BMP3 Alone and Together with TGF-β Promote the Differentiation of Human Mesenchymal Stem Cells into a Nucleus Pulposus-Like Phenotype
Abstract
:1. Introduction
2. Results
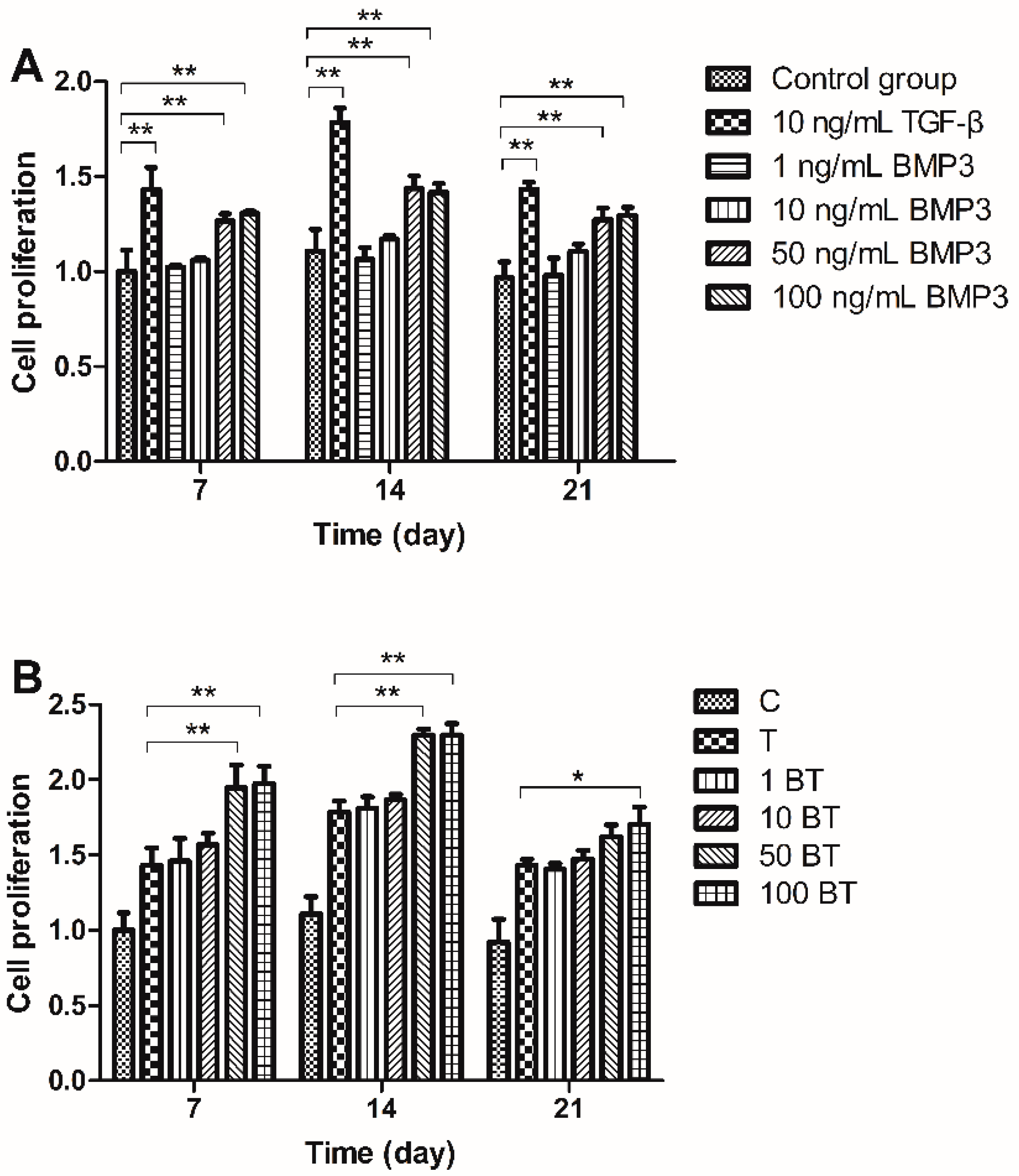
2.1. Cell Proliferation
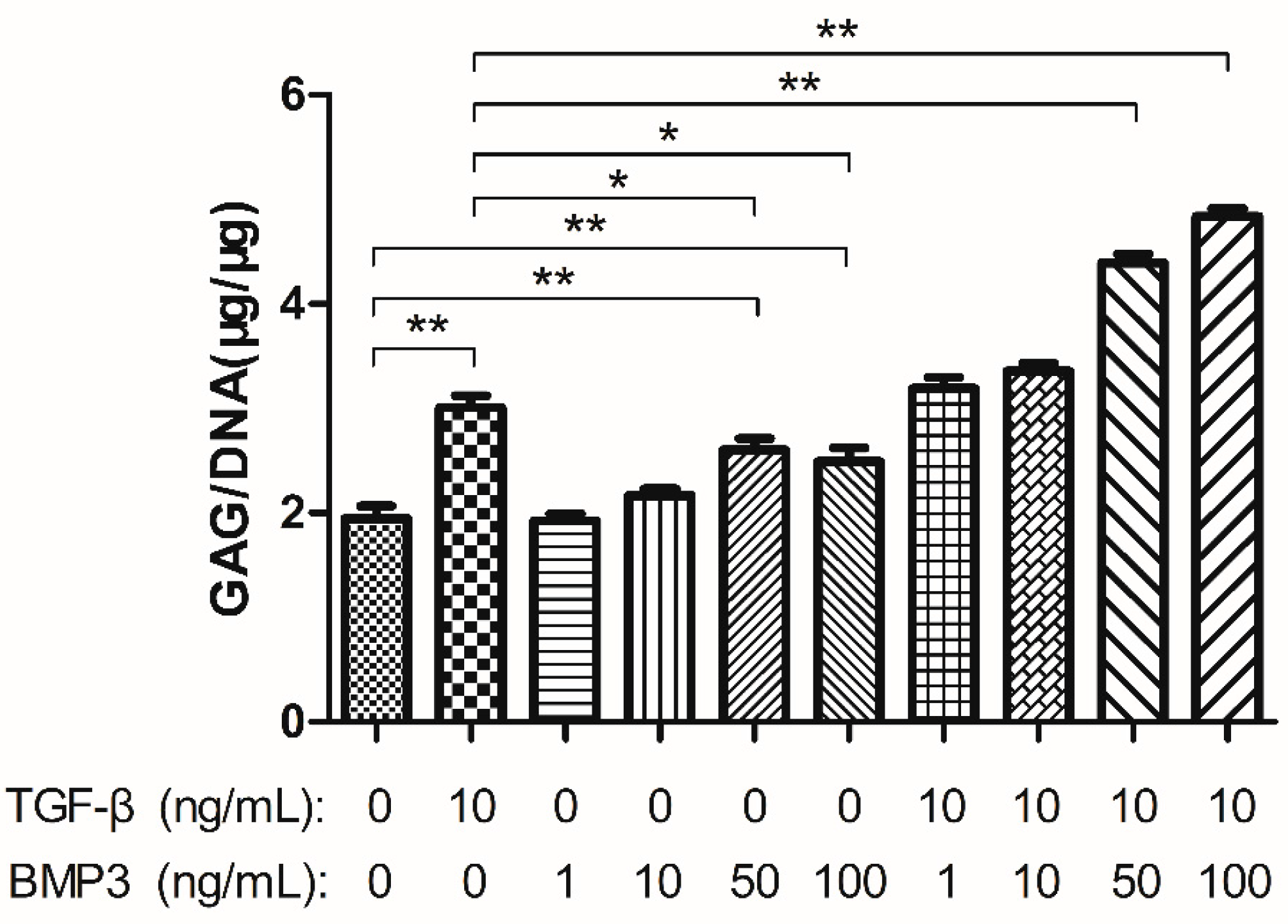
2.2. Glycosaminoglycan (GAG) Content
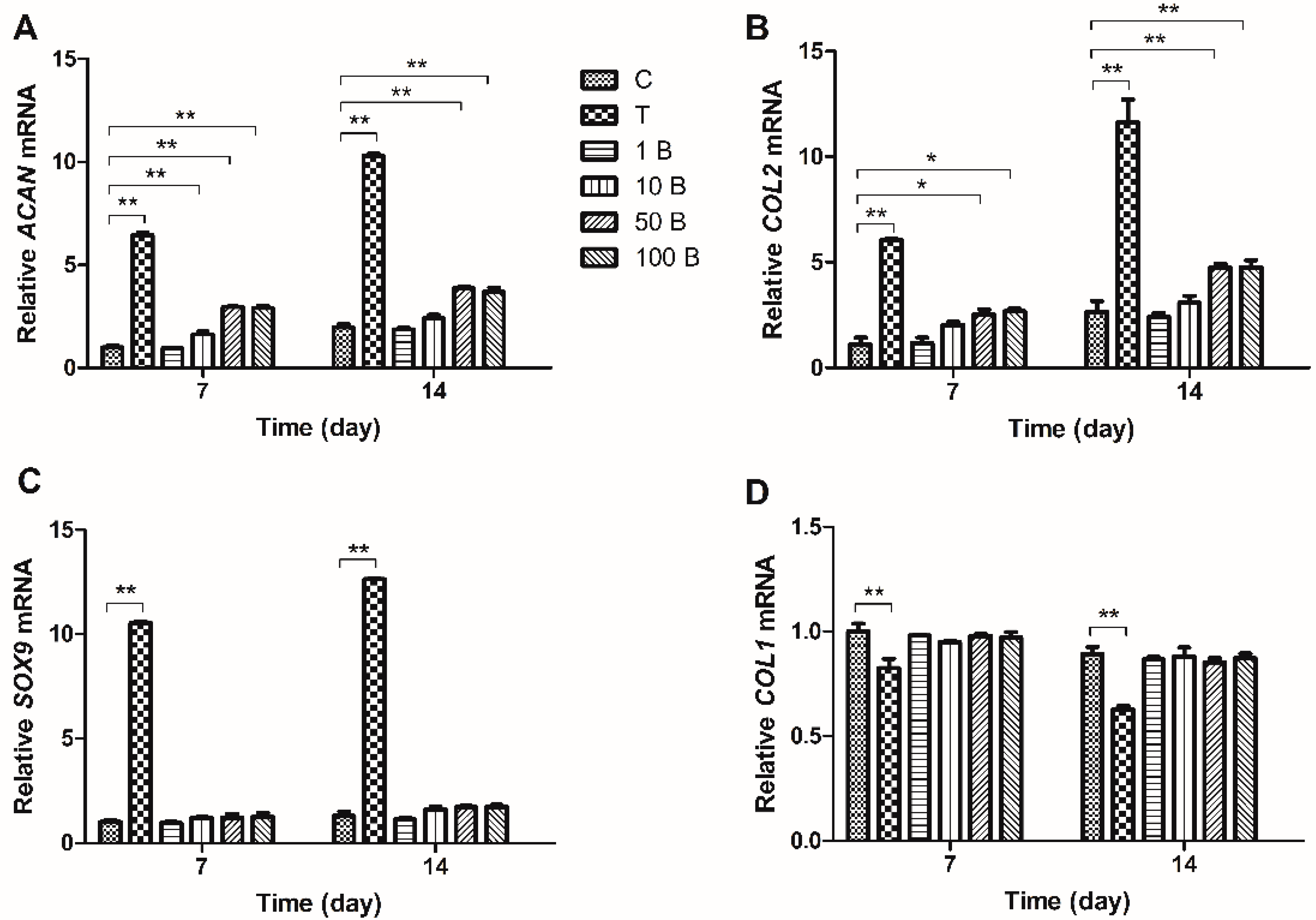
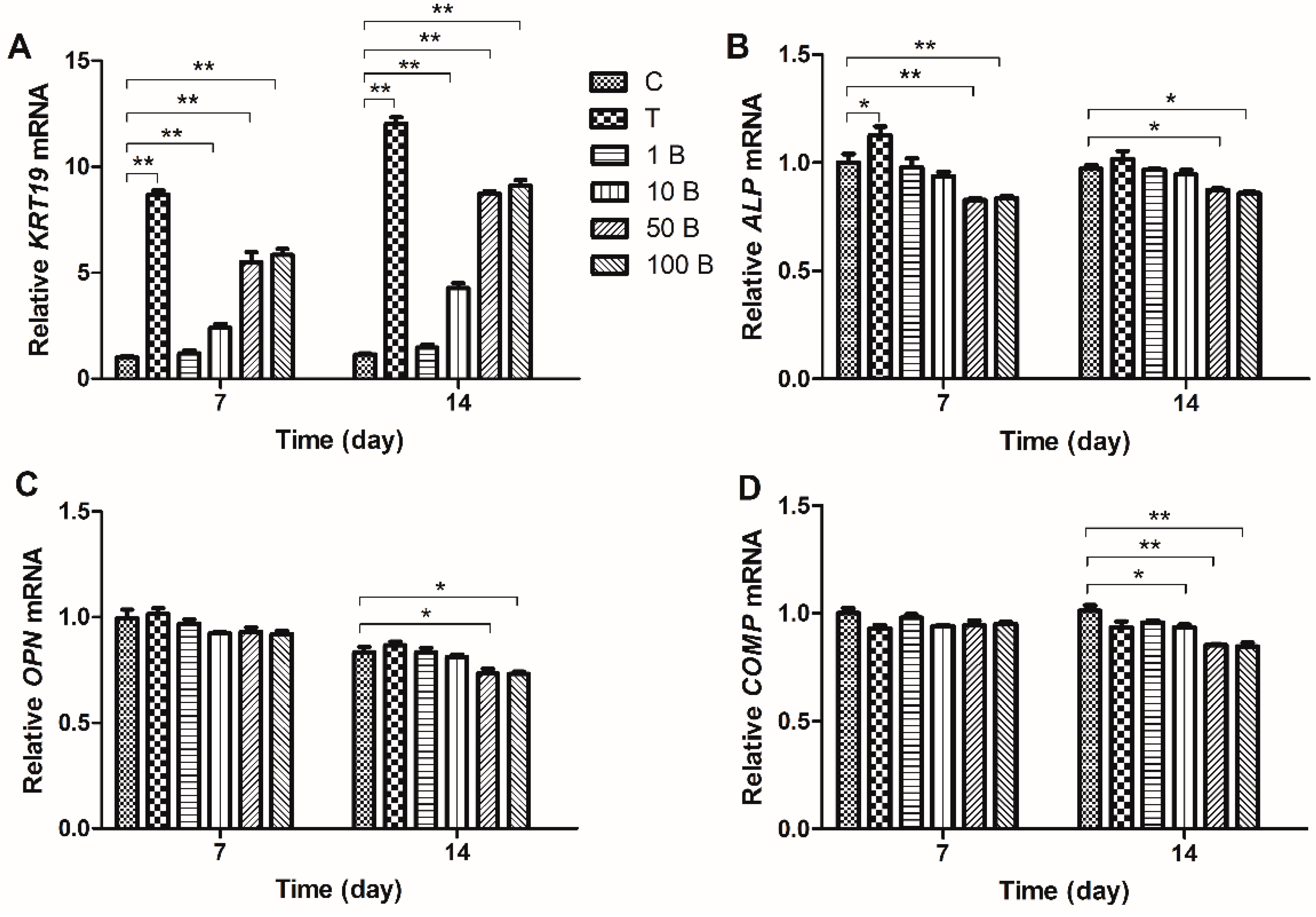
2.3. Gene Expression Analysis


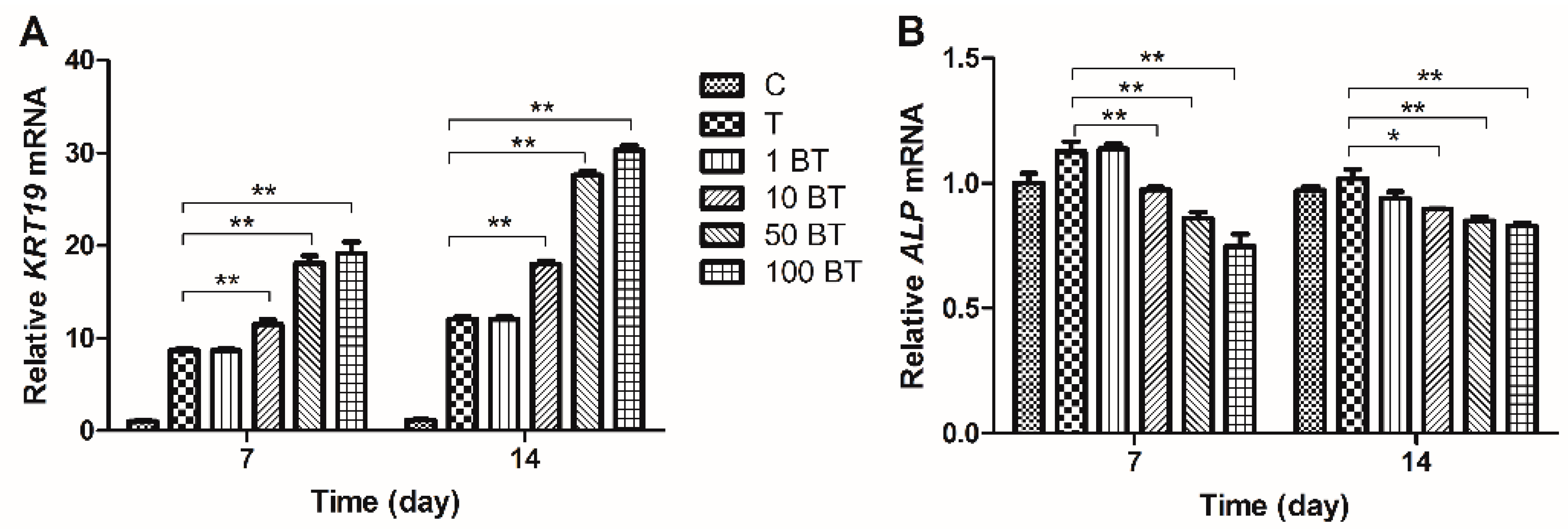
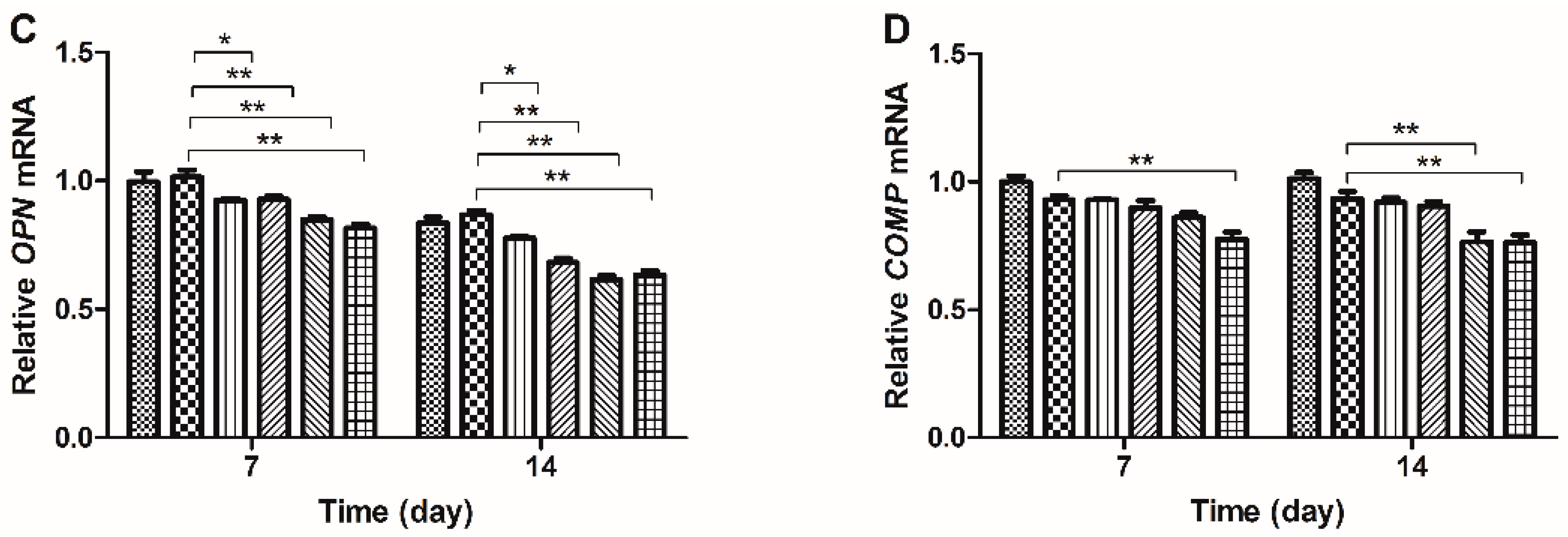
2.4. Western Blot Results
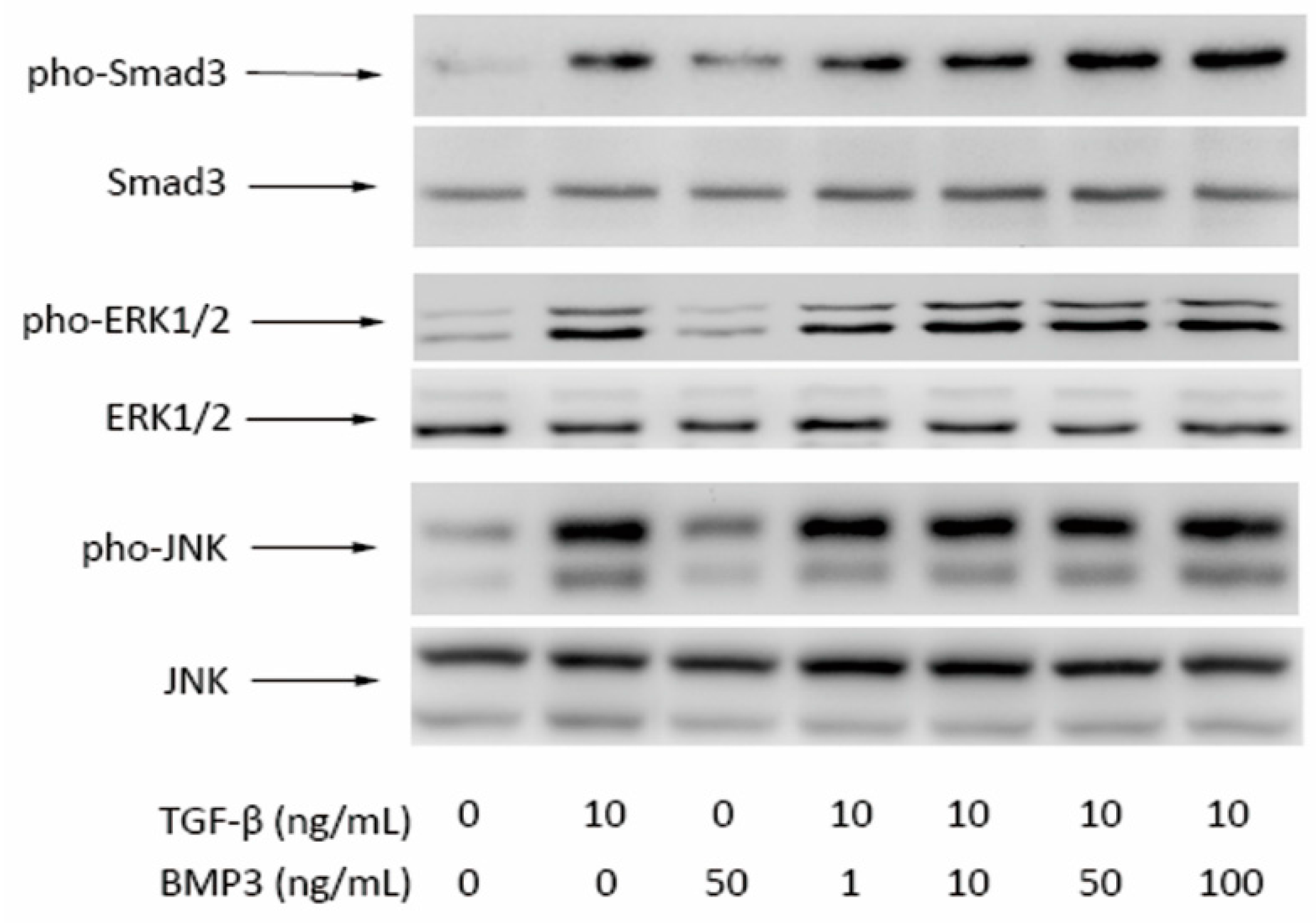
3. Discussion
4. Experimental Section
4.1. Cell Culture
4.2. Pellets and Cell Differentiation
4.3. Measurement of Cellular Proliferation
4.4. Measurement of Glycosaminoglycan Synthesis
4.5. Real Time Quantitative-PCR
| Gene | Primer Nucleotide Sequence (5′ to 3′) | Product Size (bp) |
|---|---|---|
| 18S | F-ATCCTCAGTGAGTTCTCCCG | 106 |
| R-CTTTGCCATCACTGCCATTA | ||
| ACAN | F-AGAATCAAGTGGAGCCGTGT | 115 |
| R-GGTAGTTGGGCAGTGAGACC | ||
| SOX9 | F-AGCGAACGCACATCAAGAC | 129 |
| R-CTGTAGGCGATCTGTTGGGG | ||
| KRT19 | F-GATAGTGAGCGGCAGAATCA | 178 |
| R-CCTCCAAAGGACAGCAGAAG | ||
| COL2A1 | F-CATCCCACCCTCTCACAGTT | 151 |
| R-ACCAGTTAGTTTCCTGCCTCTG | ||
| COL1A1 | F-AGTCTGTCCTGCGTCCTCTG | 183 |
| R-TGTTTGGGTCATTTCCACAT | ||
| ALP | F-TTTATAAGGCGGCGGGGGT | 142 |
| R-TTAACTGATGTTCCAATCCTGCG | ||
| COMP | F-CAACCAGGGAAGGGAGATCG | 130 |
| R-CGCATAGTCGTCATCCGTGA | ||
| OPN | F-AGCAGCTTTACAACAAATACCCAG | 100 |
| R-TTACTTGGAAGGGTCTGTGGG |
4.6. Western Blot Analysis
4.7. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Eyring, E.J. The biochemistry and physiology of the intervertebral disk. Clin. Orthop. Relat. Res. 1969, 67, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Trout, J.J.; Buckwalter, J.A.; Moore, K.C. Ultrastructure of the human intervertebral disc: II. Cells of the nucleus pulposus. Anat. Rec. 1982, 204, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yan, W.; Setton, L.A. Molecular phenotypes of notochordal cells purified from immature nucleus pulposus. Eur. Spine J. 2006, 15, S303–S311. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.M.; Hughes, N.; Hunt, J.A.; Freemont, A.J.; Hoyland, J.A. Human mesenchymal stem cell differentiation to NP-like cells in chitosan-glycerophosphate hydrogels. Biomaterials 2008, 29, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Risbud, M.V.; Albert, T.J.; Guttapalli, A.; Vresilovic, E.J.; Hillibrand, A.S.; Vaccaro, A.R.; Shapiro, I.M. Differentiation of mesenchymal stem cells towards a nucleus pulposus-like phenotype in vitro: Implications for cell-based transplantation therapy. Spine 2004, 29, 2627–2632. [Google Scholar] [CrossRef] [PubMed]
- Walsh, A.J.; Bradford, D.S.; Lotz, J.C. In vivo growth factor treatment of degenerated intervertebral discs. Spine 2004, 29, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Z.; Rui, Y.F.; Lu, J.; Wang, C. Cell and molecular biology of intervertebral disc degeneration: Current understanding and implications for potential therapeutic strategies. Cell Prolif. 2014, 47, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Xiong, W.; Qiu, M.; Lv, Y.; Li, Y.; Li, F. Differentiation of mesenchymal stem cells towards a nucleus pulposus-like phenotype utilizing simulated microgravity in vitro. J. Huazhong Univ. Sci. Technol. Med. Sci. 2011, 31, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Morigele, M.; Shao, Z.; Zhang, Z.; Kaige, M.; Zhang, Y.; Qiang, W.; Yang, S. TGF-β induces a nucleus pulposus-like phenotype in Notch 1 knockdown rabbit bone marrow mesenchymal stem cells. Cell Biol. Int. 2013, 37, 820–825. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, B.; Hering, T.M.; Caplan, A.I.; Goldberg, V.M.; Yoo, J.U. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp. Cell Res. 1998, 238, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Jiang, S.; Hantash, B.M. Transforming growth factor β1 induces osteogenic differentiation of murine bone marrow stromal cells. Tissue Eng. Part A 2010, 16, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Bahamonde, M.E.; Lyons, K.M. BMP3: To be or not to be a BMP. J. Bone Jt. Surg. 2001, 83 (Suppl. 1), S56–S62. [Google Scholar]
- Sampath, T.K.; Muthukumaran, N.; Reddi, A.H. Isolation of osteogenin, an extracellular matrix-associated, bone-inductive protein, by heparin affinity chromatography. Proc. Natl. Acad. Sci. USA 1987, 84, 7109–7113. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Alidadi, S.; Moshiri, A.; Bigham-Sadegh, A. Bone morphogenetic proteins: A powerful osteoinductive compound with non-negligible side effects and limitations. BioFactors 2014, 40, 459–481. [Google Scholar] [CrossRef] [PubMed]
- Daluiski, A.; Engstrand, T.; Bahamonde, M.E.; Gamer, L.W.; Agius, E.; Stevenson, S.L.; Cox, K.; Rosen, V.; Lyons, K.M. Bone morphogenetic protein-3 is a negative regulator of bone density. Nat. Genet. 2001, 27, 84–88. [Google Scholar] [PubMed]
- Stewart, A.; Guan, H.; Yang, K. BMP-3 promotes mesenchymal stem cell proliferation through the TGF-β/activin signaling pathway. J. Cell. Physiol. 2010, 223, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Massague, J.; Seoane, J.; Wotton, D. Smad transcription factors. Genes Dev. 2005, 19, 2783–2810. [Google Scholar] [CrossRef] [PubMed]
- Risbud, M.V.; di Martino, A.; Guttapalli, A.; Seghatoleslami, R.; Denaro, V.; Vaccaro, A.R.; Albert, T.J.; Shapiro, I.M. Toward an optimum system for intervertebral disc organ culture: TGF-β 3 enhances nucleus pulposus and anulus fibrosus survival and function through modulation of TGF-β-r expression and ERK signaling. Spine 2006, 31, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Derynck, R.; Zhang, Y.E. Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature 2003, 425, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Jin, X.; Hu, J.; Ma, H.; Gupte, M.J.; Liu, H.; Ma, P.X. Effects of hypoxias and scaffold architecture on rabbit mesenchymal stem cell differentiation towards a nucleus pulposus-like phenotype. Biomaterials 2011, 32, 8182–8189. [Google Scholar] [CrossRef] [PubMed]
- Rutges, J.; Creemers, L.B.; Dhert, W.; Milz, S.; Sakai, D.; Mochida, J.; Alini, M.; Grad, S. Variations in gene and protein expression in human nucleus pulposus in comparison with annulus fibrosus and cartilage cells: Potential associations with aging and degeneration. Osteoarthr. Cartil. 2010, 18, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Dong, Q.; Wang, Y.; Feng, Q.; Zhou, P.; Ou, X.; Meng, Q.; He, T.; Luo, J. Hedgehog signaling is involved in the BMP9-induced osteogenic differentiation of mesenchymal stem cells. Int. J. Mol. Med. 2015, 36, 1641–1650. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.; Lin, S.L.; Wen, H.; Deng, H. [crosstalk between canonical TGF-β/Smad and Wnt/β-catenin signaling pathway]. Zhejiang Da Xue Xue Bao Yi Xue Ban 2013, 42, 591–596. [Google Scholar] [PubMed]
- Zhang, T.; Wen, F.; Wu, Y.; Goh, G.S.; Ge, Z.; Tan, L.P.; Hui, J.H.; Yang, Z. Cross-talk between TGF-β/Smad and integrin signaling pathways in regulating hypertrophy of mesenchymal stem cell chondrogenesis under deferral dynamic compression. Biomaterials 2015, 38, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Ng, F.; Boucher, S.; Koh, S.; Sastry, K.S.; Chase, L.; Lakshmipathy, U.; Choong, C.; Yang, Z.; Vemuri, M.C.; Rao, M.S.; et al. PDGF, TGF-β, and FGF signaling is important for differentiation and growth of mesenchymal stem cells (MSCs): Transcriptional profiling can identify markers and signaling pathways important in differentiation of mscs into adipogenic, chondrogenic, and osteogenic lineages. Blood 2008, 112, 295–307. [Google Scholar] [PubMed]
- Kulterer, B.; Friedl, G.; Jandrositz, A.; Sanchez-Cabo, F.; Prokesch, A.; Paar, C.; Scheideler, M.; Windhager, R.; Preisegger, K.H.; Trajanoski, Z. Gene expression profiling of human mesenchymal stem cells derived from bone marrow during expansion and osteoblast differentiation. BMC Genom. 2007, 8. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Hall, R.; Pelinkovic, D.; Cassinelli, E.; Usas, A.; Gilbertson, L.; Huard, J.; Kang, J. New use of a three-dimensional pellet culture system for human intervertebral disc cells: Initial characterization and potential use for tissue engineering. Spine 2001, 26, 2316–2322. [Google Scholar] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative pcr and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Tao, Y.; Liang, C.; Zhang, Y.; Li, H.; Chen, Q. BMP3 Alone and Together with TGF-β Promote the Differentiation of Human Mesenchymal Stem Cells into a Nucleus Pulposus-Like Phenotype. Int. J. Mol. Sci. 2015, 16, 20344-20359. https://doi.org/10.3390/ijms160920344
Zhou X, Tao Y, Liang C, Zhang Y, Li H, Chen Q. BMP3 Alone and Together with TGF-β Promote the Differentiation of Human Mesenchymal Stem Cells into a Nucleus Pulposus-Like Phenotype. International Journal of Molecular Sciences. 2015; 16(9):20344-20359. https://doi.org/10.3390/ijms160920344
Chicago/Turabian StyleZhou, Xiaopeng, Yiqing Tao, Chengzhen Liang, Yujie Zhang, Hao Li, and Qixin Chen. 2015. "BMP3 Alone and Together with TGF-β Promote the Differentiation of Human Mesenchymal Stem Cells into a Nucleus Pulposus-Like Phenotype" International Journal of Molecular Sciences 16, no. 9: 20344-20359. https://doi.org/10.3390/ijms160920344





