1. Introduction
It is largely established that the regenerative power present in some regions of the body of vertebrates depends on the presence of resident stem cells localized in micro-regions known as stem cell niches [
1,
2,
3]. The number, extension, and duration of the niches during lifetimes in different vertebrates may vary and be higher in anamniotes such as amphibians and fish that grow for most of their lifespan in comparison to birds and mammals (homethermic amniotes) that instead possess a determinate growth. In heterothermic sauropsids, tissue regeneration is generally higher than in mammals and birds, and the tail can broadly regenerate, although imperfectly, in many lizards [
4,
5,
6,
7]. It is likely that skeletally mature reptiles possess stem cell niches in body regions where growth is still active, whereas stem cell niches are reduced in mammals at skeletal maturity. Sparse stem cells niches remain in mammals, and are involved with cell replacement in organs that need a rapid cell turnover (intestine, skin, bone marrow) or are the sites of reproductive cells (gonads) [
8].
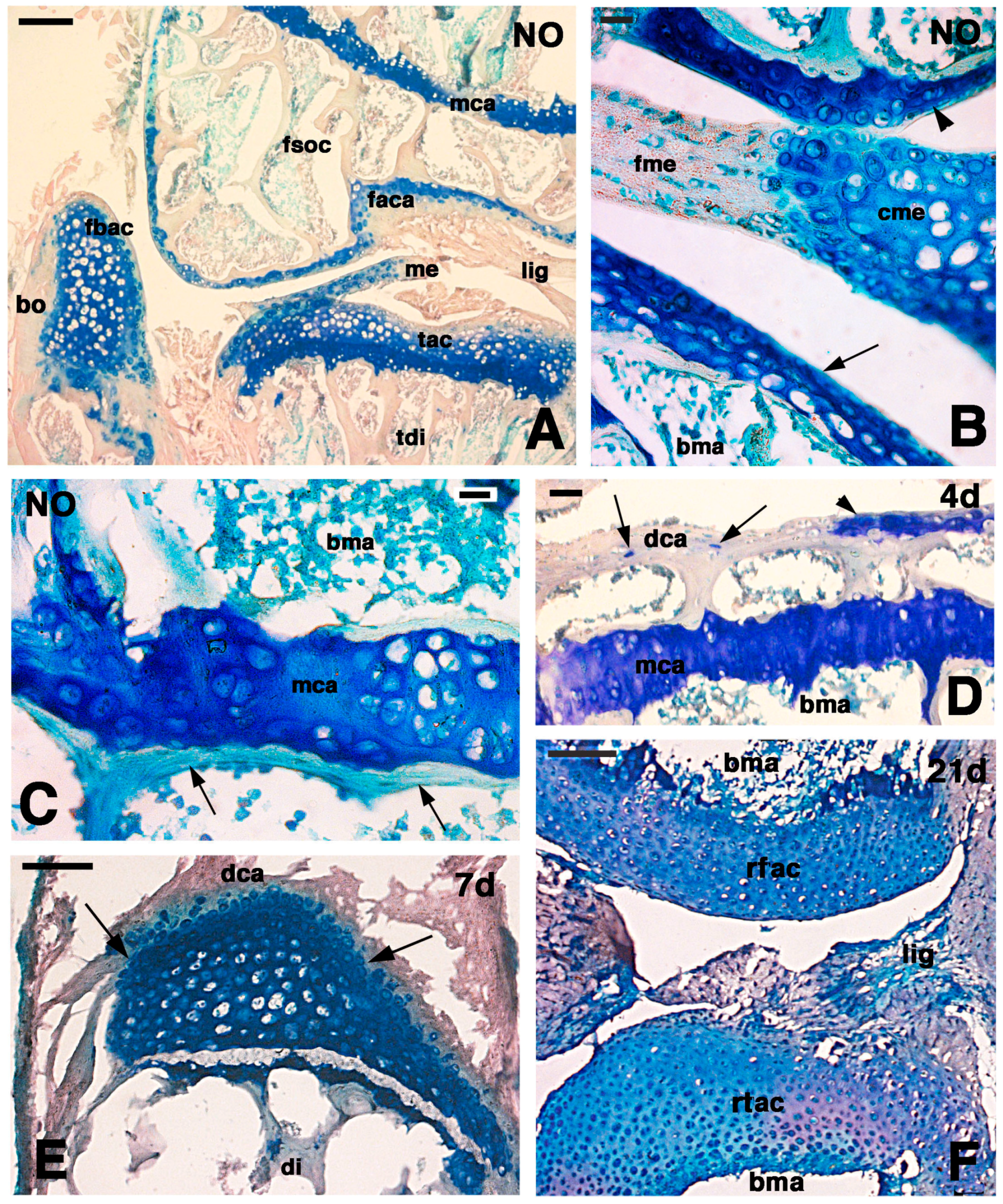
One of the tissues capable of regeneration in lizards is the cartilaginous tissue of the tail vertebrae, which recovers after lesions and gives rise to a long cartilaginous tube in the regenerated tail [
9,
10,
11,
12,
13,
14]. In contrast, instances of cartilage regeneration are uncommon in mammals, in particular the articular cartilage of the joints such as knees or elbows [
15,
16].
Recent studies have illustrated the ample regenerative potential of the epiphyses of long bones in the knee of adult lizards (
Podarcis muralis) after injury [
17]. This study showed that proliferating cells are localized in the articular cartilage and in the metaphyseal growth plate of the epiphyses of the long bones, likely as a part of the continuous growth in this species. The new cartilage forms cartilaginous epiphyses which cells are mainly derived from the proliferation of chondrocytes from the articular cartilage. The study suggested that other cartilaginous cells are also derived from the methaphyseal growth centers where numerous chondroblasts are still proliferating in normal conditions. The same study postulated that proliferating cells forming the new cartilage represent a population of transient amplifying cells derived from resident stem cells localized in the epiphyses, but no direct proof has been shown.
In order to address the above hypothesis in the present study, we have analyzed the distal femur and proximal tibia bones forming the knee, after injuries. We used immunocytochemical methods to detect the presence of putative resident stem cells that replace chondrocytes in the growing centers of normal bones. This has been done using 5-Bromo-deoxyuridine and telomerase immunolabeling as an indication of stemness [
18].
3. Discussion
The present study strongly suggests that the broad regeneration of the epiphyses in
P. muralis derives from the presence of stem/primordial cells present in the articular cartilage and in the metaphyseal growth plate of the knee joint. These regions likely represent stem cell niches and correspond to the growing centers of the bones that are variably proliferating in sexually mature lizards (adults). In fact, it is likely that these reptiles continue to grow, although slowly, also after they reach sexual maturity at about two to three years of life in the species here analyzed (
P. muralis). This appears evident from the uptake of 5BrdU in these cartilaginous areas under normal conditions, even though their epiphyses form secondary centers of ossification similar to those of mammalian epiphyses [
19,
20,
21]. Although in some cases the normal adult knees in lizards show little cartilage and an almost complete ossification around the metaphyseal cartilage (closed metaphyses, see [
15]) this definitive condition in mammalian long bones can represent only a temporary condition in other vertebrates. In fact, the apparent closure of the metaphyseal growth plate has been noted in the epiphyses of fish, amphibians and also in lepidosaurian reptiles in some periods during the year, where it is indicated as a “temporary growth cessation”, probably related with winter (resting) periods [
19,
20,
21]. In these species the process of growth can be resumed later, possibly in spring, by the proliferation of quiescent cells (likely stem cells as indicated in the present study), and is followed by the erosion of the bone tissues around the cartilage that expands into new isogenous groups of variable extension [
19].
The immunodetection of an intense immunolabeling for chondroitin Sulfate proteoglycan in regenerating chondrocytes, indicates a high cytoplasmic synthesis and secretion mainly in the ring-like, pericellular region around these cells. This observation indicates that the small chondroblasts and chondrocytes of the regenerating cartilage are actively producing the proteoglycan to be discharged in the extracellular matrix. This observation agrees with previous immunolocalization studies that showed that perlecan is especially localized in the pericellular region whereas aggregans are more widespread within the entire extracellular matrix [
22]. The latter study showed that the detection of proteoglycans also in the extracellular matrix often requires treatment with chondroitinase that unmasks antigenic sites previously masked by other molecules of the matrix [
22]. The lower immunoreactivity observed in chondrocytes of the normal epiphysis, further supports the hypothesis that a higher active synthesis of new matrix is present in regenerating chondrocytes, and not only in the piled serial cartilage.
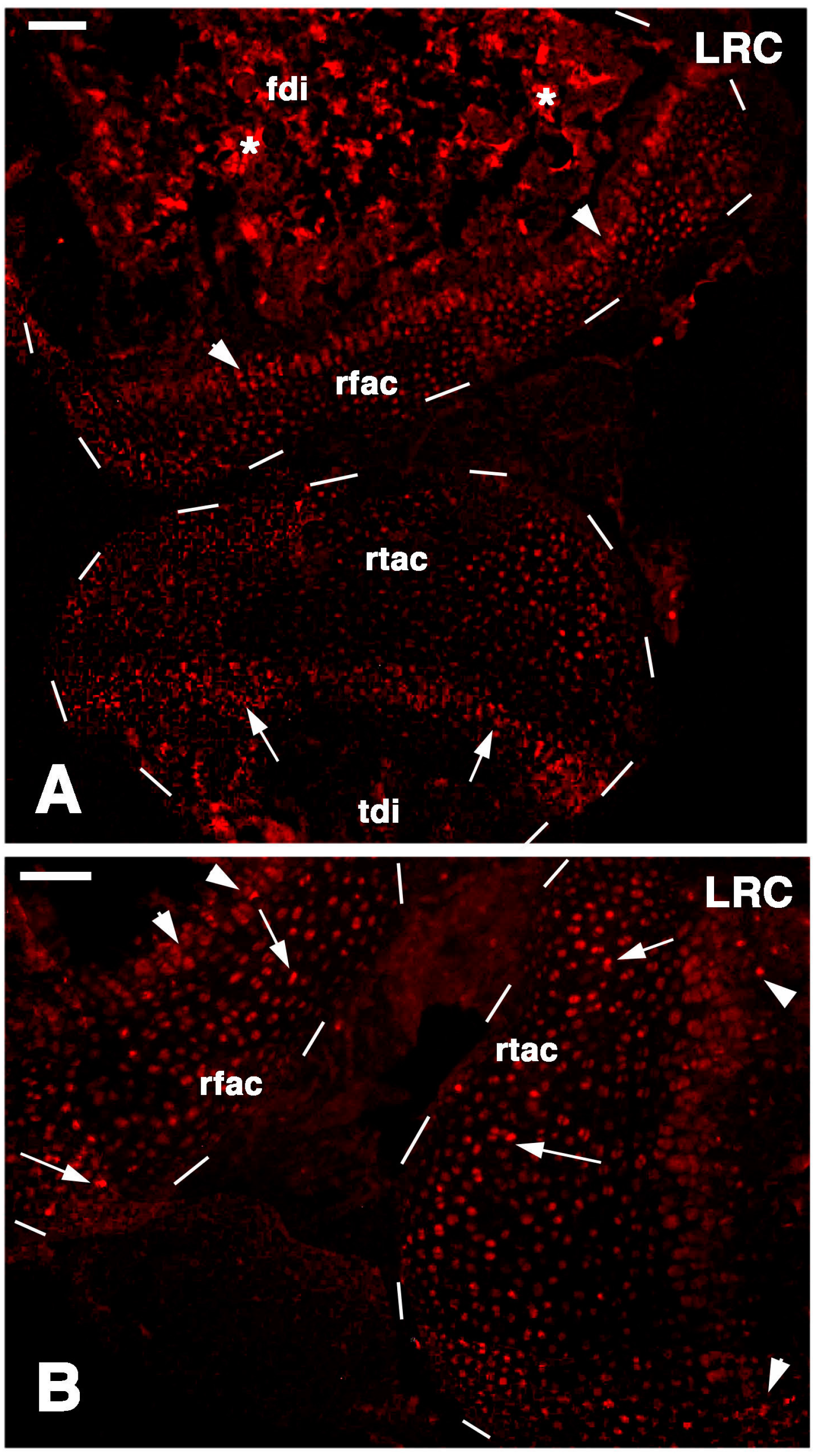
The main results of the present study on the lizard
P. muralis, are summarized in
Figure 9D,E. putative stem/progenitor cells are present on the surface of the articular cartilage and in the resting/proliferating serial chondroblasts of the metaphyseal (growth) plate. These cells likely supply new chondroblasts (transient amplifying cells) for the elongation of long bones at a high rate in the higher phase of growth taking place during the first one to three years. Unlike mammals, these cells continue to supply new chondroblasts for long bone elongation in lizards, though at a slower rate. The lizards here utilized were all adults females and males, so the main phase of growth was past, although long retaining and telomerase positive cells remained in the knees. After injury (
Figure 9A–C) these stem cells are further stimulated and give rise to a surplus of transient amplifying chondroblasts that continue to proliferate and eventually form new cartilaginous epiphyses (
Figure 9D,E). The present study suggests that putative stem cells are displaced in the new cartilaginous tissue within three weeks. It is unknown whether new secondary ossification centers are later re-formed and whether the remaining stem cells eventually reposition on the surface of the eroded articular cartilage. These open questions remain viable for future investigations. Previous studies conducted on mammalian sub-adults and adult articular cartilage also indicate that progenitor cells are localized in these areas [
23,
24,
25].
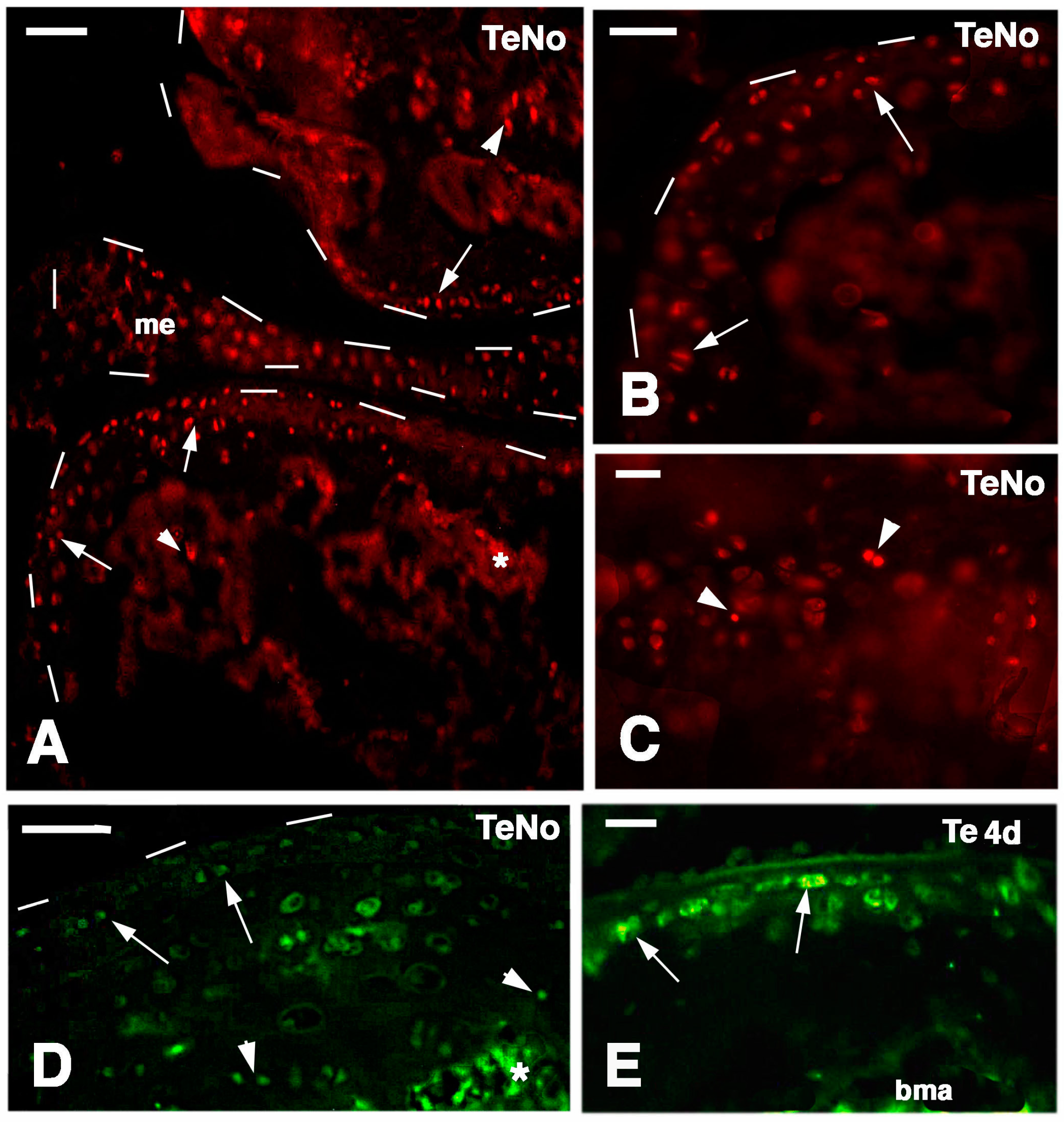
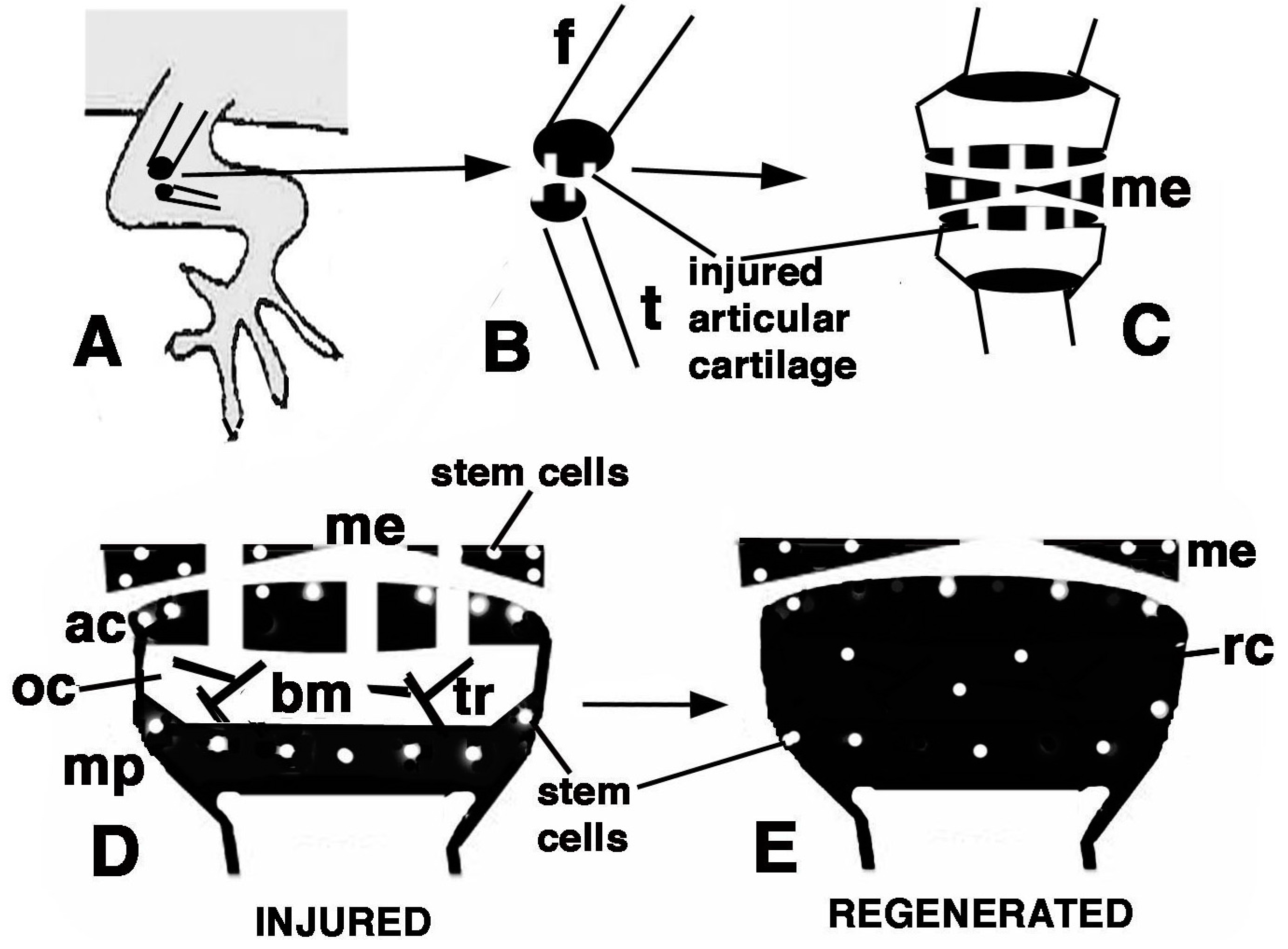
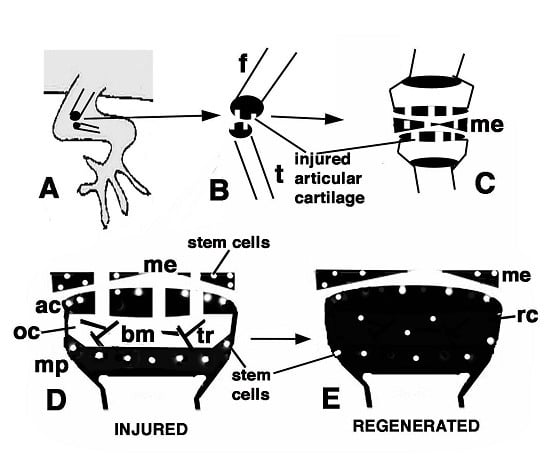
Figure 9.
Schematic drawing resuming the present observations. (A) depicts a lizard knee where the injury of the articular surfaces is indicated in (B); (C) depicts the damaged area (white) produced on the articular cartilages and meniscus of the knee; (D) further depicts the structure of an injured epiphyses and meniscus with the position of Long Retaining Cells (LRC)/telomerase positive and putative stem cells in the articular cartilage and metaphyseal plate indicated; (E) depicts that the regenerated cartilage (in black) occupies the entire epiphyses at 21−32 days (four-and-a-half weeks) post-injury while stem cells appear sparse and not localized in the surface or metaphyseal plate, and are also present in the meniscus. (ac, articular cartilage; bm, bone marrow; f, femur; me, meniscus; mp, metaphyseal (growth) plate; oc, ossification center; rc, regenerated cartilage; t, tibia; tr, bone trabeculae.)
Figure 9.
Schematic drawing resuming the present observations. (A) depicts a lizard knee where the injury of the articular surfaces is indicated in (B); (C) depicts the damaged area (white) produced on the articular cartilages and meniscus of the knee; (D) further depicts the structure of an injured epiphyses and meniscus with the position of Long Retaining Cells (LRC)/telomerase positive and putative stem cells in the articular cartilage and metaphyseal plate indicated; (E) depicts that the regenerated cartilage (in black) occupies the entire epiphyses at 21−32 days (four-and-a-half weeks) post-injury while stem cells appear sparse and not localized in the surface or metaphyseal plate, and are also present in the meniscus. (ac, articular cartilage; bm, bone marrow; f, femur; me, meniscus; mp, metaphyseal (growth) plate; oc, ossification center; rc, regenerated cartilage; t, tibia; tr, bone trabeculae.)
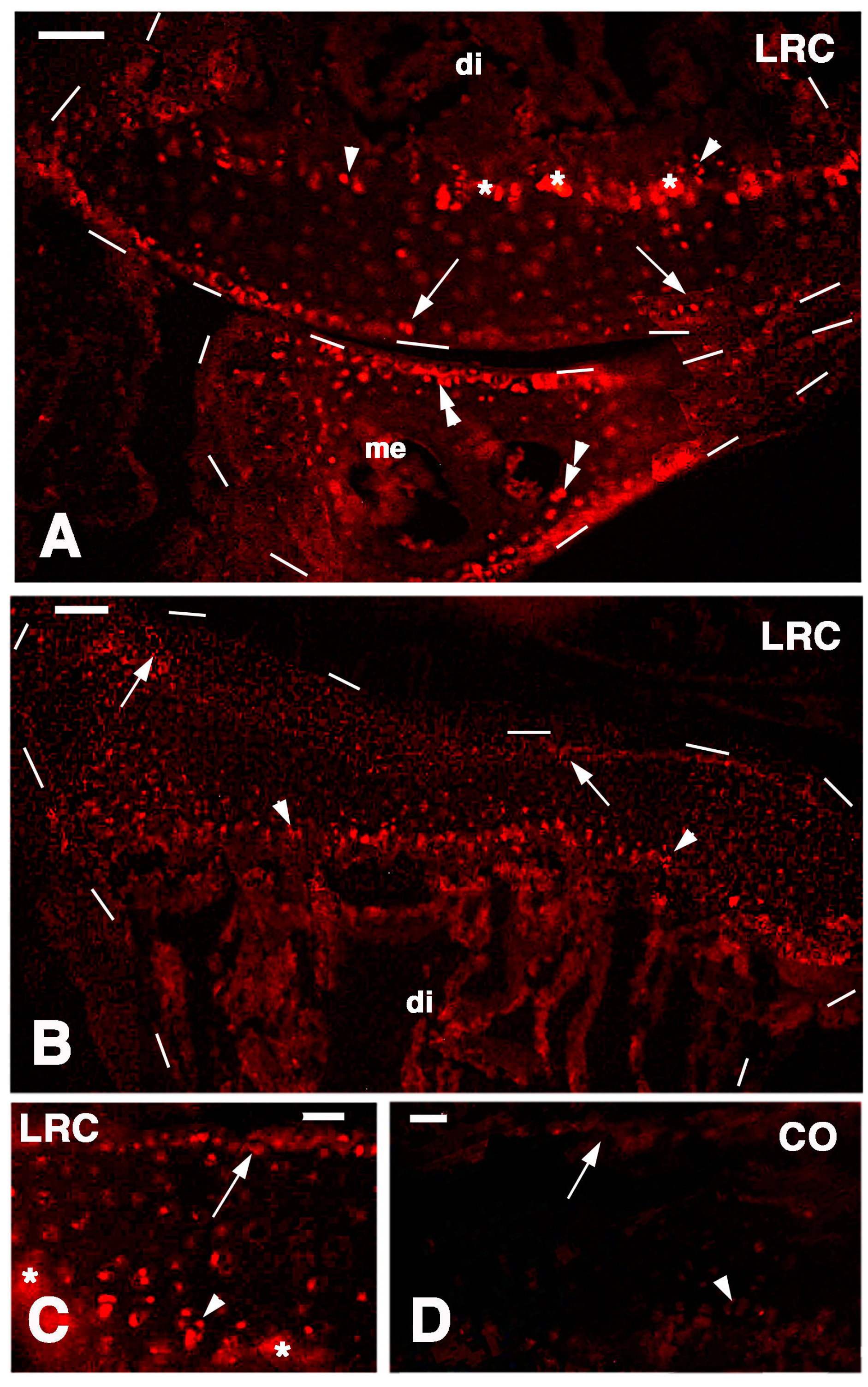
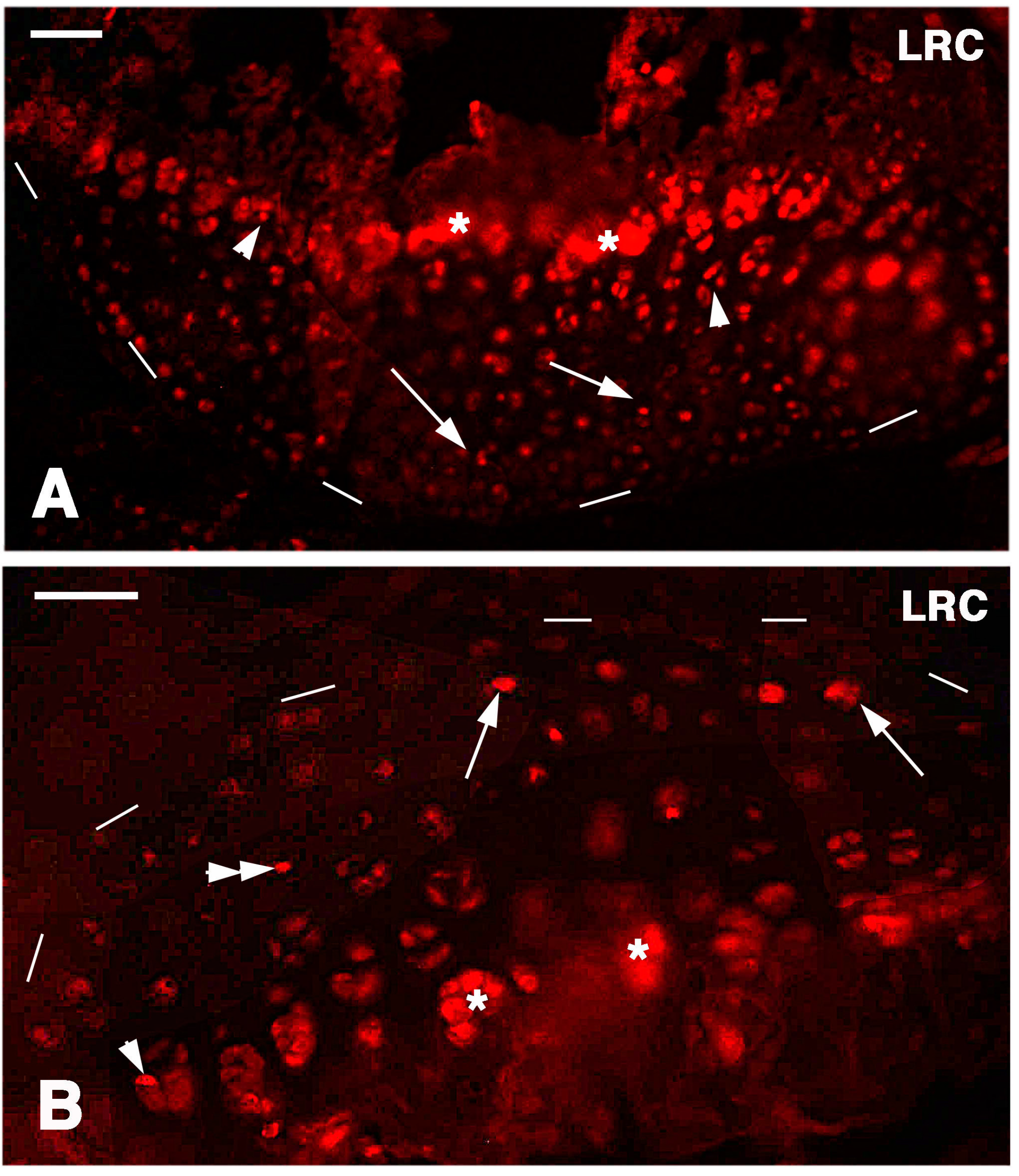
Long retaining cells, some also positive for telomerase, were also observed in the bone marrow of secondary centers but their possible contribution to the regeneration of the new epiphyses remains undetermined. A previous study [
18] indicated that the enzyme is particularly present in the nucleus of stem cells in the gonads, intestinal crypts and only sparsely in the blastema of the regenerating tail. Whatever the case, the presence of stem cells in the cartilage itself appears sufficient to explain the high regenerative response observed in our study as well as for the ample regeneration of cartilaginous tissues observed in other regions of the body of lizards [
6,
14,
25,
26]. The presence of stem/progenitor cells is not only limited to the cartilage but also to the cartilaginous and fibrous parts of the meniscus and also the ligaments appear to contain progenitor cells, but a further study is needed on this point. The understanding of the mechanism by which these vertebrates maintain stem cell niches in their long bones is important for studies of regenerative medicine on the articular cartilage [
16,
24,
25,
27].
4. Experimental Section
4.1. Experiments
We used six adult individuals of Italian wall lizard (
Podarcis muralis), including males and females longer than 12 cm. The husbandry and experimental manipulations followed the Italian guidelines for animal care and handling (Art. 5, DL 116/92). Briefly, after anesthesia using ethylic ether, the skin of the right knee of lizards was cleaned with a Lugol iodinated-solution and opened with a sharp scalpel, the muscles and connective tissues and the knee bones were exposed. The epiphyses of both femur and tibia were visually damaged under a stereomicroscope with three scissor-cuts made on the surface of the articular cartilage, taking care not to damage the femoral artery to avoid extensive bleeding (
Figure 9). After the operation, the limb tissues were recomposed over the injured knee and the skin flaps reconstituted to cover the surgical incision adding healing powder (Cicatrene, Welcome Italia, Pomezia-Rome) to help maintain the skin in place over the wound.
The six operated lizards recovered well after the operation, and regained an apparent normal movement of the operated hindlimbs. They were maintained in cages with sun exposure, and at summer temperatures reaching over 30 °C during most of the day, and keeping above 20 during the night. A few hours after surgery the lizards received injection of 50 mg/kg body weight with 5-Bromo-deoxyUridine (5BrdU, Sigma, St. Louis, MO, USA) dissolved in Ringer. The injections were repeated for a week (pulse period) and later the animals were left to recover for three-and-a half weeks before sacrifice (chase period). Therefore recovery lasted four-and-a-half weeks in total from the initial injury, including the first week of pulse experiments. This group served for detecting long retaining labeled cells (putative stem cells) in the knee epiphyses and cartilage.
Two additional operated lizards, were left to recover for 3 weeks after injury, and they were injected with 5BrdU and sacrificed after 4 h as above. These specimens served for checking the general proliferation in the cartilage after 3 weeks post-injury (for both the injured and the contralateral normal knees). The other 8 lizards with injuries at progressive periods were utilized for histology and for the immunodetection of telomerase. From these animals, the injured knees were collected and fixed as above at 4 days post-injury (n = 2), 7 day post-injury (n = 2), 14 days post-injury (n = 2), and 21 days post-injury (n = 2).
4.2. Tissue Preparation and Microscopic Techniques
The operated and the contra-lateral normal knees were collected at about half of the length of both femur and tibia, and immediately immersed in 4% paraformaldehyde at 0–4 °C for about 4 h. During this time non-bone tissue around the knee bones were removed in order to allow a better and more rapid penetration of the fixative. The samples were immersed for 18–20 h (with two changes) in a post-fixative decalcifying solution (5% formic acid, 15% formaldehyde at 35% concentration, and 80% distilled water in volumes) at room temperature. Later, the tissues were dehydrated, clarified in xylene, and embedded in wax for the following histological and immunocytochemical study.
Sections along the longitudinal plane were obtained using a microtome (Reichert, Depew, NY, USA) at 6–9 μm in thickness, and collected on glass slide pre-coated with albumin-chromoalum. After de-waxing and dehydration, some sections were stained with 1% methylene blue and 1% Eosin, for the histological study. Other sections containing the femur and tibia, and in some cases also the fibula, were instead utilized for the immunocytochemical detection.
Sections were pre-incubated for 30 min with a 0.1 M Tris buffer solution at pH 7.6 containing 2% Bovine Serum Albumin (Sigma, St. Louis, MO, USA) and 5% Normal Goat Serum (Sigma), to saturate un-specific binding sites. Part of the sections were incubated with a mouse anti-5BrdU antibody at 1:100 dilution in the buffer for 5–6 h at room temperature and then rinsed in the buffer (in control sections the primary antibody was omitted from the solution). The 5BrdU-monoclonal antiserum (mouse G3G4) was developed by Dr. S.J. Kaufmann of the University of Illinois at Urbana, USA, and was provided by the Developmental Studies Hybridoma Bank, maintained by the University of Iowa, Iowa City, USA. This immunodetection aimed to detect long retaining labeled cells, indicated as putative stem cells.
A second mouse antibody against a chondroitin sulfate proteoglycan (MAB2029, Chemicon Int., Tamecula, CA, USA) was utilized at 1:150 dilution in buffer in order to detect chondroblasts in active phase of secretion, including possible regenerating chondroblasts. Other sections were instead stained for a rabbit antibody produced against a lizard telomerase-1 component. The latter antibody has labeled germinal and intestinal stem cells, and is indicative for stemness [
18]. The sections were later incubated with a goat anti-mouse or anti-rabbit TRITC-conjugated secondary antibody (Tetramethyl Rhodamine Isothiocyanate, Sigma) or FITC (Fluoresceine Isothiocyanate, Sigma) diluted 1:100 in the above buffer solution. The immunoreacted sections were observed and photographed using an Epifluorescence microscope (Euromex, Arnhem, The Netherlands) equipped with a Rhodamine (Euromex) or Fluorescein filter (Euromex).