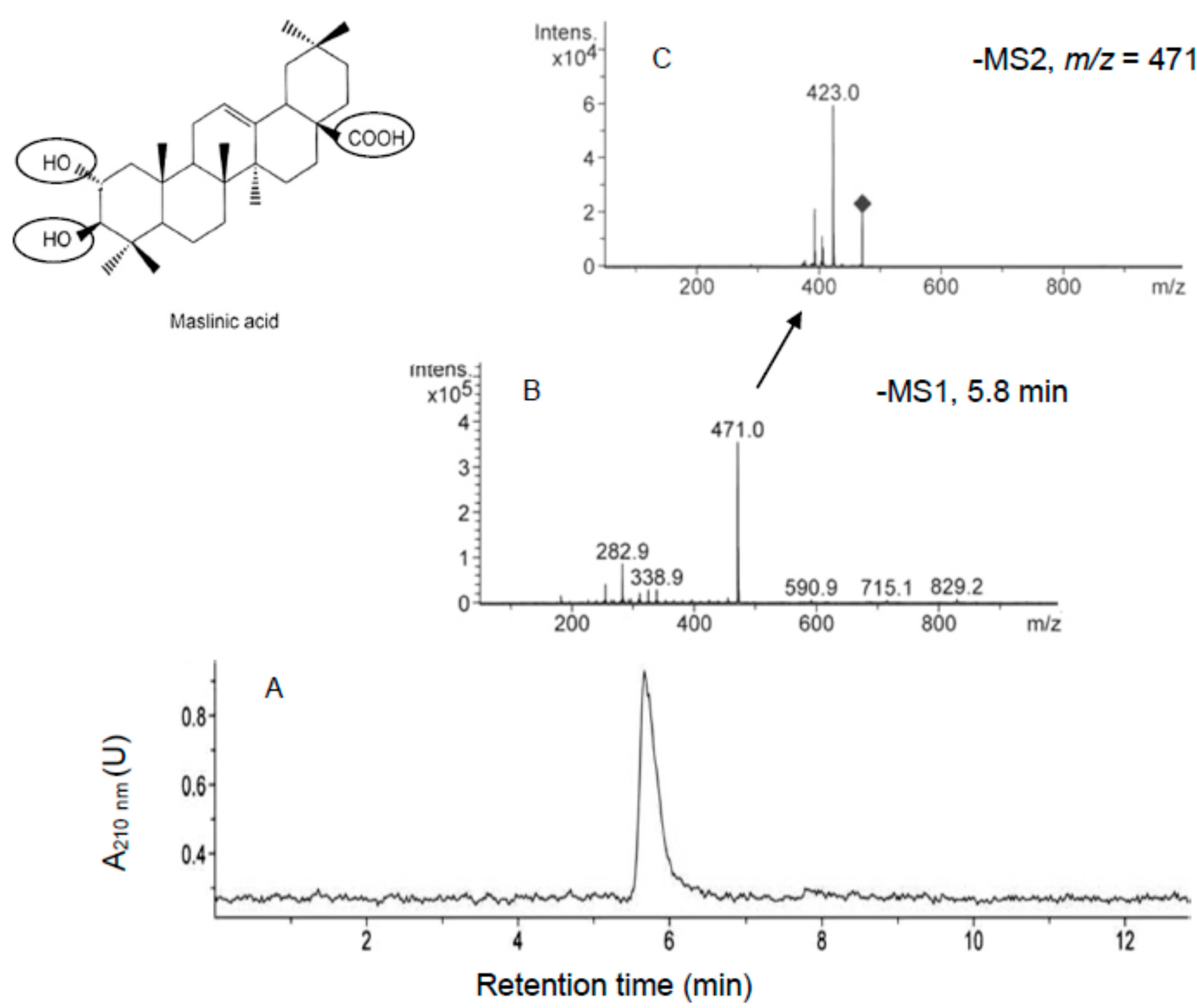
3.5. HPLC-MS and HPLC-MS/MS Analysis
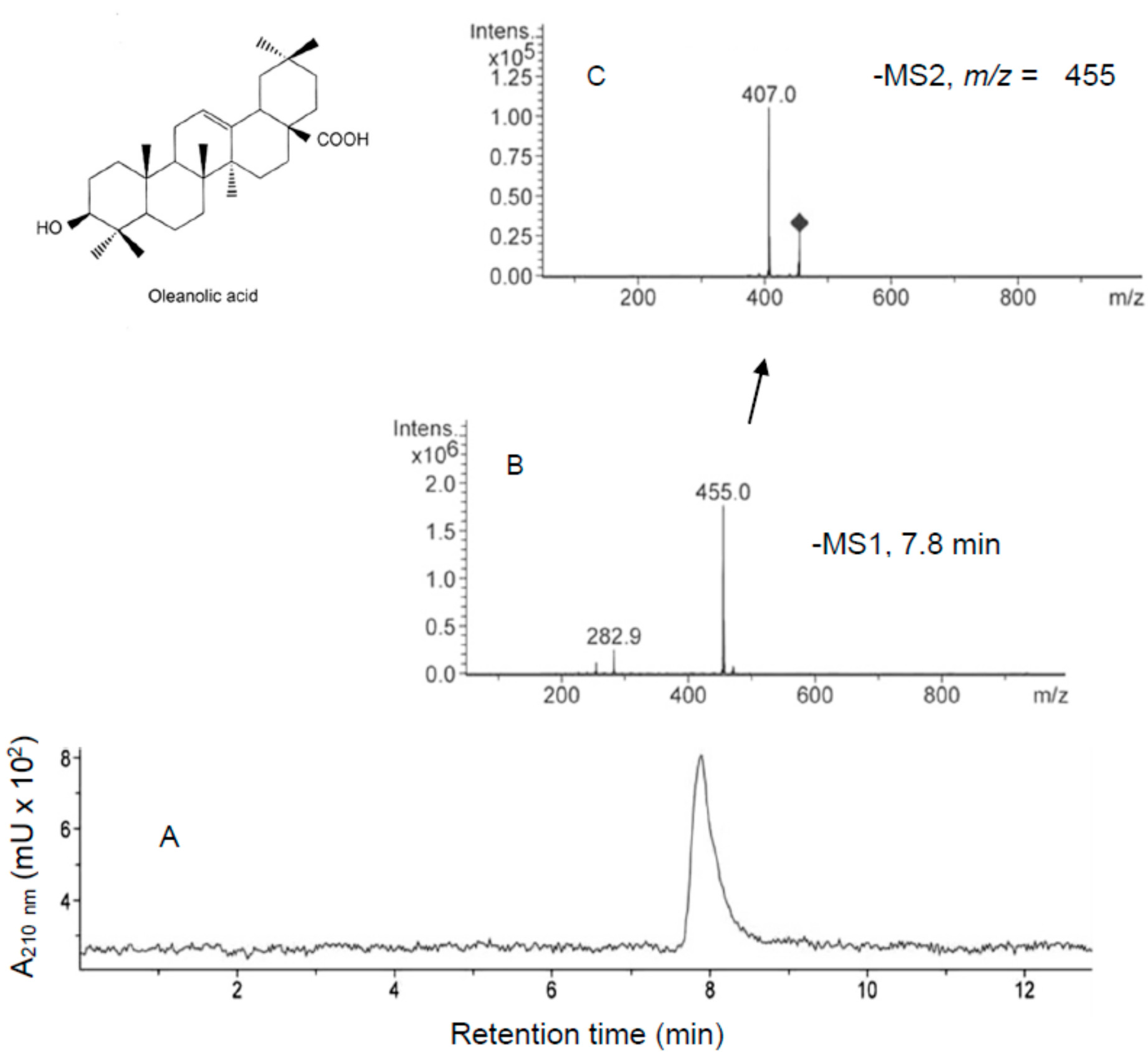
3.6. Statistical Treatment
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
References
- Peragón, J. Time course of pentacyclic triterpenoids from fruits and leaves of olive tree (Olea europaea L.) cv. Picual and cv. Cornezuelo during ripening. J. Agric. Food Chem. 2013, 61, 6671–6678. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Navarro, M.; Peragón, J.; Esteban, F.J.; de la Higuera, M.; Lupiáñez, J.A. Maslinic acid as a feed additive to stimulate growth and hepatic protein-turnover rates in rainbow trout (Onchorhynchus mykiss). Comp. Biochem. Physiol. C 2006, 144, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Navarro, M.; Peragón, J.; Amores, V.; de la Higuera, M.; Lupiáñez, J.A. Maslinic acid added to the diet increases growth and protein-turnover rates in the white muscle of rainbow trout (Oncorhynchus mykiss). Comp. Biochem. Physiol. C 2008, 147, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Rufino-Palomares, E.E.; Reyes-Zurita, F.J.; García-Salguero, L.; Peragón, J.; de la Higuera, M.; Lupiáñez, J.A. Maslinic acid, a natural triterpene, and ration size increased growth and protein turnover of white muscle in gilthead sea bream (Sparus aurata). Aquac. Nutr. 2012, 18, 568–580. [Google Scholar] [CrossRef]
- Rufino-Palomares, E.E.; Reyes-Zurita, F.J.; García-Salguero, L.; Peragón, J.; de la Higuera, M.; Lupiáñez, J.A. Maslinic acid and ration size enhanced hepatic protein-turnover rates of gilthead sea bream (Sparus aurata). Aquac. Nutr. 2012, 18, 138–151. [Google Scholar] [CrossRef]
- Rufino-Palomares, E.E.; Reyes-Zurita, F.J.; García-Salguero, L.; Peragón, J.; de la Higuera, M.; Lupiáñez, J.A. The role of maslinic acid in the pentose phosphate pathway during growth of gilthead sea bream (Sparus Aurata). Aquac. Nutr. 2013, 19, 709–720. [Google Scholar] [CrossRef]
- Wang, D.; Xia, M.; Cui, Z. New triterpenoids isolated from the root bark of Ulmus pumila L. Chem. Pharm. Bull. 2006, 54, 775–778. [Google Scholar] [CrossRef] [PubMed]
- Montilla, M.P.; Agil, A.; Navarro, M.C.; Jiménez, M.I.; García-Granados, A.; Parra, A.; Cabo, M.M. Antioxidant activity of maslinic acid, a triterpene derivative obtained from Olea Europaea. Planta Med. 2003, 69, 472–474. [Google Scholar] [PubMed]
- Yang, Z.G.; Li, H.R.; Wang, L.Y.; Li, Y.H.; Lu, S.G.; Wen, X.F.; Wang, J.; Daikonya, A.; Kitanaka, S. Triterpenoids from Hippophae rhamnoides L. and their nitric oxide production-inhibitory and DPPH radical-scavenging activities. Chem. Pharm. Bull. 2007, 55, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Mokthari, K. Antioxidant and Proliferative Characterization of Maslinic Acid in Different Tumor Cell Lines. Ph.D. Thesis, University of Granada, Granada, Spain, 2013. [Google Scholar]
- Mokhtari, K.; Rufino-Palomares, E.E.; Pérez-Jiménez, A.; Reyes-Zurita, F.J.; Figuera, C.; García-Salguero, L.; Medina, P.P.; Peragón, J.; Lupiáñez, J.A. Maslinic acid, a triterpene from olive, affects the antioxidant and mitochondrial status of B16F10 melanoma cells grown under stressful conditions. Evid.-Based Complement. Altern. Med. 2015, 2015, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Márquez-Martín, A.; de la Puerta-Vázquez, R.; Fernández-Arche, A.; Ruíz-Gutiérrez, V. Suppressive effect of maslinic acid from pmace olive oil on oxidative stress and cytokine production in stimulated murine macrophages. Free Radic. Res. 2006, 40, 295–302. [Google Scholar] [CrossRef] [PubMed]
- García-Granados, A.; Martínez, A.; Parra, A.; Rivas, F.; Osuna, A.; Mascaró, C. Use of Maslinic Acid as Inhibitor of Serine Proteases for Treatment of Diseases Caused by Cryptosporidium Parasites. Spanish Patent P9701029, 16 July 1999. [Google Scholar]
- Xu, H.X.; Zeng, F.Q.; Wan, M.; Sim, K.Y. Anti-HIV triterpene acids from Geum japonicum. J. Nat. Prod. 1996, 59, 643–645. [Google Scholar] [CrossRef] [PubMed]
- Rivas, F.; Osuna, A.; Mascaró, C.; Nájera, R. Use of Maslinic Acid as Protease Inhibitor in the Treatment of Diseases Caused by the HIV Virus. Spanish Patent P2140329, 16 February 2000. [Google Scholar]
- Reyes-Zurita, F.J.; Centelles, J.J.; Lupiáñez, J.A.; Cascante, M. (2α,3β)-2,3-Dihydroxyolean-1,2-en-28-oic acid, a new natural triterpene from Olea europaea, induces caspase dependent apoptosis selectively in colon adenocarcinoma cells. FEBS Lett. 2006, 580, 6302–6310. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Zurita, F.J.; Rufino-Palomares, E.E.; Lupiáñez, J.A.; Cascante, M. Maslinic acid, a natural triterpene from Olea europaea L., induces apoptosis in HT29 human colon-cancer cells via the mitochondrial apoptotic pathway. Cancer Lett. 2009, 273, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Tena, S.; Reyes-Zurita, F.J.; Díaz-Moralli, S.; Vinardell, M.P.; Reed, M.; García-García, F.; Dopazo, J.; Lupiáñez, J.A.; Günther, U.; Cascante, M. Maslinic Acid-enriched diet decreases intestinal tumorigenesis in ApcMin/+ mice through transcriptomic and metabolomic Reprogramming. PLoS ONE 2013, 8, 59392. [Google Scholar]
- Reyes-Zurita, F.J.; Pachón-Pena, G.; Lizárraga, D.; Rufino-Palomares, E.E.; Cascante, M.; Lupiáñez, J.A. The natural triterpene maslinic acid induces apoptosis in HT29 colon cancer cells by a JNK-p53-dependent mechanism. BMC Cancer 2011, 11, 154. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Zurita, F.J.; Rufino-Palomares, E.E.; Medina, P.P.; García-Salguero, E.L.; Peragón, J.; Cascante, M.; Lupiáñez, J.A. Antitumor activity on extrinsic apoptotic targets of the triterpenoid maslinic acid in p53-deficient Caco-2 adenocarcinoma cells. Biochimie 2013, 95, 2157–2167. [Google Scholar] [CrossRef] [PubMed]
- Rufino-Palomares, E.; Reyes-Zurita, F.; García-Salguero, L.; Mokhtari, K.; Medina, P.P.; Lupiáñez, J.A.; Peragón, J. Maslinic acid, a triterpenic anti-tumoral agent, interferes with cytoskeleton protein expression in HT29 human colon-cancer cells. J. Proteom. 2013, 83, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Yap, W.H.; Khoo, K.S.; Lim, S.H.; Yeo, C.C.; Lim, Y.M. Proteomic analysis of the molecular response of Raji cells to maslinic acid treatment. Phytomedicine 2012, 19, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Romero, C.; García, A.; Medina, E.; Ruíz-Méndez, M.V.; de Castro, A.; Brenes, M. Triterpenic acids in table olives. Food Chem. 2010, 118, 670–674. [Google Scholar] [CrossRef]
- Allouche, Y.; Jiménez, A.; Uceda, M.; Aguilera, M.P.; Gaforio, J.J.; Beltrán, G. Triterpenic content and chemometric analysis of virgin olive oils from forty olive cultivars. J. Agric. Food Chem. 2009, 57, 3604–3610. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Mena, G.; Juan, M.E.; García-Granados, A.; Planas, J.M. Determination of maslinic acid, a pentacyclic triterpene from olives, in rat plasma by high-performance liquid chromatography. J. Agric. Food Chem. 2012, 60, 10220–10225. [Google Scholar] [CrossRef] [PubMed]
- Knasmuller, S.; Parzefall, W.; Sanyal, R.; Ecker, S.; Schwab, C.; Uhl, M.; Mersch-Sundermann, V.; Williamson, G.; Hietsch, G.; Langer, T.; et al. Use of metabolically competent human hepatoma cells for the detection of mutagens and antimutagens. Mutat. Res. 1998, 402, 185–202. [Google Scholar] [CrossRef]
- Knasmuller, S.; Mersch-Sundermann, V.; Kevekordes, S.; Darroudi, F.; Huber, W.W.; Hoelzl, C.; Bichler, J.; Majer, B.J. Use of human-derived liver cell lines for the detection of environmental and dietary genotoxicants: Current state of knowledge. Toxicology 2004, 198, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Mersch-Sundermann, V.; Knasmuller, S.; Wu, X.J.; Darroudi, F.; Kassie, F. Use of a human-derived liver cell line for the detection of cytoprotective, antigenotoxic and cogenotoxic agents. Toxicology 2004, 198, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Ávila, N.; Priego-Capote, F.; Ruiz-Jimenez, J.; de Castro, M.D. Fast and selective determination of triterpenic compounds in olive leaves by liquid chromatography-tandem mass spectrometry with multiple reaction monitoring after microwave-assisted extraction. Talanta 2009, 78, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Stiti, N.; Triki, S.; Hartmann, M-A. Formation of triterpenoids throughout Olea europaea fruit ontogeny. Lipids 2007, 42, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Matito, C.; Mastorakou, F.; Centelles, J.J.; Torres, J.L.; Cascante, M. Antiproliferative effect of antioxidant polyphenols from grape in murine Hepa-1c1c7. Eur. J. Nutr. 2003, 42, 43–49. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peragón, J.; Rufino-Palomares, E.E.; Muñoz-Espada, I.; Reyes-Zurita, F.J.; Lupiáñez, J.A. A New HPLC-MS Method for Measuring Maslinic Acid and Oleanolic Acid in HT29 and HepG2 Human Cancer Cells. Int. J. Mol. Sci. 2015, 16, 21681-21694. https://doi.org/10.3390/ijms160921681
Peragón J, Rufino-Palomares EE, Muñoz-Espada I, Reyes-Zurita FJ, Lupiáñez JA. A New HPLC-MS Method for Measuring Maslinic Acid and Oleanolic Acid in HT29 and HepG2 Human Cancer Cells. International Journal of Molecular Sciences. 2015; 16(9):21681-21694. https://doi.org/10.3390/ijms160921681
Chicago/Turabian StylePeragón, Juan, Eva E. Rufino-Palomares, Irene Muñoz-Espada, Fernando J. Reyes-Zurita, and José A. Lupiáñez. 2015. "A New HPLC-MS Method for Measuring Maslinic Acid and Oleanolic Acid in HT29 and HepG2 Human Cancer Cells" International Journal of Molecular Sciences 16, no. 9: 21681-21694. https://doi.org/10.3390/ijms160921681