Interaction of High Flash Point Electrolytes and PE-Based Separators for Li-Ion Batteries
Abstract
:1. Introduction
2. Results and Discussion
2.1. Electrolyte Mixtures
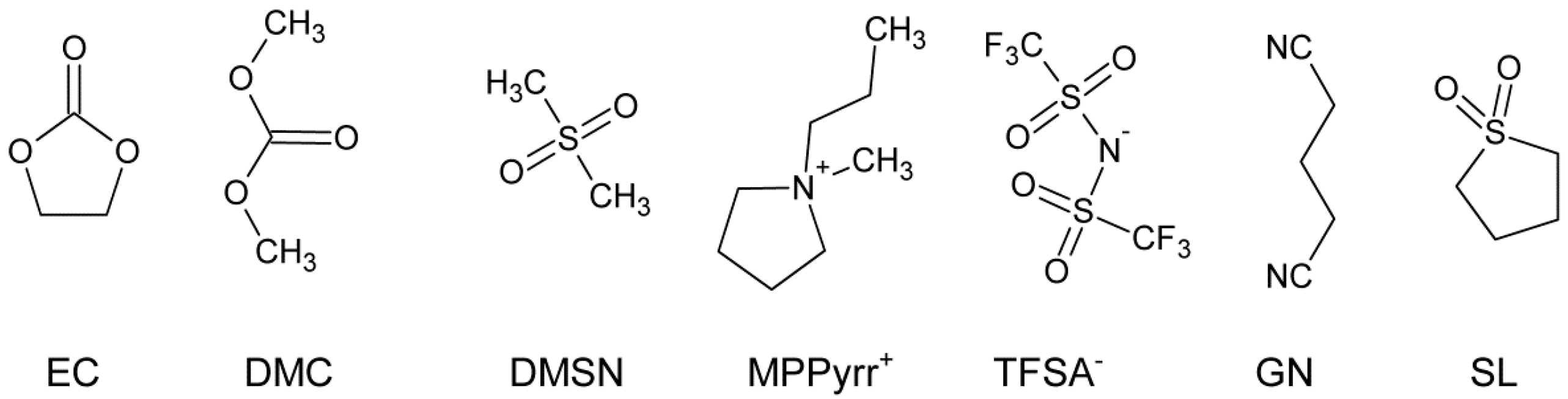
| Sample | Solvent | Ratio (wt:wt) | Conducting Salt (CS) | c (CS) [a] mol∙kg−1 |
|---|---|---|---|---|
| M-1 | EC/DMC | 50:50 | LiPF6 | 0.787 |
| M-2 | EC/DMSN [b] | 79.8:20 | LiPF6 | 0.75 |
| M-3 | EC/DMSN [b] | 79.8:20 | LiTFSA | 0.75 |
| M-4 | MPPyrr-TFSA | 100 | LiTFSA | 0.75 |
| M-5 | GN | 100 | LiTFSA | 0.75 |
| M-6 | SL | 100 | LiTFSA | 0.75 |
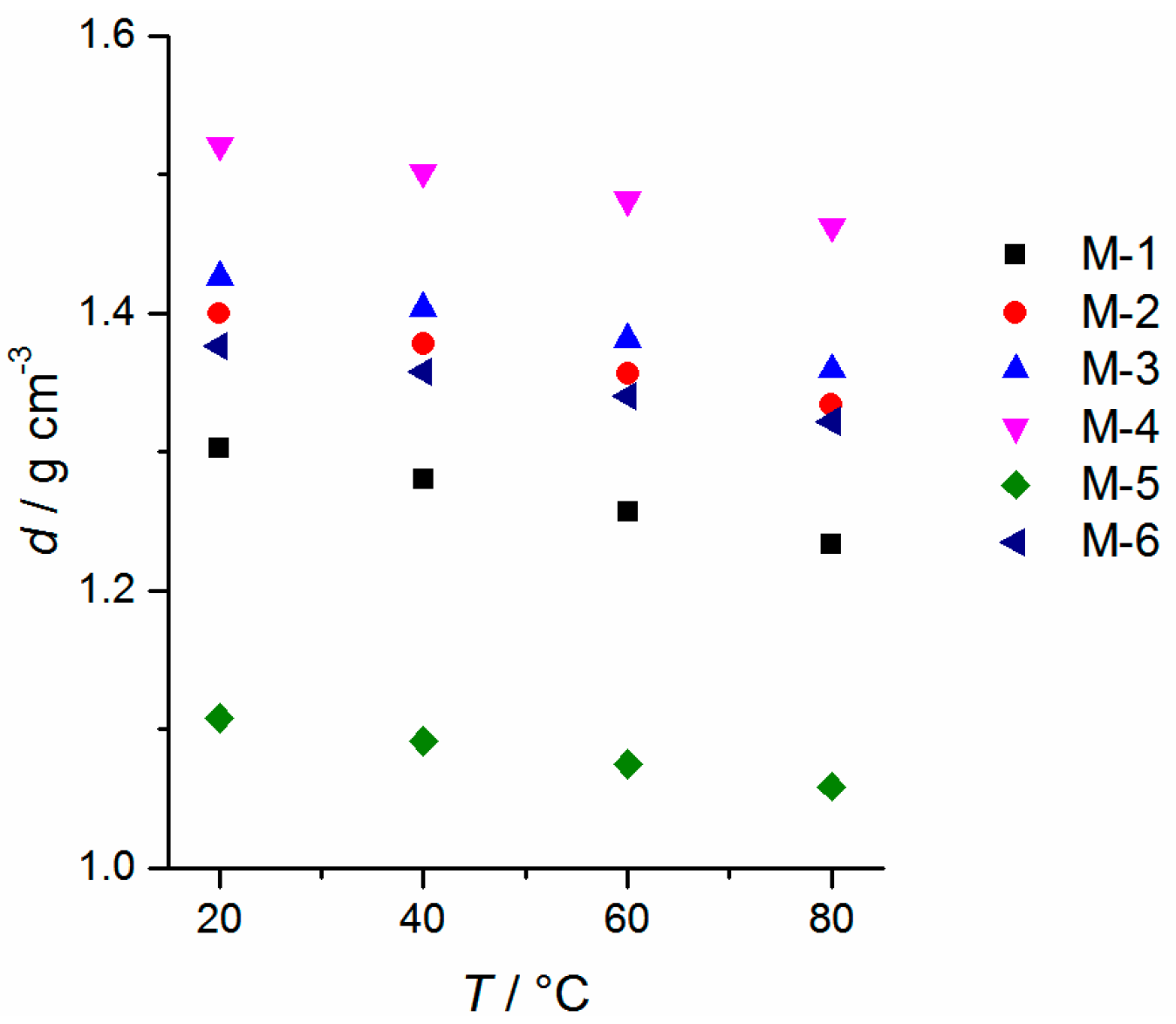
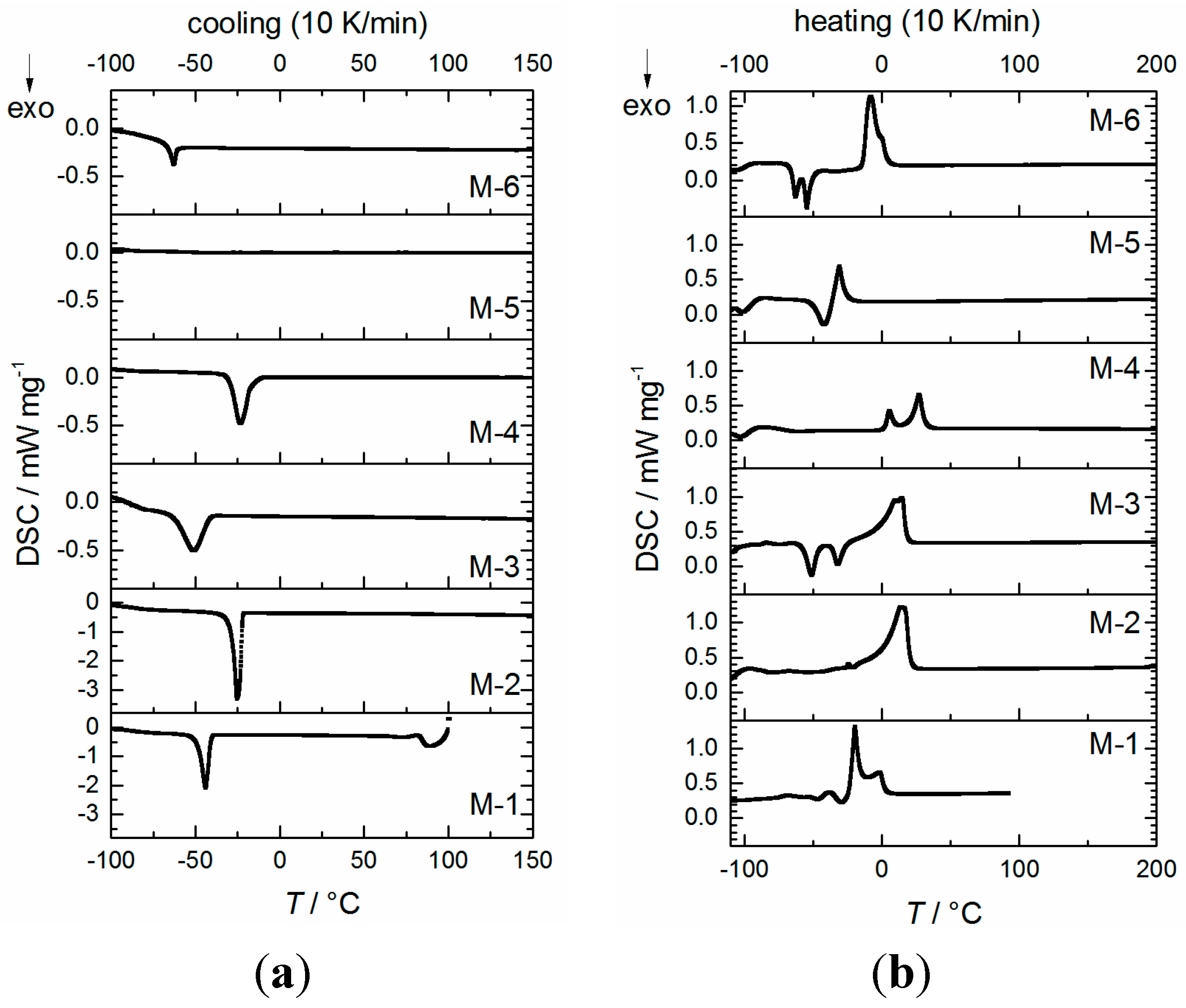
2.2. Physicochemical Properties
| Sample | Tc/°C (DSC, onset) [a] | Tm/°C (DSC, peak max.) [a] | D (25 °C) g∙cm−3 ±0.0005 | η (20 °C) mPa∙s | κ [b] (20 °C) mS∙cm−1 (n = 3) | γL[b] (25 ± 2 °C) mN∙m−1 (n = 25) | Eox (5 mV∙s−1) V vs. Li/Li+ (±0.1 V) | fp [c] (°C) |
|---|---|---|---|---|---|---|---|---|
| M-1 | −39.4 | −20.5 | 1.3030 | 4.4 ± 0.1 | 10.3 ± 0.5 | 29.4 ± 0.2 | 5.6 | 24 |
| M-2 | −20.1 | 14.6 | 1.3996 | 12.1 ± 0.2 | 6.1 ± 0.3 | 38.1 ± 0.3 | 5.6 | 142 |
| M-3 | −39.4 | 12.4 | 1.4260 | 13.4 ± 0.2 | 5.3 ± 0.2 | 34.3 ± 0.1 | 5.4 | 142 |
| M-4 | −12.1 | 27.1 | 1.5210 | 399.8 ± 0.8 | 0.53 ± 0.05 | 23.3 ± 0.2 | 5.5 | 353 [d] |
| M-5 | −42.3 [e] | −30.4 | 1.1082 | 19.0 ± 0.2 | 2.4 ± 0.1 | 32.9 ± 0.1 | 5.6 | 112 [f] |
| M-6 | −59.3 | −8.0 | 1.3760 | 37.8 ± 0.2 | 2.1 ± 0.1 | 33.1 ± 0.4 | 5.0 | 151 |

2.3. Separators and SEM Imaging
| Separator | Thickness [a] µm | Permeability [a,b] s/100 mL | Porosity [a,c] % | Heat Shrinkage [c] % at 105 °C, 1 h | |
|---|---|---|---|---|---|
| COD-20 | 19.1 | 237.6 | 42.3 | 2.4 | 42.4 |
| COATED | 20.6 | 241.0 | n/a [d] | 1.5 | 23.7 |


2.4. Contact Angle Measurements and Drop Shape Analysis of the Separators
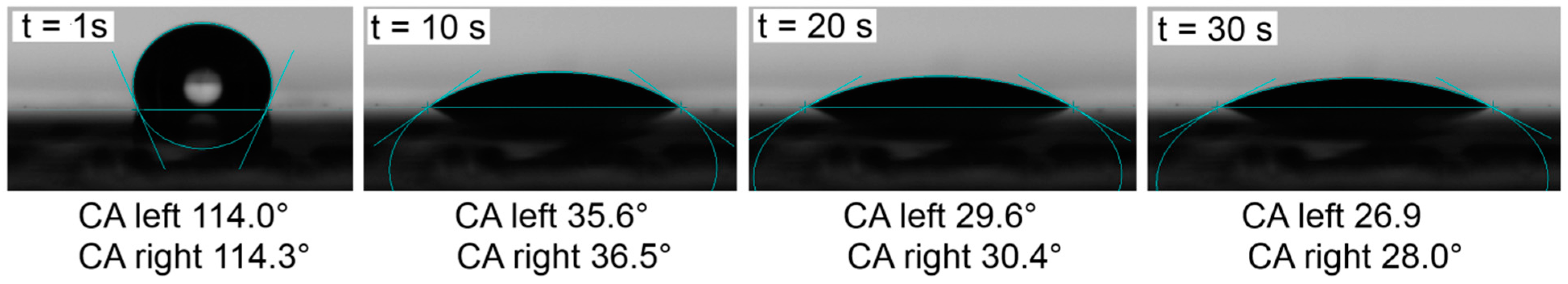
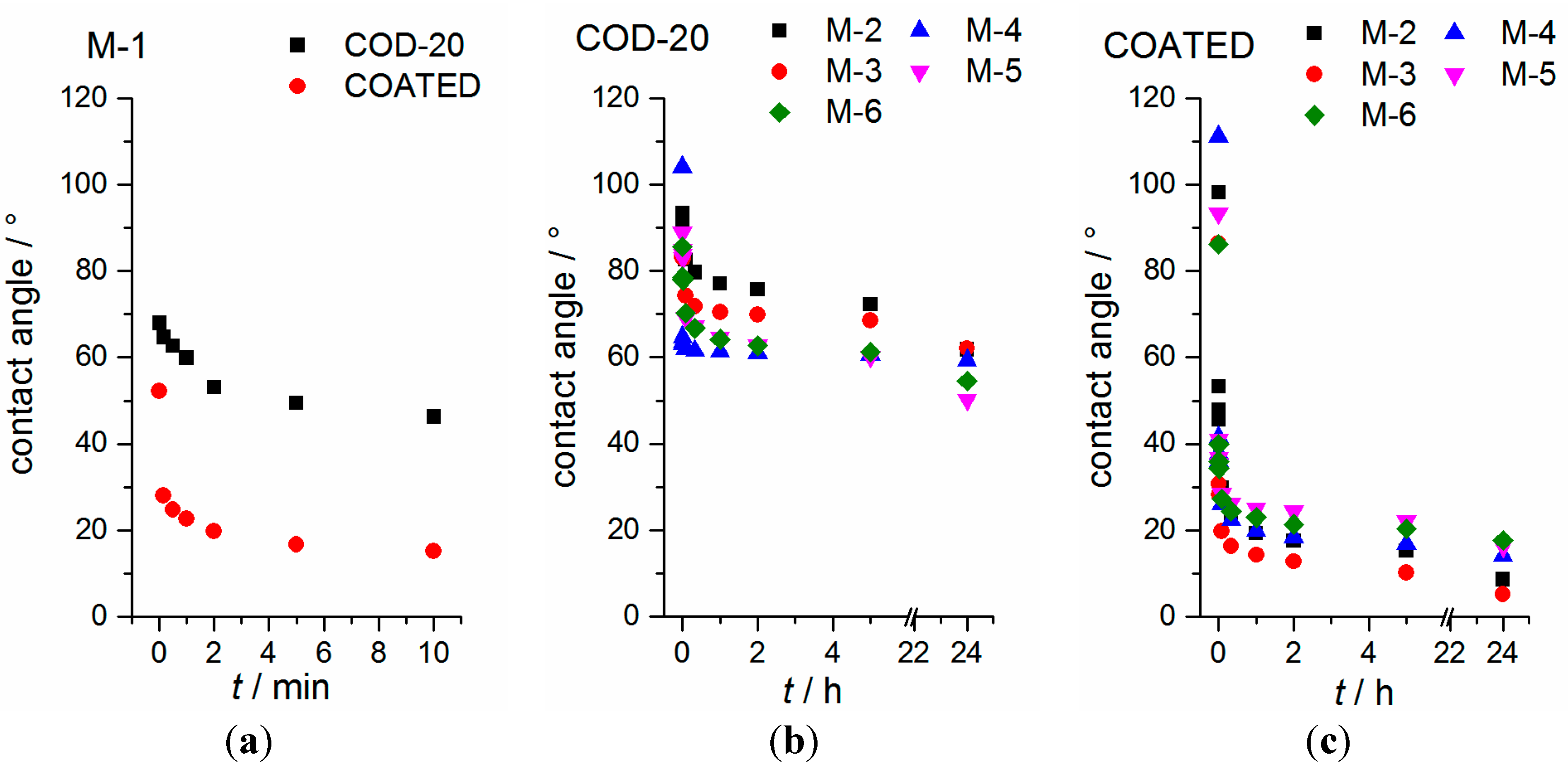
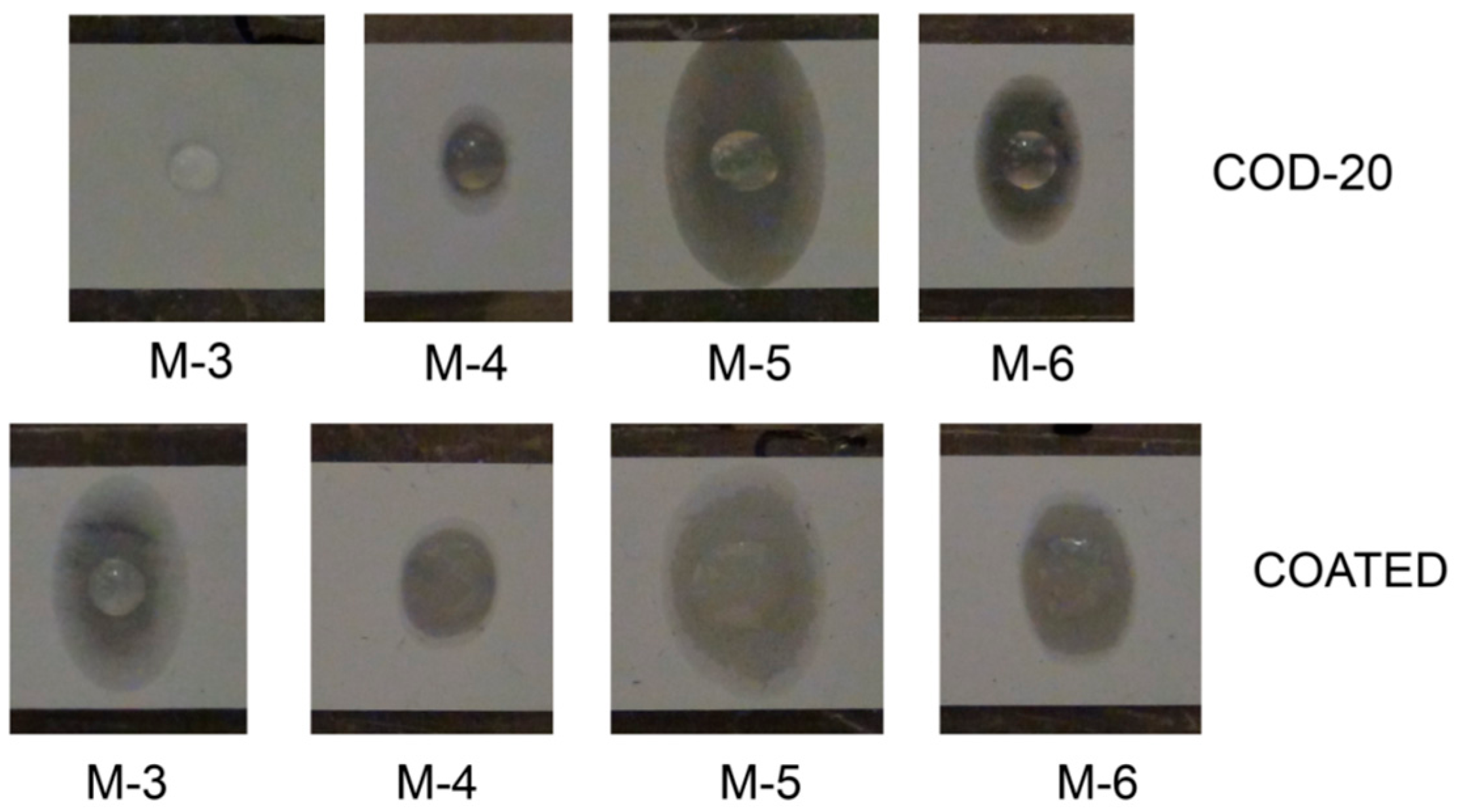
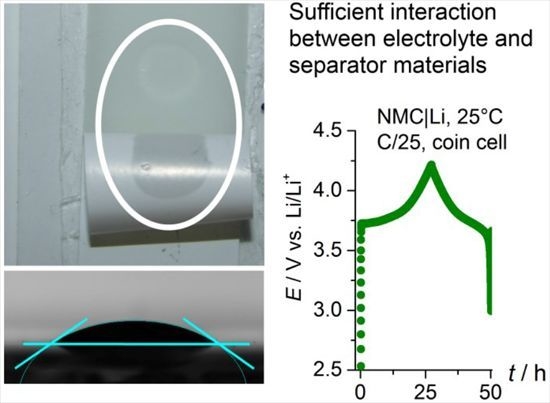
2.5. Cell Cycling (NMC|Li Coin Cells) and Separator Penetration
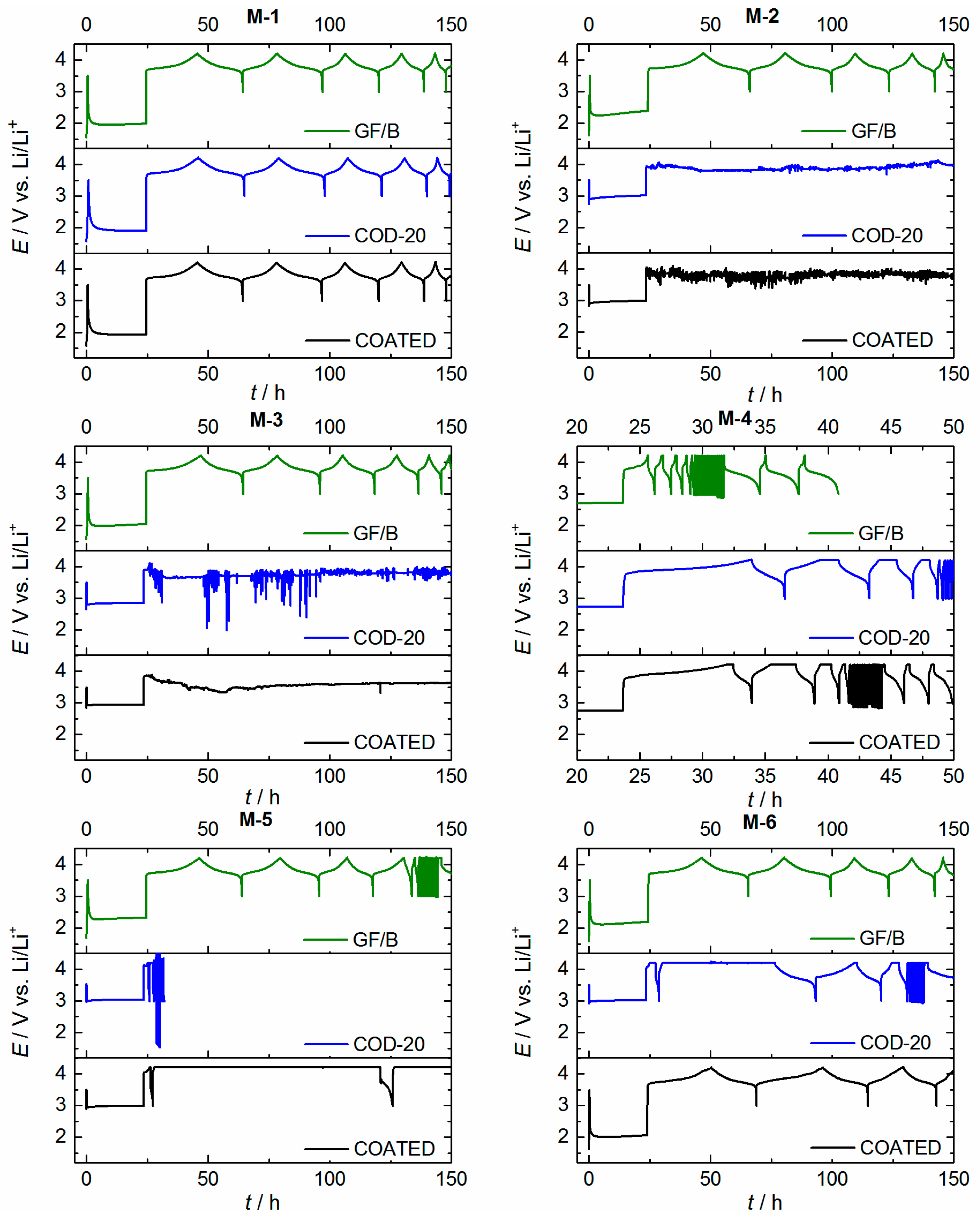
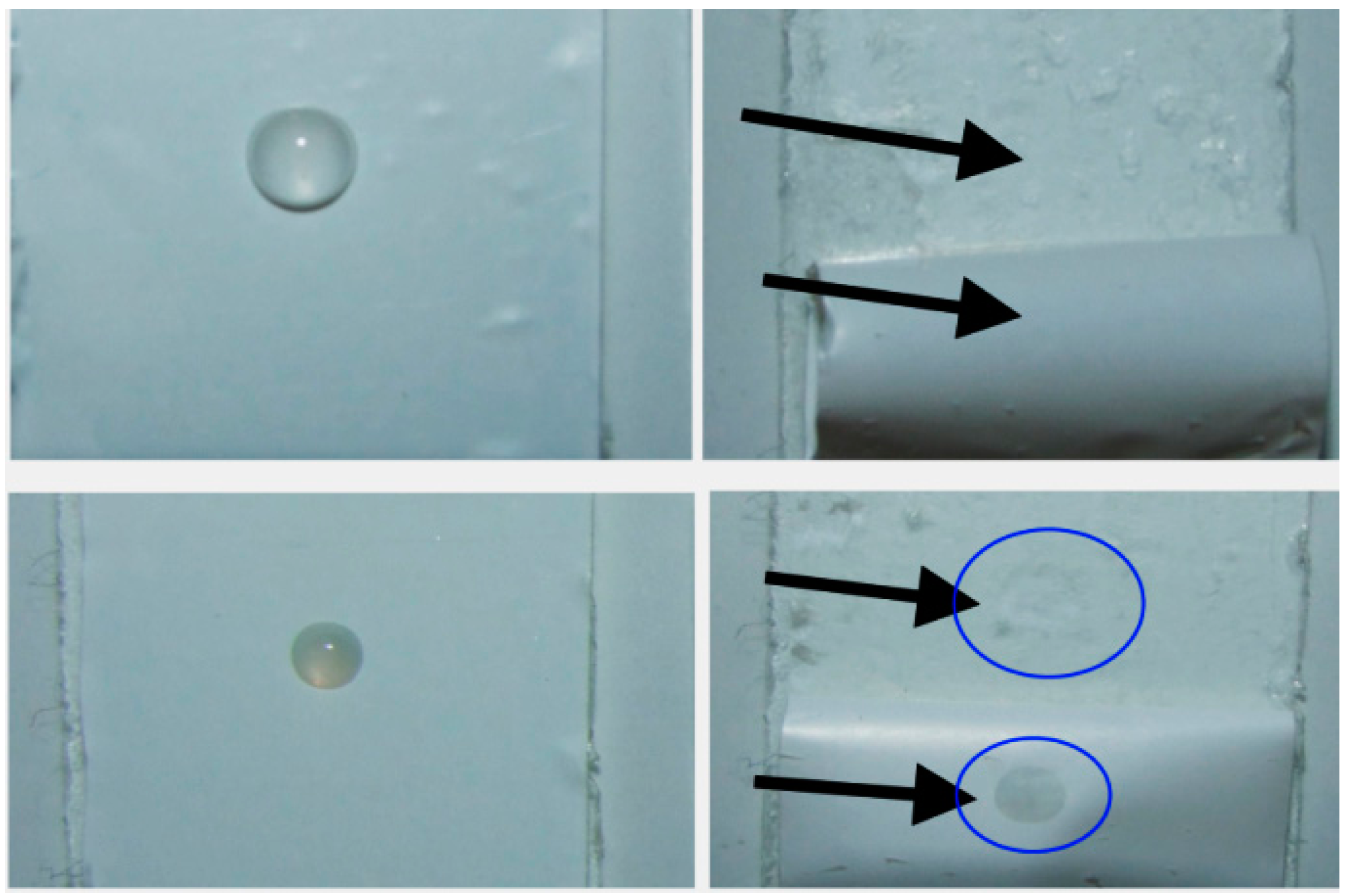
| Sample | COD-20 | COATED | COATED, Silanized [b] |
|---|---|---|---|
| M-1 | ++ | ++ | ++ |
| M-2 | − | − | − |
| M-3 | − | − | − |
| M-4 | ++ | ++ | ++ |
| M-5 | − | − | − |
| M-6 | − | o | + |
3. Experimental Section
| Solvent [a] | mp[b]/°C (Solvent) | bp[b]/°C (Solvent) | Supplier | Purity | Purification for This Study |
|---|---|---|---|---|---|
| EC | 35–37 | 244–245 | Sigma-Aldrich | 99% anhydrous | drying [c] |
| DMSN | 108–10 | 238 | Alfa Aesar | 99% | sublimation [d] |
| MPPyrr-TFSA | 12 | NN | Iolitec | 99% | drying [c] |
| GN | −29 | 286–288 | Aldrich | 99% | drying [c] |
| SL | 25–28 | 285 | Aldrich | 99% | drying [c] |
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Xu, K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem. Rev. 2004, 104, 4303–4417. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.S. A review on the separators of liquid electrolyte li-ion batteries. J. Power Sources 2007, 164, 351–364. [Google Scholar] [CrossRef]
- Fu, Z.; Feng, H.; Sun, C.; Xiang, X.; Wu, W.; Luo, J.; Hu, Q.; Feng, A.; Li, W. Influence of solvent type on porosity structure and properties of polymer separator for the Li-ion batteries. J. Solid State Electrochem. 2013, 17, 2167–2172. [Google Scholar] [CrossRef]
- Leitner, K.W.; Wolf, H.; Garsuch, A.; Chesneau, F.; Schulz-Dobrick, M. Electroactive separator for high voltage graphite/lini0.5Mn1.5O4 lithium ion batteries. J. Power Sources 2013, 244, 548–551. [Google Scholar] [CrossRef]
- Sun, Z-X.; Zhang, X.; Huang, B.; Ma, Y.-W. Effects of separator on the electrochemical performance of electrical double-layer capacitor and hybrid battery-supercapacitor. Acta Phys. Chim. Sin. 2014, 30, 485–491. [Google Scholar]
- Herle, S.P.; Gordon, J.G. Ceramic Coating on Battery Separators. Patent WO2014093519 A1, PCT/US2013/074449, 19 June 2014. [Google Scholar]
- Shin, W.-K.; Kim, D.-W. High performance ceramic-coated separators prepared with lithium ion-containing Sio2 particles for lithium-ion batteries. J. Power Sources 2013, 226, 54–60. [Google Scholar] [CrossRef]
- Imachi, N.; Nakamura, H.; Fujitani, S.; Yamaki, J. Insertion of an insulating layer between cathode and separator for improving storage characteristics of Li-ion batteries. J. Electrochem. Soc. 2012, 159, A269–A272. [Google Scholar] [CrossRef]
- Lee, T.; Kim, W.-K.; Lee, Y.; Ryou, M.-H.; Lee, Y. Effect of Al2O3 coatings prepared by RF sputtering on polyethylene separators for high-power lithium ion batteries. Macromol. Res. 2014, 22, 1190–1195. [Google Scholar] [CrossRef]
- Ko, Y.; Yoo, H.; Kim, J. Curable polymeric binder-ceramic composite-coated superior heat-resistant polyethylene separator for lithium ion batteries. RSC Adv. 2014, 4, 19229. [Google Scholar] [CrossRef]
- Shin, W.-K.; Yoo, J.-H.; Kim, D.-W. Surface-modified separators prepared with conductive polymer and aluminum fluoride for lithium-ion batteries. J. Power Sources 2015, 279, 737–744. [Google Scholar] [CrossRef]
- Kirchhöfer, M.; von Zamory, J.; Paillard, E.; Passerini, S. Separators for Li-ion and Li-metal battery including ionic liquid based electrolytes based on the TFSI− and FSI− anions. Int. J. Mol. Sci. 2014, 15, 14868–14890. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Zhang, P.; Chen, L.; Yang, P.; Zhao, J. Effect of a thin ceramic-coating layer on thermal and electrochemical properties of polyethylene separator for lithium-ion batteries. J. Power Sources 2014, 270, 547–553. [Google Scholar] [CrossRef]
- Guan, H.-Y.; Lian, F.; Ren, Y.; Wen, Y.; Pan, X.-R.; Sun, J.-L. Comparative study of different membranes as separators for rechargeable lithium-ion batteries. Int. J. Miner. Metall. Mater. 2013, 20, 598–603. [Google Scholar] [CrossRef]
- Song, J.; Ryou, M.-H.; Son, B.; Lee, J.-N.; Lee, D.J.; Lee, Y.M.; Choi, J.W.; Park, J.-K. Co-polyimide-coated polyethylene separators for enhanced thermal stability of lithium ion batteries. Electrochim. Acta 2012, 85, 524–530. [Google Scholar] [CrossRef]
- Krämer, E.; Passerini, S.; Winter, M. Dependency of aluminum collector corrosion in lithium ion batteries on the electrolyte solvent. ECS Electrochem. Lett. 2012, 1, C9–C11. [Google Scholar] [CrossRef]
- Isken, P.; Dippel, C.; Schmitz, R.; Schmitz, R.W.; Kunze, M.; Passerini, S.; Winter, M.; Lex-Balducci, A. High flash point electrolyte for use in lithium-ion batteries. Electrochim. Acta 2011, 56, 7530–7535. [Google Scholar] [CrossRef]
- Xu, K. Electrolytes and interphases in Li-ion batteries and beyond. Chem. Rev. 2014, 114, 11503–11618. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, A.; Migeot, M.; Thißen, E.; Schulz, M.; Heinzmann, R.; Indris, S.; Bergfeldt, T.; Lei, B.; Ziebert, C.; Hanemann, T. Electrolyte mixtures based on ethylene carbonate and dimethyl sulfone for Li-ion batteries with improved safety characteristics. ChemSusChem 2015, 8, 1892–1900. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, A.; Schulz, M.; Indris, S.; Heinzmann, R.; Hanemann, T. Mixtures of ionic liquid and sulfolane as electrolytes for Li-ion batteries. Electrochim. Acta 2014, 147, 704–711. [Google Scholar] [CrossRef]
- Indris, S.; Heinzmann, R.; Schulz, M.; Hofmann, A. Ionic liquid based electrolytes: Correlating Li diffusion coefficients and battery performance. J. Electrochem. Soc. 2014, 161, A2036–A2041. [Google Scholar] [CrossRef]
- Kühnel, R.S.; Böckenfeld, N.; Passerini, S.; Winter, M.; Balducci, A. Mixtures of ionic liquid and organic carbonate as electrolyte with improved safety and performance for rechargeable lithium batteries. Electrochim. Acta 2011, 56, 4092–4099. [Google Scholar] [CrossRef]
- Sato, T.; Maruo, T.; Marukane, S.; Takagi, K. Ionic liquids containing carbonate solvent as electrolytes for lithium ion cells. J. Power Sources 2004, 138, 253–261. [Google Scholar] [CrossRef]
- Xiang, H.F.; Yin, B.; Wang, H.; Lin, H.W.; Ge, X.W.; Xie, S.; Chen, C.H. Improving electrochemical properties of room temperature ionic liquid (RTIL) based electrolyte for Li-ion batteries. Electrochim. Acta 2010, 55, 5204–5209. [Google Scholar] [CrossRef]
- Yang, B.; Li, C.; Zhou, J.; Liu, J.; Zhang, Q. Pyrrolidinium-based ionic liquid electrolyte with organic additive and LiTFSI for high-safety lithium-ion batteries. Electrochim. Acta 2014, 148, 39–45. [Google Scholar] [CrossRef]
- Alessandrini, F.; Appetecchi, G.B.; Conte, M.; Passerini, S. Ionic Liquid Based Electrolytes For High Energy Electrochemical Storage Devices, In Proceedings of the 208th Meeting of The Electrochemical Society, Los Angeles, CA, USA, 16–21 October 2005.
- Kim, G.T.; Jeong, S.S.; Xue, M.Z.; Balducci, A.; Winter, M.; Passerini, S.; Alessandrini, F.; Appetecchi, G.B. Development of ionic liquid-based lithium battery prototypes. J. Power Sources 2012, 199, 239–246. [Google Scholar] [CrossRef]
- Borodin, O.; Smith, G.D.; Henderson, W. Li+ cation environment, transport, and mechanical properties of the litfsi doped n-methyl-n-alkylpyrrolidinium+TFSI− ionic liquids. J. Phys. Chem. B 2006, 110, 16879–16886. [Google Scholar] [CrossRef] [PubMed]
- Wilson, G.J.; Hollenkamp, A.F.; Pandolfo, A.G. Resolving ambiguous naming for an ionic liquid anion. Chem. Int. 2007, 29, 16–18. [Google Scholar]
- Johansson, P.; Jacobsson, P. New lithium salts on the computer: Fiction or fact? Electrochim. Acta 2001, 46, 1545–1552. [Google Scholar] [CrossRef]
- Hofmann, A.; Merklein, L.; Schulz, M.; Hanemann, T. Anodic aluminum dissolution of LiTFSA containing electrolytes for Li-ion-batteries. Electrochim. Acta 2014, 116, 388–395. [Google Scholar] [CrossRef]
- Dahbi, M.; Ghamouss, F.; Tran-Van, F.; Lemordant, D.; Anouti, M. Comparative study of EC/DMC litfsi and LiPF6 electrolytes for electrochemical storage. J. Power Sources 2011, 196, 9743–9750. [Google Scholar] [CrossRef]
- Huang, X. Separator technologies for lithium-ion batteries. J. Solid State Electrochem. 2010, 15, 649–662. [Google Scholar] [CrossRef]
- Dahbi, M.; Violleau, D.; Ghamouss, F.; Jacquemin, J.; Tran-Van, F.; Lemordant, D.; Anouti, M. Interfacial properties of LiTFSI and LiPF6-based electrolytes in binary and ternary mixtures of alkylcarbonates on graphite electrodes and celgard separator. Ind. Eng. Chem. Res. 2012, 51, 5240–5245. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.K.; Khare, P.; Singh, J.K.; Verma, N. Preparation of novel carbon microfiber/carbon nanofiber-dispersed polyvinyl alcohol-based nanocomposite material for lithium-ion electrolyte battery separator. Mater. Sci. Eng. C 2013, 33, 1702–1709. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Pang, X.; Zhou, Z. Recent progress in high-voltage lithium ion batteries. J. Power Sources 2013, 237, 229–242. [Google Scholar] [CrossRef]
- Capsoni, D.; Bini, M.; Ferrari, S.; Quartarone, E.; Mustarelli, P. Recent advances in the development of Li–air batteries. J. Power Sources 2012, 220, 253–263. [Google Scholar] [CrossRef]
- Ue, M. Ionic radius of (CF3SO2)3C−and applicability of stokes law to its propylene carbonate solution. J. Electrochem. Soc. 1996, 143, L270–L272. [Google Scholar] [CrossRef]
- Saito, Y.; Yamamoto, H.; Kageyama, H.; Nakamura, O.; Miyoshi, T.; Matsuoka, M. Investigation of the solution condition of lithium electrolyte solutions with LiCF3SO3 salt. J. Mater. Sci. 2000, 35, 809–812. [Google Scholar] [CrossRef]
- Aihara, Y.; Bando, T.; Nakagawa, H.; Yoshida, H.; Hayamizu, K.; Akiba, E.; Price, W.S. Ion transport properties of six lithium salts dissolved in γ-butyrolactone studied by self-diffusion and ionic conductivity measurements. J. Electrochem. Soc. 2004, 151, A119. [Google Scholar] [CrossRef]
- Seo, D.M.; Boyle, P.D.; Sommer, R.D.; Daubert, J.S.; Borodin, O.; Henderson, W.A. Solvate structures and spectroscopic characterization of LiTFSI electrolytes. J. Phys. Chem. B 2014, 118, 13601–13608. [Google Scholar] [CrossRef] [PubMed]
- Lassègues, J.-C.; Grondin, J.; Talaga, D. Lithium solvation in bis (trifluoromethanesulfonyl) imide-based ionic liquids. Phys. Chem. Chem. Phys. 2006, 8, 5629. [Google Scholar] [CrossRef] [PubMed]
- Duluard, S.; Grondin, J.; Bruneel, J.-L.; Pianet, I.; Grélard, A.; Campet, G.; Delville, M.-H.; Lassègues, J.-C. Lithium solvation and diffusion in the 1-butyl-3-methylimidazolium bis (trifluoromethanesulfonyl) imide ionic liquid. J. Raman Spectrosc. 2008, 39, 627–632. [Google Scholar] [CrossRef]
- Hardwick, L.J.; Holzapfel, M.; Wokaun, A.; Novak, P. Raman study of lithium coordination in EMI-TFSI additive systems as lithium-ion battery ionic liquid electrolytes. J. Raman Spectrosc. 2007, 38, 110–112. [Google Scholar] [CrossRef]
- Le, M.L.P.; Alloin, F.; Strobel, P.; Leprétre, J.-C.; Valle, C.P.; Judeinstein, P. Structure-properties relationships of lithium electrolytes based on ionic liquid. J. Phys. Chem. B 2010, 114, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-K.; Matic, A.; Ahn, J.-H.; Jacobsson, P. An imidazolium based ionic liquid electrolyte for lithium batteries. J. Power Sources 2010, 195, 7639–7643. [Google Scholar] [CrossRef]
- Osinska, M.; Walkowiak, M.; Zalewska, A.; Jesionowski, T. Study of the role of ceramic filler in composite gel electrolytes based on microporous polymer membranes. J. Membr. Sci. 2009, 326, 582–588. [Google Scholar] [CrossRef]
- Bauer, W.; Nötzel, D.; Wenzel, V.; Nirschl, H. Influence of dry mixing and distribution of conductive additives in cathodes for lithium ion batteries. J. Power Sources 2015, 288, 359–367. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hofmann, A.; Kaufmann, C.; Müller, M.; Hanemann, T. Interaction of High Flash Point Electrolytes and PE-Based Separators for Li-Ion Batteries. Int. J. Mol. Sci. 2015, 16, 20258-20276. https://doi.org/10.3390/ijms160920258
Hofmann A, Kaufmann C, Müller M, Hanemann T. Interaction of High Flash Point Electrolytes and PE-Based Separators for Li-Ion Batteries. International Journal of Molecular Sciences. 2015; 16(9):20258-20276. https://doi.org/10.3390/ijms160920258
Chicago/Turabian StyleHofmann, Andreas, Christoph Kaufmann, Marcus Müller, and Thomas Hanemann. 2015. "Interaction of High Flash Point Electrolytes and PE-Based Separators for Li-Ion Batteries" International Journal of Molecular Sciences 16, no. 9: 20258-20276. https://doi.org/10.3390/ijms160920258