Weekly Treatment of 2-Hydroxypropyl-β-cyclodextrin Improves Intracellular Cholesterol Levels in LDL Receptor Knockout Mice
Abstract
:1. Introduction
2. Results
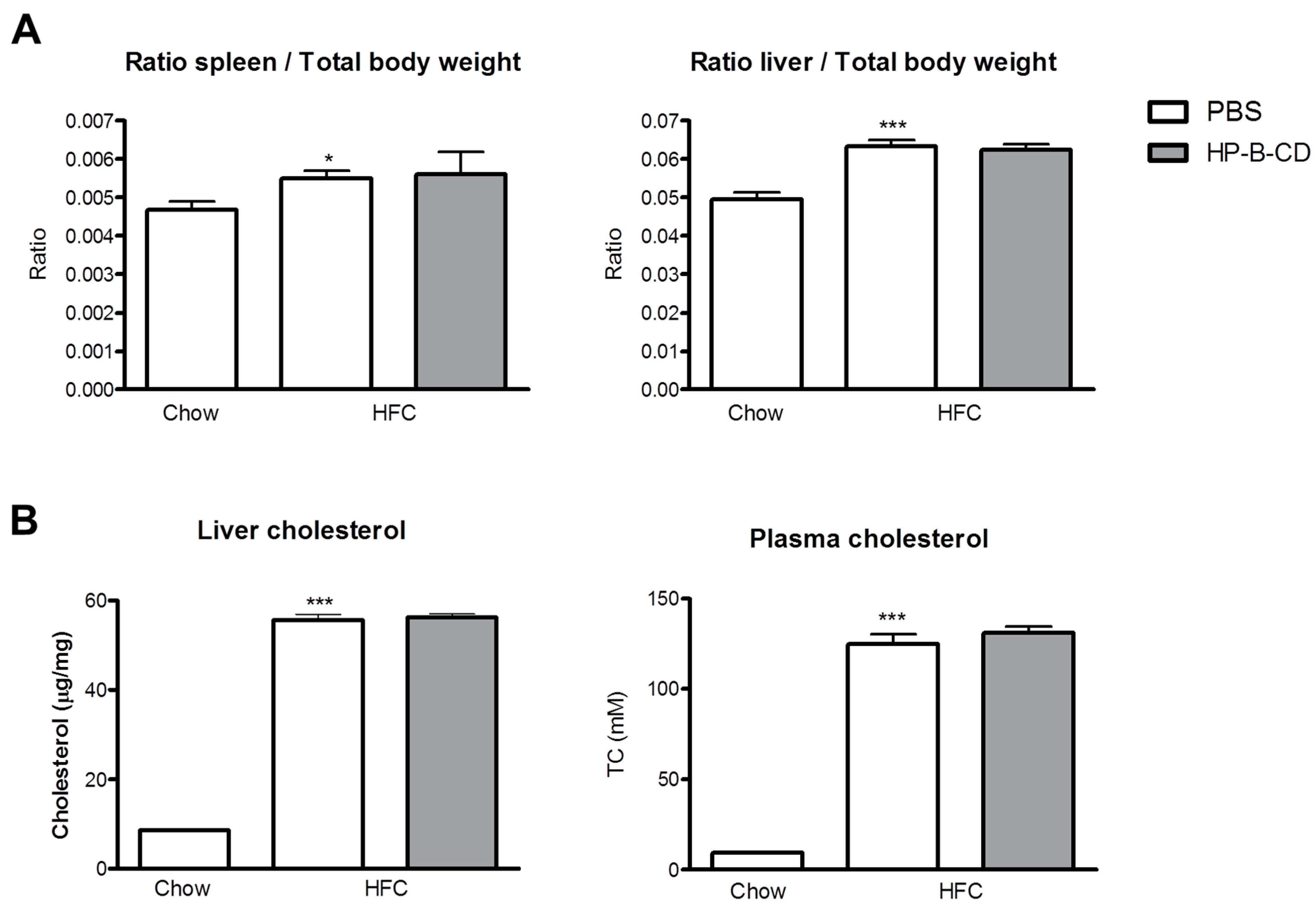
2.1. No Difference in Liver and Plasma Cholesterol Levels upon HP-B-CD Treatment
2.2. Foamy KC Appearance Is Similar between Control- and HP-B-CD-Injected Mice

2.3. HP-B-CD-Treated Mice Demonstrate Decreased Lysosomal Cholesterol Accumulation and Cholesterol Crystallization

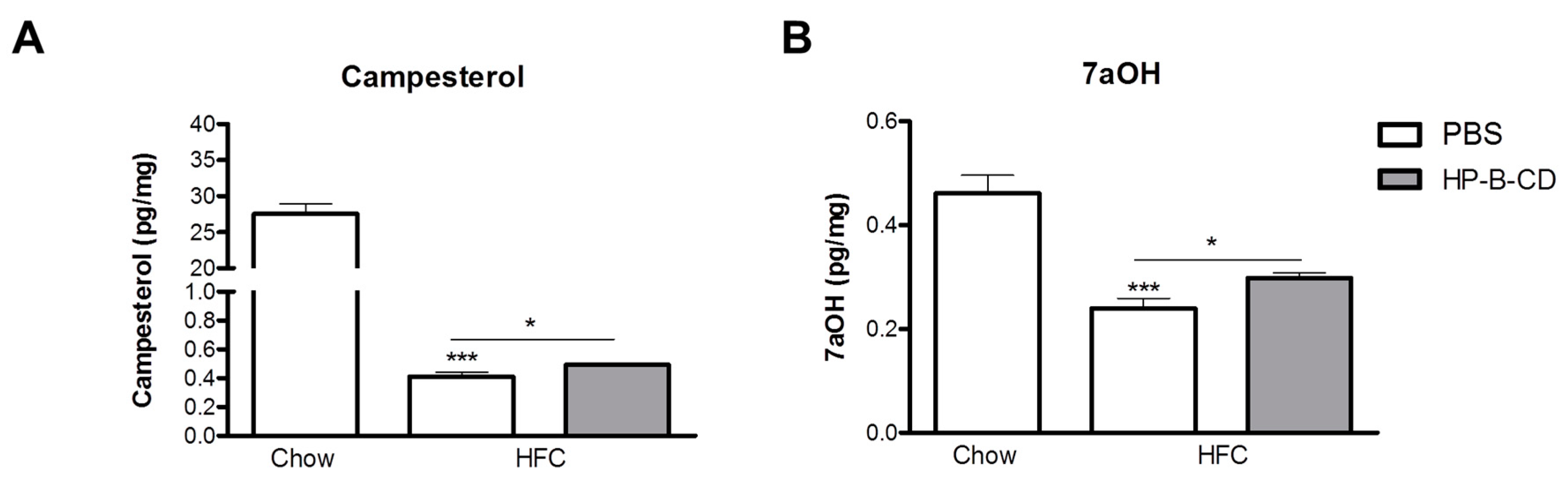
2.4. Campesterol and 7α-Hydroxycholesterol Are Increased after HP-B-CD Treatment
3. Discussion
4. Experimental Section
4.1. Mice, Diet and Injections
4.2. CD68 Staining
4.3. Scoring of Lysosomal Lipid Droplets, Cytoplasmic CE Droplets and Cholesterol Crystals
4.4. Bone Marrow-Derived Macrophages
4.5. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
References
- Lonardo, A.; Ballestri, S.; Marchesini, G.; Angulo, P.; Loria, P. Nonalcoholic fatty liver disease: A precursor of the metabolic syndrome. Dig. Liver Dis. 2015, 47, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Angulo, P. Nonalcoholic fatty liver disease. N. Engl. J. Med. 2002, 346, 1221–1231. [Google Scholar] [CrossRef] [PubMed]
- Starley, B.Q.; Calcagno, C.J.; Harrison, S.A. Nonalcoholic fatty liver disease and hepatocellular carcinoma: A weighty connection. Hepatology 2010, 51, 1820–1832. [Google Scholar] [CrossRef] [PubMed]
- Bieghs, V.; Hendrikx, T.; van Gorp, P.J.; Verheyen, F.; Guichot, Y.D.; Walenbergh, S.M.; Jeurissen, M.L.; Gijbels, M.; Rensen, S.S.; Bast, A.; et al. The cholesterol derivative 27-hydroxycholesterol reduces steatohepatitis in mice. Gastroenterology 2013, 144, 167–178.e1. [Google Scholar] [CrossRef] [PubMed]
- Bieghs, V.; van Gorp, P.J.; Walenbergh, S.M.; Gijbels, M.J.; Verheyen, F.; Buurman, W.A.; Briles, D.E.; Hofker, M.H.; Binder, C.J.; Shiri-Sverdlov, R. Specific immunization strategies against oxidized low-density lipoprotein: A novel way to reduce nonalcoholic steatohepatitis in mice. Hepatology 2012, 56, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Duewell, P.; Kono, H.; Rayner, K.J.; Sirois, C.M.; Vladimer, G.; Bauernfeind, F.G.; Abela, G.S.; Franchi, L.; Nunez, G.; Schnurr, M.; et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 2010, 464, 1357–1361. [Google Scholar] [CrossRef] [PubMed]
- Hendrikx, T.; Walenbergh, S.M.; Hofker, M.H.; Shiri-Sverdlov, R. Lysosomal cholesterol accumulation: Driver on the road to inflammation during atherosclerosis and non-alcoholic steatohepatitis. Obes. Rev. 2014, 15, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Jerome, W.G. Advanced atherosclerotic foam cell formation has features of an acquired lysosomal storage disorder. Rejuv. Res. 2006, 9, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Vanier, M.T. Niemann-Pick disease type C. Orphanet J. Rare Dis. 2010, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Abi-Mosleh, L.; Infante, R.E.; Radhakrishnan, A.; Goldstein, J.L.; Brown, M.S. Cyclodextrin overcomes deficient lysosome-to-endoplasmic reticulum transport of cholesterol in Niemann-Pick type C cells. Proc. Natl. Acad. Sci. USA 2009, 106, 19316–19321. [Google Scholar] [CrossRef] [PubMed]
- Davidson, C.D.; Ali, N.F.; Micsenyi, M.C.; Stephney, G.; Renault, S.; Dobrenis, K.; Ory, D.S.; Vanier, M.T.; Walkley, S.U. Chronic cyclodextrin treatment of murine Niemann-Pick C disease ameliorates neuronal cholesterol and glycosphingolipid storage and disease progression. PLoS ONE 2009, 4, e6951. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Ramirez, C.M.; Miller, A.M.; Repa, J.J.; Turley, S.D.; Dietschy, J.M. Cyclodextrin overcomes the transport defect in nearly every organ of NPC1 mice leading to excretion of sequestered cholesterol as bile acid. J. Lipid Res. 2010, 51, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Turley, S.D.; Burns, D.K.; Miller, A.M.; Repa, J.J.; Dietschy, J.M. Reversal of defective lysosomal transport in NPC disease ameliorates liver dysfunction and neurodegeneration in the npc1−/− mouse. Proc. Natl. Acad. Sci. USA 2009, 106, 2377–2382. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, C.M.; Liu, B.; Taylor, A.M.; Repa, J.J.; Burns, D.K.; Weinberg, A.G.; Turley, S.D.; Dietschy, J.M. Weekly cyclodextrin administration normalizes cholesterol metabolism in nearly every organ of the Niemann-Pick type C1 mouse and markedly prolongs life. Pediatr. Res. 2010, 68, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, S.; Brown, M.S.; Goldstein, J.L.; Gerard, R.D.; Hammer, R.E.; Herz, J. Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J. Clin. Investig. 1993, 92, 883–893. [Google Scholar] [CrossRef] [PubMed]
- Bieghs, V.; van Gorp, P.J.; Wouters, K.; Hendrikx, T.; Gijbels, M.J.; van Bilsen, M.; Bakker, J.; Binder, C.J.; Lutjohann, D.; Staels, B.; et al. LDL receptor knock-out mice are a physiological model particularly vulnerable to study the onset of inflammation in non-alcoholic fatty liver disease. PLoS ONE 2012, 7, e30668. [Google Scholar] [CrossRef] [PubMed]
- Bieghs, V.; Walenbergh, S.M.; Hendrikx, T.; van Gorp, P.J.; Verheyen, F.; Olde Damink, S.W.; Masclee, A.A.; Koek, G.H.; Hofker, M.H.; Binder, C.J.; et al. Trapping of oxidized LDL in lysosomes of Kupffer cells is a trigger for hepatic inflammation. Liver Int. 2013, 33, 1056–1061. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, C.M.; Liu, B.; Aqul, A.; Taylor, A.M.; Repa, J.J.; Turley, S.D.; Dietschy, J.M. Quantitative role of LAL, NPC2, and NPC1 in lysosomal cholesterol processing defined by genetic and pharmacological manipulations. J. Lipid Res. 2011, 52, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Jerome, W.G.; Cash, C.; Webber, R.; Horton, R.; Yancey, P.G. Lysosomal lipid accumulation from oxidized low density lipoprotein is correlated with hypertrophy of the Golgi apparatus and trans-Golgi network. J. Lipid Res. 1998, 39, 1362–1371. [Google Scholar] [PubMed]
- Schmitz, G.; Grandl, M. Endolysosomal phospholipidosis and cytosolic lipid droplet storage and release in macrophages. Biochim. Biophys. Acta 2009, 1791, 524–539. [Google Scholar] [CrossRef] [PubMed]
- Sims-Robinson, C.; Bakeman, A.; Rosko, A.; Glasser, R.; Feldman, E.L. The role of oxidized cholesterol in diabetes-induced lysosomal dysfunction in the brain. Mol. Neurobiol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Yancey, P.G.; Jerome, W.G. Lysosomal cholesterol derived from mildly oxidized low density lipoprotein is resistant to efflux. J. Lipid Res. 2001, 42, 317–327. [Google Scholar] [PubMed]
- Lougheed, M.; Zhang, H.F.; Steinbrecher, U.P. Oxidized low density lipoprotein is resistant to cathepsins and accumulates within macrophages. J. Biol. Chem. 1991, 266, 14519–14525. [Google Scholar] [PubMed]
- Peake, K.B.; Vance, J.E. Normalization of cholesterol homeostasis by 2-hydroxypropyl-β-cyclodextrin in neurons and glia from Niemann-Pick C1 (NPC1)-deficient mice. J. Biol. Chem. 2012, 287, 9290–9298. [Google Scholar] [CrossRef] [PubMed]
- Stella, V.J.; He, Q. Cyclodextrins. Toxicol. Pathol. 2008, 36, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.M.; Terpack, S.J.; Posey, K.S.; Liu, B.; Ramirez, C.M.; Turley, S.D. Systemic administration of 2-hydroxypropyl-β-cyclodextrin to symptomatic NPC1-deficient mice slows cholesterol sequestration in the major organs and improves liver function. Clin. Exp. Pharmacol. Physiol. 2014, 41, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.M.; Liu, B.; Mari, Y.; Liu, B.; Repa, J.J. Cyclodextrin mediates rapid changes in lipid balance in Npc1−/− mice without carrying cholesterol through the bloodstream. J. Lipid Res. 2012, 53, 2331–2342. [Google Scholar] [CrossRef] [PubMed]
- Grebe, A.; Latz, E. Cholesterol crystals and inflammation. Curr. Rheumatol. Rep. 2013, 15, 313. [Google Scholar] [CrossRef] [PubMed]
- Hendrikx, T.; Bieghs, V.; Walenbergh, S.M.; van Gorp, P.J.; Verheyen, F.; Jeurissen, M.L.; Steinbusch, M.M.; Vaes, N.; Binder, C.J.; Koek, G.H.; et al. Macrophage specific caspase-1/11 deficiency protects against cholesterol crystallization and hepatic inflammation in hyperlipidemic mice. PLoS ONE 2013, 8, e78792. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, G.N.; Haigh, W.G.; Thorning, D.; Savard, C. Hepatic cholesterol crystals and crown-like structures distinguish nash from simple steatosis. J. Lipid Res. 2013, 54, 1326–1334. [Google Scholar] [CrossRef] [PubMed]
- Tangirala, R.K.; Jerome, W.G.; Jones, N.L.; Small, D.M.; Johnson, W.J.; Glick, J.M.; Mahlberg, F.H.; Rothblat, G.H. Formation of cholesterol monohydrate crystals in macrophage-derived foam cells. J. Lipid Res. 1994, 35, 93–104. [Google Scholar] [PubMed]
- Rosenbaum, A.I.; Zhang, G.; Warren, J.D.; Maxfield, F.R. Endocytosis of β-cyclodextrins is responsible for cholesterol reduction in Niemann-Pick type C mutant cells. Proc. Natl. Acad. Sci. USA 2010, 107, 5477–5482. [Google Scholar] [CrossRef] [PubMed]
- Simonen, P.; Gylling, H.; Howard, A.N.; Miettinen, T.A. Introducing a new component of the metabolic syndrome: Low cholesterol absorption. Am. J. Clin. Nutr. 2000, 72, 82–88. [Google Scholar] [PubMed]
- Pinedo, S.; Vissers, M.N.; von Bergmann, K.; Elharchaoui, K.; Lutjohann, D.; Luben, R.; Wareham, N.J.; Kastelein, J.J.; Khaw, K.T.; Boekholdt, S.M. Plasma levels of plant sterols and the risk of coronary artery disease: The prospective EPIC-Norfolk Population Study. J. Lipid Res. 2007, 48, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.C.; Watts, G.F.; Barrett, P.H.; O’Neill, F.H.; Thompson, G.R. Plasma markers of cholesterol homeostasis and apolipoprotein B-100 kinetics in the metabolic syndrome. Obes. Res. 2003, 11, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Rigamonti, E.; Helin, L.; Lestavel, S.; Mutka, A.L.; Lepore, M.; Fontaine, C.; Bouhlel, M.A.; Bultel, S.; Fruchart, J.C.; Ikonen, E.; et al. Liver X receptor activation controls intracellular cholesterol trafficking and esterification in human macrophages. Circ. Res. 2005, 97, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Ruiu, G.; Pinach, S.; Veglia, F.; Gambino, R.; Marena, S.; Uberti, B.; Alemanno, N.; Burt, D.; Pagano, G.; Cassader, M. Phytosterol-enriched yogurt increases LDL affinity and reduces CD36 expression in polygenic hypercholesterolemia. Lipids 2009, 44, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Bieghs, V.; Verheyen, F.; van Gorp, P.J.; Hendrikx, T.; Wouters, K.; Lutjohann, D.; Gijbels, M.J.; Febbraio, M.; Binder, C.J.; Hofker, M.H.; et al. Internalization of modified lipids by CD36 and SR-A leads to hepatic inflammation and lysosomal cholesterol storage in kupffer cells. PLoS ONE 2012, 7, e34378. [Google Scholar] [CrossRef] [PubMed]
- Bieghs, V.; Wouters, K.; van Gorp, P.J.; Gijbels, M.J.; de Winther, M.P.; Binder, C.J.; Lutjohann, D.; Febbraio, M.; Moore, K.J.; van Bilsen, M.; et al. Role of scavenger receptor A and CD36 in diet-induced nonalcoholic steatohepatitis in hyperlipidemic mice. Gastroenterology 2010, 138, 2477–2486.e3. [Google Scholar] [CrossRef] [PubMed]
- Wisse, E.; Braet, F.; Duimel, H.; Vreuls, C.; Koek, G.; Olde Damink, S.W.; van den Broek, M.A.; de Geest, B.; Dejong, C.H.; Tateno, C.; et al. Fixation methods for electron microscopy of human and other liver. World J. Gastroenterol. 2010, 16, 2851–2866. [Google Scholar] [CrossRef] [PubMed]
- Wouters, K.; van Gorp, P.J.; Bieghs, V.; Gijbels, M.J.; Duimel, H.; Lutjohann, D.; Kerksiek, A.; van Kruchten, R.; Maeda, N.; Staels, B.; et al. Dietary cholesterol, rather than liver steatosis, leads to hepatic inflammation in hyperlipidemic mouse models of nonalcoholic steatohepatitis. Hepatology 2008, 48, 474–486. [Google Scholar] [CrossRef] [PubMed]
- Lutjohann, D.; Stroick, M.; Bertsch, T.; Kuhl, S.; Lindenthal, B.; Thelen, K.; Andersson, U.; Bjorkhem, I.; Bergmann Kv, K.; Fassbender, K. High doses of simvastatin, pravastatin, and cholesterol reduce brain cholesterol synthesis in guinea pigs. Steroids 2004, 69, 431–438. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walenbergh, S.M.A.; Houben, T.; Hendrikx, T.; Jeurissen, M.L.J.; Van Gorp, P.J.; Vaes, N.; Damink, S.W.M.O.; Verheyen, F.; Koek, G.H.; Lütjohann, D.; et al. Weekly Treatment of 2-Hydroxypropyl-β-cyclodextrin Improves Intracellular Cholesterol Levels in LDL Receptor Knockout Mice. Int. J. Mol. Sci. 2015, 16, 21056-21069. https://doi.org/10.3390/ijms160921056
Walenbergh SMA, Houben T, Hendrikx T, Jeurissen MLJ, Van Gorp PJ, Vaes N, Damink SWMO, Verheyen F, Koek GH, Lütjohann D, et al. Weekly Treatment of 2-Hydroxypropyl-β-cyclodextrin Improves Intracellular Cholesterol Levels in LDL Receptor Knockout Mice. International Journal of Molecular Sciences. 2015; 16(9):21056-21069. https://doi.org/10.3390/ijms160921056
Chicago/Turabian StyleWalenbergh, Sofie M. A., Tom Houben, Tim Hendrikx, Mike L. J. Jeurissen, Patrick J. Van Gorp, Nathalie Vaes, Steven W. M. Olde Damink, Fons Verheyen, Ger H. Koek, Dieter Lütjohann, and et al. 2015. "Weekly Treatment of 2-Hydroxypropyl-β-cyclodextrin Improves Intracellular Cholesterol Levels in LDL Receptor Knockout Mice" International Journal of Molecular Sciences 16, no. 9: 21056-21069. https://doi.org/10.3390/ijms160921056






