Physiological and Molecular Aspects of Tolerance to Environmental Constraints in Grain and Forage Legumes
Abstract
:1. Introduction
2. Legumes with Nutrient Deficiencies: Case of P-Deficiency and Examples of Adaptive Strategies
2.1. Acid Phosphatases: Expression and Activity
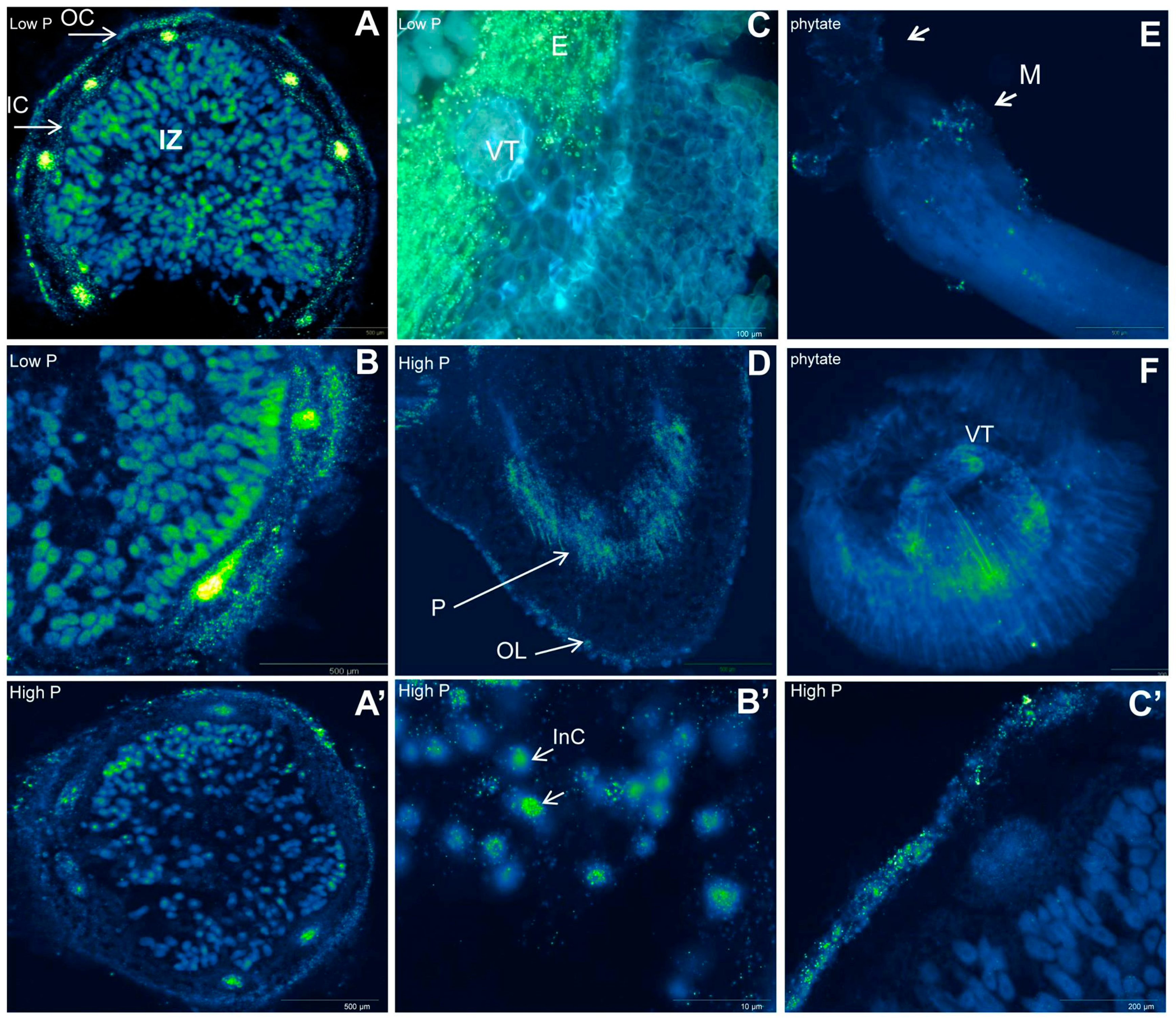
2.2. Organic Acid Exudation
2.3. Phosphorus Use Efficiency Involves Complex Quantitative Traits
3. N2-Fixing Legume and Micronutrient Deficiencies
3.1. Tolerance Mechanisms Associated with Fe Deficiency
3.2. Boron and Molybdenum
4. Drought Tolerance in N2 Fixing Legumes
4.1. Overview on the Impact of Drought on Legumes
4.2. Overview of the Regulation of SNF under Drought
5. Salinity Tolerance in N2 Fixing Legumes
5.1. Intracellular Sequestration of Sodium
5.2. Biosynthesis of Osmoprotectants
5.3. Responses of Antioxidant-Gene Enzymes

5.4. Acid Phosphatases under Salinity
5.5. Phytohormones in Regulation of Salinity Tolerance
6. Concluding Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, W.X.; Vinocur, B.; Altman, A. Plant responses to drought, salinity and extreme temperatures: Towards genetic engineering for stress tolerance. Planta 2003, 218, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Vance, C.P. Symbiotic nitrogen fixation and phosphorus acquisition. Plant nutrition in a world of declining renewable resources. Plant Physiol. 2001, 127, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Graham, P.H.; Vance, C.P. Legumes: Importance and constraints to greater use. Plant Physiol. 2003, 31, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.P.; Shan, L.; Inanaga, S.; Inoue, M. Water-saving approaches for improving wheat production. J. Sci. Food. Agric. 2005, 85, 1379–1388. [Google Scholar] [CrossRef]
- Shenoy, V.V.; Kalagudi, G.M. Enhancing plant phosphorus use efficiency for sustainable cropping. Biotechnol. Adv. 2005, 23, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Vadez, V.; Rodier, F.; Payre, H.; Drevon, J.J. Nodule permeability to O2 and nitrogenase-linked respiration in bean genotypes varying in the tolerance of N2 fixation to P deficiency. Plant Physiol. Biochem. 1996, 34, 871–878. [Google Scholar]
- Serraj, R.; Adu-Gyamfi, J. Role of symbiotic nitrogen fixation in the improvement of legume productivity under stressed environments. West Afr. J. App. Ecol. 2004, 6, 95–109. [Google Scholar] [CrossRef]
- Schulze, J.; Drevon, J.J. P-deficiency increases the O2 uptake per N2 reduced in alfalfa. J. Exp. Bot. 2005, 56, 1779–1784. [Google Scholar] [CrossRef] [PubMed]
- Vance, C.P.; Uhde-Stone, C.; Allan, D.L. Phosphorus acquisition and use: Critical adaptations by plants for securing a nonrenewable resource. New Phytol. 2003, 157, 423–447. [Google Scholar] [CrossRef]
- Franco-Zorrilla, J.M.; Gonzalez, E.; Bustos, R.; Linhares, F.; Leyva, A.; Paz-Ares, J. The transcriptional control of plant responses to phosphate limitation. J. Exp. Bot. 2004, 55, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Hammond, J.P.; Bennett, M.J.; Bowen, H.C.; Broadley, M.R.; Eastwood, D.C.; May, S.T.; Rahn, C.; Swarup, R.; Woolaway, K.E.; White, P.J. Changes in gene expression in Arabidopsis shoots during phosphate starvation and the potential for developing smart plants. Plant Physiol. 2003, 132, 578–596. [Google Scholar] [CrossRef] [PubMed]
- Duff, S.M.G.; Sarath, G.; Plaxton, W.C. The role of acid phosphatases in plant phosphorus metabolism. Physiol. Plant. 1994, 90, 791–800. [Google Scholar] [CrossRef]
- Hinsinger, P.; Betencourt, E.; Bernard, L.; Brauman, A.; Passard, C.; Shen, C.; Tang, X. Phosphorus for two, sharing a scarce resource: Soil phosphorus acquisition in the rhizosphere of intercropped species. Plant Physiol. 2011, 156, 1078–1086. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, Z.; Qian, W.; Guo, W.; Gao, X.; Huang, L.; Wang, H. The Arabidopsis purple acid phosphatase AtPAP10 is predominantly associated with the root surface and plays an important role in plant tolerance to phosphate limitation. Plant Physiol. 2011, 157, 1283–1299. [Google Scholar] [CrossRef] [PubMed]
- Penheiter, A.R.; Duff, S.M.G.; Sarath, G. Soybean root nodule acid phosphatase. Plant Physiol. 1997, 14, 597–604. [Google Scholar] [CrossRef]
- Tang, H.; Li, X.; Zu, C.; Zhang, F.; Shen, J. Spatial distribution and expression of intracellular and extracellular acid phosphatases of cluster roots at different developmental stages in white lupine. J. Plant Physiol. 2013, 170, 1243–1250. [Google Scholar] [CrossRef] [PubMed]
- Dinkelaker, B.; Römheld, V.; Marshner, H. Citric acid excretion and precipitation of calcium citrate in the rhizosphere of white lupin (Lupinus albus L.). Plant Cell Environ. 1989, 12, 285–292. [Google Scholar] [CrossRef]
- Veneklaas, E.J.; Stevens, J.; Cawthray, G.; Rturner, S.; Grigg, A.M.; Lambers, H. Chickpea and white lupin rhizosphere carboxylates vary with soil properties and enhance phosphorus uptake. Plant Soil 2003, 248, 187–197. [Google Scholar] [CrossRef]
- Lambers, H.; Clements, C.J.; Nelson, N.M. How a phosphorus-acquisition strategy based on carboxylate exudations powers the success and agronomical potential of lupines (Lupinus, Fabaceae). Am. J. Bot. 2013, 100, 263–288. [Google Scholar] [CrossRef] [PubMed]
- Benedito, V.A.; Torres-Jerez, I.; Murray, J.D. A gene expression atlas of the model legume Medicago truncatula. Plant J. 2008, 55, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Libault, M.; Farmer, A.; Joshi, T.; Takahashi, K.; Langley, R.; Franklin, LD.; He, J.; Xu, D.; May, G.; Stacey, G. An integrated transcriptome atlas of the crop model Glycine max, and its use in comparative analyses in plants. Plant J. 2010, 63, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Severin, A.J.; Woody, J.L.; Bolon, Y.T.; Joseph, B.; Diers, B.; Farmer, A.; Muehlbauer, G.; Nelson, R.; Grant, D.; Specht, J.; et al. RNA-seq atlas of Glycine max: A guide to the soybean transcriptome. BMC Plant Biol. 2010, 10, 160. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Guil, S.; Yang, T.; Walk, T.; Wang, X.; Liao, H. Identification of soybean purple acid phosphatase genes and their expression responses to phosphorus availability and symbiosis. Ann. Bot. 2012, 109, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Roussis, A.; Flemetakis, E.; Dimou, M. A putative phosphatase whose expression is induced during Phaseolus vulgaris nodule development. Plant Physiol. Biochem. 2003, 41, 719–725. [Google Scholar] [CrossRef]
- Araujo, A.P.; Plassard, C.; Drevon, J.-J. Phosphatase and phytase activities in nodules of common bean genotypes at different levels of phosphorus supply. Plant Soil 2008, 312, 129–138. [Google Scholar] [CrossRef]
- Bargaz, A.; Ghoulam, C.; Amenc, L.; Lazali, M.; Faghire, M.; Abadie, J.; Drevon, J.J. A phosphoenol pyruvate phosphatase gene transcript is induced in the root nodule cortex of Phaseolus vulgaris under P deficiency. J. Exp. Bot. 2012, 63, 4723–4730. [Google Scholar] [CrossRef] [PubMed]
- Bargaz, A.; Lazali, M.; Amenc, M.; Abadie, J.; Ghoulam, C.; Farissi, M.; Faghire, M.; Drevon, J.J. Differential expression of trehalose 6-P phosphatase and ascorbate peroxidase transcripts in nodule cortex of Phaseolus vulgaris and regulation of nodule O2 permeability. Planta 2013, 238, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Lazali, M.; Louadj, L.; Ounane, G.; Abadie, J.; Amenc, L.; Bargaz, A.; Lullien-Pellerin, V.; Drevon, J.-J. Localization of phytase transcripts in germinating seeds of the common bean (Phaseolus vulgaris L.). Planta 2014, 240, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Turner, B.L.; Paphazy, M.J.; Haygarth, P.M.; McKelvie, I.D. Inositol phosphates in the environment. Phil. Trans. R. Soc. B 2002, 357, 449–469. [Google Scholar] [CrossRef] [PubMed]
- Richardson, A.E.; Hadobas, P.A.; Hayes, J.E. Extracellular secretion of Aspergillus phytase from Arabidopsis roots enables plants to obtain phosphorus from phytate. Plant J. 2001, 25, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Lott, J.N.A.; Ockenden, I.; Raboy, V.; Batten, G.D. Phytic acid and phosphorus in crop seeds and fruits: A global estimate. Seed Sci. Res. 2000, 10, 11–33. [Google Scholar]
- Raboy, V. Molecules of interest: Myo-inositol 1,2,3,4,5,6-hexakisphosphate. Phytochemistry 2003, 64, 1033–1043. [Google Scholar] [CrossRef]
- Loewus, F.A.; Loewus, M.W. Myo-inositol: Its biosynthesis and metabolism. Annu. Rev. Plant Physiol. 1983, 34, 137–161. [Google Scholar] [CrossRef]
- Valverde, C.; Ferrari, A.; Wall, L.G. Phosphorus and the regulation of nodulation in the actinorhizal symbiosis between Discaria trinervis (Rhamnaceae) and Frankia BCU110501. New Phytol. 2002, 153, 43–51. [Google Scholar] [CrossRef]
- Moraghan, J.T.; Etchevers, J.D.; Padilla, J. Contrasting accumulations of calcium and magnesium in seed coats and embryos of common bean and soybean. Food Chem. 2006, 95, 554–561. [Google Scholar] [CrossRef]
- Cvitanich, C.; Przybylowicz, W.J.; Mesjasz-Przybylowicz, J.; Blair, M.W.; Astudillo, C.; Orlowska, E.; Jurkiewicz, A.M.; Jensen, E.O.; Stougaard, J. Micro-PIXE investigation of bean seeds to assist micronutrient biofortification. Nucl. Instrum. Meth. B 2011, 269, 2297–2302. [Google Scholar] [CrossRef]
- Ribeiro, N.D.; Mazierio, S.M.; Prigol, M.; Nogueira, C.W.; Rosa, D.P.; Possobom, M.T.D.F. Mineral concentrations in the embryo and seed coat of common bean cultivars. J. Food Compos. Anal. 2012, 26, 89–95. [Google Scholar] [CrossRef]
- Vardien, W.; Mesjasz-Przybylowicz, J.; Przybylowicz, J.W.; Wang, Y.; Steenkamp, E.T.; Valentine, A.J. Nodules from Fynbos legume Virgilia divaricata have high functional plasticity under variable P supply levels. J. Plant Physiol. 2014, 171, 1732–1739. [Google Scholar] [CrossRef] [PubMed]
- Unno, Y.; Kenzo, O.; Jun, W.; Takuro, S.; Mitsuru, O. Plant growth promotion abilities and microscale bacterial dynamics in the rhizosphere of lupin analysed by phytate utilization ability. Environ. Microbiol. 2005, 7, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Richardson, A.E.; Hadobas, P.A. Soil isolates of Pseudomonas spp. that utilize inositol phosphates. Can. J. Microbiol. 1997, 43, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.J.; Choi, Y.J.; Min, H.K.; Cho, K.K.; Kim, J.W.; Lee, S.C.; Jung, Y.H. Isolation and identification of phytase producing bacterium, Enterobacter sp. 4, and enzymatic properties of phytase enzyme. Enzym. Microb. Technol. 1996, 18, 449–454. [Google Scholar] [CrossRef]
- Jorquera, M.A.; Hernández, M.T.; Rengel, Z.; Marschner, P.; Mora, M.L. Isolation of culturable phosphobacteria with both phytate-mineralization and phosphate solubilization activity from the rhizosphere of plants grown in a volcanic soil. Biol. Fertil. Soils 2008, 44, 1025–1034. [Google Scholar] [CrossRef]
- Maougal, R.T.; Bargaz, A.; Sahel, C.; Amenc, L.; Djekoun, A.; Plassard, C.; Drevon, J.J. Localization of the Bacillus subtilis beta-propeller phytase transcripts in nodulated roots of Phaseolus vulgaris supplied with phytate. Planta 2014, 239, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Lazali, M.; Zaman-Allah, M.; Amenc, L.; Ounane, G.; Abadie, J.; Drevon, J.J. A phytase gene is over-expressed in root nodules cortex of Phaseolus vulgaris-rhizobia symbiosis under phosphorus deficiency. Planta 2013, 238, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Gruszewski, H.A.; Chevone, B.I.; Nessler, C.L. An Arabidopsis purple acid phosphatase with phytase activity increases foliar ascorbate. Plant Physiol. 2008, 146, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhu, H.; Liu, K. Purple acid phosphatases of Arabidopsis thaliana: Comparative analysis and differential regulation by phosphate deprivation. J. Biol. Chem. 2002, 277, 27772–27781. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.T.; Hurley, B.A.; Plaxton, W.C. Feeding hungry plants: The role of purple acid phosphatases in phosphate nutrition. Plant Sci. 2010, 179, 14–27. [Google Scholar] [CrossRef]
- Pereira, P.A.A.; Bliss, F.A. Nitrogen fixation and plant growth of common bean (Phaseolus vulgaris L.) at different levels of phosphorus availability. Plant Soil 1987, 104, 79–84. [Google Scholar] [CrossRef]
- Fox, T.R.; Comerford, N.B.; MacFee, W.W. Phosphorous and aluminum release from a spodic horizon mediated by organic acids. Soil Sci. Soc. Am. J. 1990, 54, 1763–1767. [Google Scholar] [CrossRef]
- Tomasi, N.; Kretzschmar, T.; Espen, L.; Weisskopf, L.; Fuglsang, A.; Palmgren, M.G.; Neumann, G. Plasma membrane H+-ATPase dependent citrate exudation from cluster roots of phosphate-deficient white lupin. Plant Cell Environ. 2009, 32, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Gardner, W.K.; Barber, D.A.; Parbery, D.G. The acquisition of phosphorus by Lupinus albus L. III. The probable mechanism by which phosphorus movement in the soil/root interface is enhanced. Plant Soil 1983, 70, 107–124. [Google Scholar] [CrossRef]
- Lambers, H.; Raven, J.A.; Shaver, G.R.; Smith, S.E. Plant nutrient-acquisition strategies change with soil age. Trends Ecol. Evol. 2008, 23, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Hagström, J.; James, W.M.; Skene, K.R. A comparison of structure, development and function in cluster roots of Lupinus albus L. under phosphate and iron stress. Plant Soil 2001, 232, 81–90. [Google Scholar] [CrossRef]
- Shane, M.W.; Lambers, H. Manganese accumulation in leaves of Hakea prostrata (Proteaceae) and the signifi cance of cluster roots for micronutrient uptake as dependent on phosphorus supply. Physiol. Plant. 2005, 124, 441–450. [Google Scholar] [CrossRef]
- Duffner, A.; Hoffland, E.; Temminghof, E.J.M. Bioavailability of zinc and phosphorus in calcareous soils as affected by citrate exudation. Plant Soil 2012, 361, 165–175. [Google Scholar] [CrossRef]
- Vance, C.P. The molecular biology of N metabolism. In Plant Metabolism; Dennis, D.T., Turpin, D.H., Lefebrre, D.D., Layzell, D.B., Eds.; Longman Scientific: London, UK, 1997; pp. 449–477. [Google Scholar]
- Miller, S.S.; Driscoll, B.T.; Gregerson, R.G.; Gantt, J.S.; Vance, C.P. Alfalfa malate dehydrogenaase (MDH): Cloning and characterization of five different forms reveals a unique nodule-enhanced MDH. Plant J. 1998, 15, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Tesfaye, M.; Temple, S.J.; Allan, D.L.; Vance, C.P.; Samac, D.A. Overexpression of malate dehydrogenase in transgenic alfalfa enhances organic acid synthesis and confers tolerance to aluminum. Plant Physiol. 2001, 127, 1836–1844. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Pineros, A.M.; Tian, J.; Yao, Z.; Sun, L.; Liu, J.; Shaff, J.; Coluccio, A.; Kochian, V.L.; Liao, H. Low pH, aluminum, and phosphorus coordinately regulate malate exudation through GmALMT1 to improve soybean adaptation to acid soils. Plant Physiol. 2013, 161, 1347–1361. [Google Scholar] [CrossRef] [PubMed]
- Hoffland, E.; van den Boogard, R.; Nelemans, J.; Findenegg, G. Biosynthesis and root exudation of citric and malic acids in phosphatestarved rape plants. New Phytol. 1992, 122, 675–680. [Google Scholar] [CrossRef]
- Martínez-Camacho, J.L.; la Vara, L.G.; Hamabata, A.; Mora-Escobedo, R.; Calderón-Salinas, V. A pH-stating mechanism in isolated wheat (Triticum aestivum) aleurone layers involves malic acid transport. J. Plant Physiol. 2004, 161, 1289–1298. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Yamamoto, Y.; Ezaki, B.; Katsuhara, M.; Ahn, S.J.; Ryan, P.R.; Delhaize, E.; Matsumoto, H. A wheat gene encoding an aluminum-activated malate transporter. Plant J. 2004, 37, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Kovermann, P.; Meyer, S.; Hörtensteiner, S.; Picco, C.; Scholz-Starke, J.; Ravera, S.; Lee, Y. Martinoia, E. The Arabidopsis vacuolar malate channel is a member of the ALMT family. Plant J. 2007, 52, 1169–1180. [Google Scholar] [CrossRef] [PubMed]
- Gruber, B.D.; Ryan, P.R.; Richardson, A.E.; Tyerman, S.D.; Ramesh, S.; Hebb, D.M.; Howitt, S.M.; Delhaize, E. HvALMT1 from barley is involved in the transport of organic anions. J. Exp. Bot. 2010, 61, 1455–1467. [Google Scholar] [CrossRef] [PubMed]
- Meyer, S.; Mumm, P.; Imes, D.; Endler, A.; Weder, B.; Al-Rasheid, K.A.S.; Geiger, D.; Marten, I.; Martinoia, E.; Hedrich, R. AtALMT12 represents an R-type anion channel required for stomatal movement in Arabidopsis guard cells. Plant J. 2010, 63, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Krotzky, A.; Berggold, R.; Werner, D. Plant characteristics limiting associative N2-fixation (C2H2 reduction) with two cultivars of Sorghum mutants. Soil Biol. Biochem. 1988, 20, 157–162. [Google Scholar] [CrossRef]
- Tomasi, N.; Weisskopf, L.; Renella, G.; Landi, L.; Pinton, R.; Varanini, Z.; Nannipieri, P. Flavonoids of white lupin roots participate in phosphorus mobilization from soil. Soil Biol. Biochem. 2008, 40, 1971–1974. [Google Scholar] [CrossRef]
- Krotzky, A.; Berggold, R.; Werner, D. Analysis of factors limiting associative N2-fixation (C2H2 reduction) with two cultivars of Sorghum mutants. Soil Biol. Biochem. 1986, 18, 201–207. [Google Scholar] [CrossRef]
- Harborne, J.B.; Iingham, J.L.; King, L.; Payne, M. The isopentenyl isofl avone luteone as a pre-infectional antifungal agent in the genus Lupinus. Phytochemistry 1976, 15, 1485–1487. [Google Scholar] [CrossRef]
- Shen, G.; Huhman, D.; Lei, Z.; Snyder, J.; Sumner, L.W.; Dixon, R.A. Characterization of an Isoflavonoid-Specific Prenyltransferase from Lupinus albus. Plant Physiol. 2012, 159, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Neumann, G.; Römheld, V. The release of root exudates as affected by the plant physiological status. In The Rhizosphere Biochemistry and Organic Substances at the Soil–Plant Interface; Pinton, R., Varanini, Z., Nannipieri, P., Eds.; CRC Press/Taylor and Francis: New York, NY, USA, 2007; pp. 23–72. [Google Scholar]
- Rudrappa, T.; Czymmek, K.J.; Pare, P.W.; Bais, H.P. Root-secreted malic acid recruits beneficial soil bacteria. Plant Physiol. 2008, 148, 1547–1556. [Google Scholar] [CrossRef] [PubMed]
- Beebe, S.E.; Rojas-Pierce, M.; Yan, X.; Blair, M.W.; Pedraza, F.; Munoz, F.; Tohme, J.; Lynch, J.P. Quantitative trait loci for root architecture traits correlated with phosphorus acquisition in common bean. Crop Sci. 2006, 46, 413–423. [Google Scholar] [CrossRef]
- Ochoa, I.E.; Blair, M.W.; Lynch, J.P. QTL analysis of adventitious root formation in common bean under contrasting phosphorus availability. Crop Sci. 2006, 46, 1609–1621. [Google Scholar] [CrossRef]
- Yan, X.; Liao, H.; Beebe, S.E.; Blair, M.W.; Lynch, J.P. QTL mapping of root hair and acid exudation traits and their relationship to phosphorus uptake in common bean. Plant Soil 2004, 265, 17–29. [Google Scholar] [CrossRef]
- Liao, H.; Yan, X.L.; Rubio, G.; Beebe, S.E.; Blair, M.W.; Lynch, J.P. Genetic mapping of basal root gravitropism and phosphorus acquisition efficiency in common bean. Funct. Plant Biol. 2004, 31, 959–970. [Google Scholar] [CrossRef]
- Li, Y.D.; Wang, Y.J.; Tong, Y.P.; Gao, J.G.; Zhang, J.S.; Chen, S.Y. QTL mapping of phosphorus deficiency tolerance in soybean (Glycine max L. Merr.). Euphytica 2005, 142, 137–142. [Google Scholar] [CrossRef]
- Ni, J.J.; Wu, P.; Senadhira, D.; Huang, M. Mapping QTLs for phosphorus deficiency tolerance in rice (Oryza sativa L.). Theor. Appl. Genet. 1998, 97, 1361–1369. [Google Scholar] [CrossRef]
- Cichy, K.A.; Blair, M.W.; Galeano Mendoza, C.H.G.; Snapp, S.S.; Kelly, J.D. QTL analysis of root architecture traits and low phosphorus tolerance in an Andean bean population. Crop Sci. 2009, 49, 59–68. [Google Scholar] [CrossRef]
- Su, J.; Xiao, Y.; Li, M.; Liu, Q.; Li, B.; Tong, Y.; Jia, J.; Li, Z. Mapping QTLs for phosphorus-deficiency tolerance at wheat seedling stage. Plant Soil 2006, 281, 25–36. [Google Scholar] [CrossRef]
- Chen, J.; Xu, L.; Cai, Y.; Xu, J. Identification of QTLs for phosphorus utilization efficiency in maize (Zea mays L.) across P levels. Euphytica 2009, 167, 245–252. [Google Scholar] [CrossRef]
- Ming, F.; Zheng, X.; Mi, G.; He, P.; Zhu, L. Identification of quantitative trait loci affecting tolerance to low phosphorus in rice (Oryza Sativa L.). Chin. Sci. Bull. 2000, 45, 520–525. [Google Scholar] [CrossRef]
- Reymond, M.; Svistoonoff, S.; Loudet, O.; Nussaume, L.; Desnos, T. Identification of QTL controlling root growth response to phosphate starvation in Arabidopsis thaliana. Plant Cell Environ. 2006, 29, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Song, H.; Cheng, H.; Hao, D.; Wang, H.; Kan, G.; Jin, H.; Yu, D. The acid hosphatase-encoding gene GmACP1 contributes to soybean tolerance to low-phosphorus stress. PLoS Genet. 2014, 10, e1004061. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Yin, Z.; Chao, M.; Ning, L.; Zhang, D.; Yu, D. Functional properties and expression quantitative trait loci for phosphate transporter GmPT1 in soybean. Plant Cell Environ. 2014, 37, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Rae, A.L.; Cybinski, D.H.; Jarmey, J.M.; Smith, F.W. Characterization of two phosphate transporters from barley; evidence for diverse function and kinetic properties among members of the Pht1 family. Plant Mol. Biol. 2003, 53, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, V.K.; Jain, A.; Poling, M.D.; Lewis, A.J.; Raghothama, K.G.; Smith, A.P. Arabidopsis Pht1; 5 mobilizes phosphate between source and sink organs and influences the interaction between phosphate homeostasis and ethylene signaling. Plant Physiol. 2011, 156, 1149–1163. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Zheng, S.J.; Qiao, Y.F.; Wang, G.H.; Han, X.Z. Interactions between high pH and iron supply on nodulation and iron nutrition of Lupinus albus L. genotypes differing in sensitivity to iron deficiency. Plant Soil 2006, 279, 153–162. [Google Scholar] [CrossRef]
- Rotaru, V.; Sinclair, T.R. Interactive influence of phosphorus and iron on nitrogen fixation by soybean. Environ. Exp. Bot. 2009, 66, 94–99. [Google Scholar] [CrossRef]
- Brear, E.M.; Day, D.A.; Smith, P.M. Iron: An essential micronutrient for the legume–rhizobium symbiosis. Front. Plant Sci. Plant Nutr. 2013, 4, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Slatni, T.; Krouma, A.; Aydi, S.; Chaiffi, C.; Gouia, H.; Abdelly, C. Growth, nitrogen fixation and ammonium assimilation in common bean (Phaseolus vulgaris L.) subjected to iron deficiency. Plant Soil 2008, 312, 49–57. [Google Scholar] [CrossRef]
- Delgado, M.J.; Bedmar, E.J. Genes Involved in the Formation and Assembly of Rhizobial Cytochromes and their Role in Symbiotic Nitrogen Fixation. Adv. Microb. Physiol. 1998, 40, 191–231. [Google Scholar] [PubMed]
- O’Hara, G.W. Nutritional constraints on root nodule bacteria affecting symbiotic nitrogen fixation: A review. Aust. J. Exp. Agric. 2001, 41, 417–433. [Google Scholar] [CrossRef]
- Dixon, R.; Kahn, D. Genetic regulation of biological nitrogen fixation. Nat. Rev. Microbiol. 2004, 2, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.W.; Szilagyi, R.K. Exploring new frontiers of nitrogenase structure and mechanism. Curr. Opin. Chem. Biol. 2006, 10, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, I.; Mergold-Villasenor, C.; Campos, M.E.; Sanchez, N.; Perez, H.; Lopez, L.; Castrejon, L.; Sanchez, F.; Cassab, G.I. The aberrant cell walls of boron-deficient bean root nodules have no covalently bound hydroxyprolin-/proline-rich proteins. Plant Physiol. 1997, 115, 1329–1340. [Google Scholar] [CrossRef] [PubMed]
- Redondo-Nieto, M.; Rivilla, R.; El-Hamdaoui, A.; Bonilla, I.; Bolaños, L. Boron deficiency affects early infection events in the pea-Rhizobium symbiotic interaction. Aust. J. Plant Physiol. 2001, 28, 819–823. [Google Scholar] [CrossRef]
- Bolaños, L.; Cebrian, A.; Redondo-Nieto, M.; Rivilla, R.; Bonilla, I. Lectin-like glycoprotein PsNLEC-1 is not correctly glycosylated and targeted in boron deficient pea nodules. Mol. Plant Microb. Interact. 2001, 14, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Wiedenhoeft, A.C. Introduction to plants and plant nutrition. In Plant Nutrition; Wiedenhoeft, A.C., Hopkins, W.G., Eds.; Infobase Publishing: New York, NY, USA, 2006; pp. 2–13. [Google Scholar]
- Hansch, R.; Mendel, R.R. Physiological functions of mineral micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl). Curr. Opin. Plant Biol. 2009, 12, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Grusak, M.A.; Pearson, J.N.; Marentes, E. The physiology of micronutrient homeostasis in field crops. Field Crops Res. 1999, 60, 41–56. [Google Scholar] [CrossRef]
- Graham, R.D.; Welch, R.M. Plant food micronutrient composition and human nutrition. Commun. Soil Sci. Plant Anal. 2000, 31, 1627–1640. [Google Scholar] [CrossRef]
- Rai, R.; Singh, S.N.; Prasad, V. Effect of presmued amended pyrite on symbiotic N2-fixation, active iron content of nodules, grain yield and quality of chickpea (Cicer arietinum L.) genotypes in calcareous soil. J. Plant Nutr. 1982, 5, 905–913. [Google Scholar] [CrossRef]
- Hemantaranjan, A.; Garg, O.K. Introduction of nitrogen fiing nodules through iron and zinc fertilization in the non nodule forming French bean (Phaseolus vulgaris L.). J. Plant Nutr. 1986, 9, 281–288. [Google Scholar] [CrossRef]
- O’Hara, G.W.; Hartzook, A.; Bell, R.W.; Loneragan, J.F. Response to Bradyrhizobium strains of peanut cultivars grown under iron stress. J. Plant Nutr. 1988, 11, 843–852. [Google Scholar] [CrossRef]
- Tang, C.; Robson, A.D.; Dilworth, M.J. The role of iron in nodulation and nitrogen fixation in Lupinus angustifolius L. New Phytol. 1990, 114, 173–182. [Google Scholar] [CrossRef]
- Soerensen, K.U.; Terry, R.E.; Jolley, V.D.; Brown, J.C.; Vargas, M.E. The interaction of iron-stress response and root nodules in iron efficient and inefficient soybeans. J. Plant Nutr. 1988, 11, 853–862. [Google Scholar] [CrossRef]
- Abadia, J.; López-Millán, A.F.; Rombolà, A.; Abadia, A. Organic acids and Fe deficiency: A review. Plant Soil 2002, 241, 75–86. [Google Scholar] [CrossRef]
- Jiménez, S.; Ollat, N.; Deborde, C.; Maucourt, M.; Rellán-Álvarez, R.; Moreno, M.A.; Gogorcena, Y. Metabolic response in roots of Prunus rootstocks submitted to iron chlorosis. J. Plant Physiol. 2011, 168, 415–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landsberg, E.-C. Organic acid synthesis and release of hydrogen ions in response to Fe deficiency stress of mono- and dicotyledonous plant species. J. Plant Nutr. 1981, 3, 579–591. [Google Scholar] [CrossRef]
- Longnecker, N.; Welch, R.M. Accumulation of apoplastic iron in plant roots. A factor in the resistance of soybeans to iron-deficiency induced chlorosis? Plant Physiol. 1990, 92, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Römheld, V.; Kramer, D. Relationship between proton efflux and rhizodermal transfer cells induced by iron deficiency. Z. Pflanzenphysiol. 1983, 113, 73–83. [Google Scholar] [CrossRef]
- Marschner, H. Marshner’s Mineral Nutrition of Higher Plants; Academic: London, UK, 2012; pp. 1–649. [Google Scholar]
- Kramer, D.; Römheld, V.; Landsberg, E.; Marschner, H. Induction of transfer-cell formation by iron deficiency in the root epidermis of Helianthus annuus. Planta 1980, 147, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Landsberg, E.-C. Proton efflux and transfer cell formation as response to Fe deficiency of soybean in nutrient solution culture. Plant Soil 1989, 114, 53–61. [Google Scholar] [CrossRef]
- Patrick, J.W.; Offler, C.E. Compartmentation of transport and transfer events in developing seeds. J. Exp. Bot. 2001, 52, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Terry, R.E.; Jolley, V.D. Nitrogenase activity is required for the activation of iron-stress response in iron-inefficient T203 soybean. J. Plant Nutr. 1994, 17, 1417–1428. [Google Scholar] [CrossRef]
- Slatni, T.; Dell’Orto, M.; Ben Salah, I.; Vigani, G.; Smaoui, A.; Gouia, H.; Zocchi, G.; Abdelly, C. Immunolocalization of H+-ATPase and IRT1 enzymes in N2-fixing common bean nodules subjected to iron deficiency. J. Plant Physiol. 2012, 169, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, W. Iron solutions: Acquisition strategies and signaling pathways in plants. Trends Plant Sci. 2003, 8, 188–193. [Google Scholar] [CrossRef]
- Lopez-Millan, A.F.; Grusak, M.A.; Abadia, J. Carboxylate metabolism changes induced by Fe deficiency in barley, a strategy II plant species. J. Plant Physiol. 2012, 169, 1121–1124. [Google Scholar] [CrossRef] [PubMed]
- Persmark, M.; Pittman, P.; Buyer, J.S.; Schwyn, B.; Gill, P.R.; Neilands, J.B. Isolation and structure of rhizobactin 1021, a siderophore from the alfalfa symbiont Rhizobium meliloti 1021. J. Am. Chem. Soc. 1993, 115, 3950–3956. [Google Scholar] [CrossRef]
- Jadhav, R.S.; Desai, A.J. Isolation and characterization of siderophore from cowpea Rhizobium (peanut isolate). Curr. Microbiol. 1992, 24, 137–141. [Google Scholar] [CrossRef]
- Berraho, E.; Lesuere, D.; Diem, H.G.; Sasson, A. Iron requirement and siderophore production in Rhizobium cicero during growth on an iron-deficient medium. World J. Microbiol. Biotechnol. 1997, 13, 501–510. [Google Scholar] [CrossRef]
- Lucena, C.; Waters, B.M.; Romera, F.J.; Garcia, M.J.; Morales, M.; Alcantara, E.; Pérez-Vicente, R. Ethylene could influence ferric reductase, iron transporter, and H+-ATPase gene expression by affecting FER (or FER-like) gene activity. J. Exp. Bot. 2006, 57, 4145–4154. [Google Scholar] [CrossRef] [PubMed]
- Waters, B.M.; Lucena, C.; Romera, F.J.; Jester, G.G.; Wynn, A.N.; Rojas, C.L.; Alcántara, E.; Pérez-Vicente, R. Ethylene involvement in the regulation of the H+-ATPase CsHA1 gene and of the new isolated ferric reductase CsFRO1 and iron transporter CsIRT1 genes in cucumber plants. Plant Physiol. Biochem. 2007, 45, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Séguéla, M.; Briat, J.F.; Vert, G.; Curie, C. Cytokinins negatively regulate the root iron uptake machinery in Arabidopsis through a growth-dependent pathway. Plant J. 2008, 55, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.L.; Li, Y.S.; Zhang, W.H. Brassinosteroids are involved in response of cucumber (Cucumis sativus) to iron deficiency. Ann. Bot. 2012, 110, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Kroumaa, A.; Drevon, J.J.; Abdelly, C. Genotypic variation of N2-fixing common bean (Phaseolus vulgaris L.) in response to iron deficiency. J. Plant Physiol. 2006, 163, 1094–1100. [Google Scholar] [CrossRef] [PubMed]
- Redondo-Nieto, M.; Pulido, L.; Reguera, M.; Bonilla, I.; Bolanos, L. Developmentally regulated membrane glycoproteins sharing antigenicity with rhamnogalacturonan II are not detected in nodulated boron deficient Pisum sativum. Plant Cell Environ. 2007, 30, 1436–1443. [Google Scholar] [CrossRef] [PubMed]
- Reguera, M.; Villarejo, A.; Abreu, A.; Bonilla, I.; Bolanos, L. Protein N-glycosylation patterns are altered during development of legume symbiotic nodules under boron deficiency. Biol. Nitrogen Fixing Plant Assoc. Microorg. 2009, 15, 169–172. [Google Scholar]
- Brewin, N.J. Plant cell wall remodelling in the rhizobium-legume symbiosis. Crit. Rev. Plant Sci. 2004, 23, 293–316. [Google Scholar] [CrossRef]
- Reguera, M.; Wimmer, M.; Bbustos, P.; Goldbach, H.E.; Bolanos, L.; Bonilla, I. Ligands of boron in Pisum sativum nodules are involved in regulation of oxygen concentration and rhizobial infection. Plant Cell Environ. 2010, 33, 1039–1048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, M.; Mutoh, T.; Matoh, T. Boron nutrition of cultured tobacco BY-2 cells. IV. Genes induced under low boron supply. J. Exp. Bot. 2004, 55, 1441–1443. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Tan, Q.; Nie, Z.; Hu, C.; An, Y. Differential expression of proteins in response to molybdenum deficiency in winter wheat leaves under low-temperature stress. Plant Mol. Biol. Rep. 2014, 32, 1057–1069. [Google Scholar] [CrossRef]
- Shah, V.K.; Rangarg, P.; Chatterjee, R.; Allen, R.M.; Roll, J.T.; Roberts, G.P.; Ludden, P.W. Requirement of NifX and other nif proteins for in vitro biosynthesis of the iron-molybdenum cofactor of nitrogenase. J. Bacteriol. 1999, 181, 2797–2801. [Google Scholar] [PubMed]
- Delgado, M.J.; Alvaro, T.A.; Chouhara, T.; Eulogio, J.B. Functional characterization of Bradyrhizobium japonicum modA and modB genes involved in molybdenum transport. Microbiology 2006, 152, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Nie, Z.; Hu, C.; Liu, H.; Tan, Q.; Sun, X. Differential expression of molybdenum transport and assimilation genes between two winter wheat cultivars (Triticum aestivum). Plant Physiol. Biochem. 2014, 82, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Zahran, H.H. Rhizobium–legume symbiosis and nitrogen fixation under severe conditions and in arid climate. Microbiol. Mol. Biol. Rev. 1999, 63, 968–989. [Google Scholar] [PubMed]
- Williams, P.M.; de Mallorca, M.S. Effect of osmotically induced leaf moisture stress on nodulation and nitrogenase activity of Glycine max. Plant Soil 1984, 80, 267–283. [Google Scholar] [CrossRef]
- Leport, L.; Turner, N.C.; Davies, S.L.; Siddique, K.H.M. Variation in pod production and abortion among chickpea cultivars under terminal drought. Eur. J. Agron. 2006, 24, 236–246. [Google Scholar] [CrossRef]
- Fang, X.; Turner, N.C.; Guijun, Y.; Fengmin, L.; Siddique, K.H.M. Flower numbers, pod production, pollen viability, and pistil function are reduced and flower and pod abortion increased in chickpea (Cicer arietinum L.) under terminal drought. J. Exp. Bot. 2010, 61, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Merah, O. Potential importance of water status traits for durum wheat improvement under Mediterranean conditions. J. Agric. Sci. 2001, 137, 139–145. [Google Scholar] [CrossRef]
- Kato, Y.; Kamoshita, A.; Yamagishi, J.P. Reflowering abortion reduces spikelet number in upland rice (Oryza sativa L.) under water stress. Crop Sci. 2008, 48, 2389–2395. [Google Scholar] [CrossRef]
- Turner, N.C.; Wright, G.C.; Siddique, K.H.M. Adaptation of grain legumes (pulses) to water-limited environments. Adv. Agron. 2001, 71, 193–231. [Google Scholar]
- Kashiwagi, J.; Krishnamurthy, L.; Upadhyaya, H.D.; Krishna, H.; Chandra, S.; Vadez, V.; Serraj, R. Genetic variability of drought-avoidance root traits in the mini-core germplasm collection of chickpea (Cicer arietinum L.). Euphytica 2005, 146, 213–222. [Google Scholar] [CrossRef] [Green Version]
- Sengupta, D.; Reddy, A.R. Water deficit as a regulatory switch for legume root responses. Plant Signal. Behav. 2011, 6, 914–917. [Google Scholar] [CrossRef] [PubMed]
- Akcay, U.C.; Eercan, O.; Kavas, M.; Yildiz, L.; Yilmaz, C.; Octem, H.A.; Yucel, M. Drought-induced oxidative damage and antioxidant responses in peanut (Arachis hypogaea L.) seedlings. Plant Growth Regul. 2010, 61, 21–28. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox homeostasis and antioxidant signaling: A metabolic interface between stress perception and physiological responses. Plant Cell 2005, 17, 1866–1875. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J. Root architecture and plant productivity. Plant Physiol. 1995, 109, 7–13. [Google Scholar] [PubMed]
- Maurel, C.; Chrispeels, M.J. Aquaporins. A Molecular Entry into Plant Water Relations. Plant Physiol. 2001, 125, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.K.L.; Penna, S.; Nguyen, D.V.; Tran, L.S. Multifaceted roles of aquaporins as molecular conduits in plant responses to abiotic stresses. Crit. Rev. Biotechnol. 2014, 28, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gil-Quintana, E.; Larrainzar, E.; Seminario, A.; Díaz-Leal, J.L.; Alamillo, JM.; Pineda, M.; Arrese-Igor, C.; Wienkoop, S.; González, E.M. Local inhibition of nitrogen fixation and nodule metabolism in drought-stressed soybean. J. Exp. Bot. 2013, 64, 2171–2182. [Google Scholar] [CrossRef] [PubMed]
- Gordon, A.J.; Minchin, F.R.; Skot, L.; James, C.L. Stress-induced declines in soybean N2 fixation are related to nodule sucrose synthase activity. Plant Physiol. 1997, 114, 937–946. [Google Scholar] [PubMed]
- Arrese-Igor, C.; González, E.M.; Gordon, A.J.; Minchin, F.R.; Gálvez, L.; Royuela, M.; Cabrerizo, P.M.; Aparicio-Tejo, P.M. Sucrose synthase and nodule nitrogen fixation under drought and other environmental stresses. Symbiosis 1999, 27, 189–212. [Google Scholar]
- Serraj, R.; Vadez, V.; Sinclair, T.R. Feedback regulation of symbiotic N2 fixation under drought stress. Agronomie 2001, 21, 621–626. [Google Scholar] [CrossRef]
- King, C.A.; Purcell, L.C. Inhibition of N2 fixation in soybean is associated with elevated ureides and amino acids. Plant Physiol. 2005, 137, 1389–1396. [Google Scholar] [CrossRef] [PubMed]
- Ladrera, R.; Marino, D.; Larrainzar, E.; Gonzalez, E.M.; Arrese-Igor, C. Reduced carbon availability to bacteroids and elevated ureides in nodules, but not in shoots, are involved in the nitrogen fixation response to early drought in soybean. Plant Physiol 2007, 145, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Marino, D.; Frendo, P.; Ladrera, R.; Zabalza, A.; Puppo, A.; Arrese-Igor, C.; Gonzalez, E.M. Nitrogen fixation control under drought stress. Localized or systemic? Plant Physiol. 2007, 143, 1968–1974. [Google Scholar] [CrossRef] [PubMed]
- Alamillo, J.M.; Diaz-Leal, J.L.; Sanchez-Moran, M.V.; Pineda, M. Molecular analysis of ureide accumulation under drought stress in Phaseolus vulgaris L. Plant Cell Environ. 2010, 33, 1828–1837. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Leal, J.L.; Gálvez, V.G.; Fernandez, J.; Pineda, M.; Alamillo, J.M. Developmental effects on ureide levels are mediated by tissue-specific regulation of allantoinase in Phaseolus vulgaris L. J. Exp. Bot. 2012, 63, 4095–4106. [Google Scholar] [CrossRef] [PubMed]
- Purcell, LC.; Serraj, R.; de Silva, M.; Sinclair, T.R.; Bona, S. Ureide concentration of field-grown soybean in response to drought and the relationship to nitrogen fixation. J. Plant Nutr. 1998, 21, 949–966. [Google Scholar] [CrossRef]
- Serraj, R.; Sinclair, T.R.; Purcell, L.C. Symbiotic N2 fixation response to drought. J. Exp. Bot. 1999, 50, 143–155. [Google Scholar] [CrossRef]
- Vadez, V.; Sinclair, T.R.; Serraj, R. Asparagine and ureide accumulation in nodules and shoots as feedback inhibitors of N2 fixation in soybean. Physiol. Plant. 2000, 110, 215–223. [Google Scholar] [CrossRef]
- Pate, J.S.; Gunning, B.E.S.; Briarty, L.G. Ultrastructure and functioning of transport system of leguminous root nodule. Planta 1969, 85, 8–34. [Google Scholar] [CrossRef] [PubMed]
- Parsons, R.; Sprent, J.I.; Raven, J.A. Humidity and light affect the growth, development and nitrogenase activity of stem nodules of Sesbanio rostrata (Brem). New Phytol. 1993, 125, 744–755. [Google Scholar] [CrossRef]
- Valentine, A.J.; Benedito, V.A.; Kang, Y. Legume nitrogen fixation and soil abiotic stress: From physiology to genomics and beyond. Annu. Plant Rev. 2011, 42, 207–248. [Google Scholar]
- Larrainzar, E.; Wienkoop, S.; Scherling, C.; Kempa, S.; Ladrera, R.; Arrese-Igor, C.; Weckwerth, W.; Gonzalez, E.M. Carbon metabolism and bacteroid functioning are involved in the regulation of nitrogen fixation in Medicago truncatula under drought and recovery. Mol. Plant Microbe Interact. 2009, 22, 1565–1576. [Google Scholar] [CrossRef] [PubMed]
- Esfahani, M.N.; Sulieman, S.; Schulze, J.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Tran, L.S.P. Mechanisms of physiological adjustment of N2 fixation in Cicer arietinum L. (chickpea) during early stages of water deficit: Single or multi-factor controls. Plant J. 2014, 79, 964–980. [Google Scholar] [CrossRef] [PubMed]
- Furlan, A.L.; Bianucci, E.; del Carmen Tordable, M.; Castro, S.; Dietz, K.-J. Antioxidant enzyme activities and gene expression patterns in peanut nodules during a drought and rehydration cycle. Funct. Plant Biol. 2014, 41, 704–713. [Google Scholar] [CrossRef]
- Talbi, C.; Sanchez, C.; Hidalgo-Garcia, A.; Gonzalez, EM.; Arrese-Igor, C.; Girard, L.; Bedmar, E.J.; Delgado, M. Enhanced expression of Rhizobium etli cbb3 oxidase improves drought tolerance of common bean symbiotic nitrogen fixation. J. Exp. Bot. 2012, 63, 5035–5043. [Google Scholar] [CrossRef] [PubMed]
- Mandon, K.; Kaminski, P.A.; Elmerich, C. Functional analysis of the fixNOQP region of Azorhizobium caulinodans. J. Bacteriol. 1994, 176, 2560–2568. [Google Scholar] [PubMed]
- Farissi, M.; Bouizgaren, A.; Faghire, M.; Bargaz, A.; Ghoulam, C. Agro-physiological responses of Moroccan alfalfa (Medicago sativa L.) populations to salt stress during germination and early seedling stages. Seed Sci. Technol. 2011, 39, 389–401. [Google Scholar] [CrossRef]
- Farissi, M.; Faghire, M.; Bouizgaren, A.; Bargaz, A.; Makoudi, B.; Ghoulam, C. Growth, nutrients concentrations and enzymes involved in plants nutrition of alfalfa populations under saline conditions. J. Agric. Sci. Technol. 2014, 16, 301–314. [Google Scholar]
- Latrach, L.; Farissi, M.; Mouradi, M.; Makoudi, B.; Bouizgaren, A.; Ghoulam, C. Growth and nodulation in alfalfa-rhizobia symbiosis under salinity: Effect on electrolyte leakage, stomatal conductance and chlorophyll fluorescence. Turk. J. Agric. For. 2014, 38, 320–326. [Google Scholar] [CrossRef]
- Farissi, M.; Ghoulam, C.; Bouizgaren, A. Changes in water deficit saturation and photosynthetic pigments of Alfalfa populations under salinity and assessment of proline role in salt tolerance. Agric. Sci. Res. J. 2013, 3, 29–35. [Google Scholar]
- Salwa, A.O.; Mekki, B.B.; Faida, A.S. Alleviation of adverse effects of salt stress on faba bean (Vicia faba L.) plants by exogenous application of salicylic acid. World Appl. Sci. J. 2013, 27, 418–427. [Google Scholar]
- Faghire, M.; Farissi, M.; Bargaz, A.; Mandri, B.; Oufdou, K.; Amenc, L.; Cherki, G.; Drevon, J.J. Genotypic variation of nodules’ enzymatic activities in symbiotic nitrogen fixation among common bean (Phaseolus vulgaris L.) genotypes grown under salinity constraint. Symbiosis 2013, 60, 115–122. [Google Scholar] [CrossRef]
- Zhu, J.K. Regulation of ion homeostasis under salt stress. Curr. Opin. Plant Biol. 2003, 6, 441–445. [Google Scholar] [CrossRef]
- Aydi, S.; Sassi, S.; Debouba, M.; Hessini, K.; Larrainzar, E.; Gouia, H.; Abdelly, C. Resistance of Medicago truncatula to salt stress is related to glutamine synthetase activity and sodium sequestration. Plant Nutr. Soil Sci. 2010, 173, 892–899. [Google Scholar] [CrossRef]
- Krouma, A. Physiological and nutritional responses of chickpea (Cicer arietinum L.) to salinity. Turk. J. Agric. For. 2009, 33, 503–512. [Google Scholar]
- Fukuda, A.; Chiba, K.; Maeda, M.; Nakamura, A.; Maeshima, M.; Tanaka, Y. Effect of salt and osmotic stresses on the expression of genes for the vacuolar H+-pyrophosphatase, H+-ATPase subunit A, and Na+/H+ antiporter from barley. J. Exp. Bot. 2004, 55, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Faghire, M.; Bargaz, A.; Farissi, M.; Palma, F.; Mandri, B.; Lluch, C. Effect of salinity on nodulation, nitrogen fixation and growth of common bean (Phaseolus vulgaris L.) inoculated with rhizobial strains isolated from the Haouz region of Morocco. Symbiosis 2011, 55, 69–75. [Google Scholar] [CrossRef]
- Mezni, M.; Albouchi, A.; Bizid, E.; Hamza, M. Minerals uptake, organic osmotica contents and water balance in alfalfa under salt stress. J. Phytol. 2010, 2, 1–12. [Google Scholar]
- Trinchant, J.C.; Boscari, A.; Spennato, G.; van de Sype, G.; le Rudulier, D. Proline betaine accumulation and metabolism in alfalfa plants under sodium chloride stress. Exploring its compartmentalization in nodules. Plant Physiol. 2004, 135, 1583–1594. [Google Scholar] [CrossRef] [PubMed]
- Özge, Ç.; Atak, Ç. Evaluation of Proline Accumulation and Δ1-pyrroline-5-carboxylate Synthetase (P5CS) Gene Expression during Salinity Stress in Two Soybean (Glycine max L. Merr.) Varieties. Pol. J. Environ. Stud. 2012, 3, 559–564. [Google Scholar]
- Verdoy, D.; de la Pena, T.C.; Redondo, F.J.; Lucas, M.M.; Pueyo, J.J. Transgenic Medicago truncatula L. plants that accumulate proline display nitrogen fixing activity with enhanced tolerance to osmotic stress. Plant Cell Environ. 2006, 29, 1913–1923. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.B.; Wang, S.M.; Jing, R.L.; Mao, X.G. Cloning the PvP5CS gene from common bean (Phaseolus vulgaris) and its expression patterns under abiotic stresses. J. Plant Physiol. 2009, 166, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Slama, I.; Ghnaya, T.; Savouré, A.; Abdelly, C. Combined effects of long-term salinity and soil drying on growth, water relations, nutrient status and proline accumulation of Sesuvium portulacastrum. C. R. Biol. 2008, 331, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Ottow, E.; Brinker, M.; Fritz, E.; Teichmann, T.; Kaiser, W.; Brosche, M.; Kangasjarvi, J.; Jiang, X.; Polle, A. Populus euphratica displays apoplastic sodium accumulation, osmotic adjustment by decreases in calcium and soluble carbohydrates, and develops leaf succulence under salt stress. Plant Physiol. 2005, 139, 1762–1772. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.Y.; Harris, P.J.C. Potential biochemical indicators of salinity tolerance in plants. Plant Sci. 2004, 166, 3–16. [Google Scholar] [CrossRef]
- Supaporn, H.; Valentine, N.; Kanyaratt, S.; Masahiro, M.; Ikuo, N. Expression of Indica rice OsBADH1 gene under salinity stress in transgenic tobacco. Plant Biotechnol. Rep. 2010, 4, 75–83. [Google Scholar]
- Tuteja, N. Mechanisms of high salinity tolerance in plants. Methods Enzymol. 2007, 428, 419–438. [Google Scholar] [PubMed]
- Phillips, D.A.; Joseph, C.M.; Maxwell, C.A. Trigonelline and stachydrine released from alfalfa seeds activate NodD2 protein in Rhizobium meliloti. Plant Physiol. 1992, 99, 1526–1531. [Google Scholar] [CrossRef] [PubMed]
- Le Rudulier, D.; Bernard, T. Salt tolerance in Rhizobium: A possible role for betaines. FEMS Microbiol. Rev. 1986, 39, 67–72. [Google Scholar] [CrossRef]
- Smith, L.T.; Pocard, J.A.; Bernard, T.; le Rudulier, D. Osmotic control of glycine betaine biosynthesis and degradation in Rhizobium meliloti. J. Bacteriol. 1988, 170, 3142–3149. [Google Scholar] [PubMed]
- Boscari, A.; Mandon, K.; Dupont, L.; Poggi, M.-C.; le Rudulier, D. BetS Is a Major Glycine Betaine/Proline Betaine Transporter Required for Early Osmotic Adjustment in Sinorhizobium meliloti. J. Bacteriol. 2002, 184, 2654–2663. [Google Scholar] [CrossRef] [PubMed]
- Bayuelo-Jimenez, J.S.; Jasso-Plata, N.; Ochoa, I. Growth and Physiological Responses of Phaseolus Species to Salinity Stress. Int. J. Agro. 2012, 2012, 527673–527685. [Google Scholar]
- Zhu, J.K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 2002, 53, 247–273. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Kaur, N. Sugar signalling and gene expression in relation to carbohydrate metabolism under abiotic stresses in plants. J. Biosci. 2005, 30, 761–776. [Google Scholar] [CrossRef] [PubMed]
- Pallavi, S.; Ambuj Bhushan, J.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 217027–217052. [Google Scholar]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Wang, W.B.; Kim, Y.H.; Lee, H.S.; Kim, K.Y.; Deng, X.P.; Kwak, S.S. Analysis of antioxidant enzyme activity during germination of alfalfa under salt and drought stresses. Plant Physiol. Biochem. 2009, 4, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Ghoulam, C.; Foursy, A.; Fares, K. Effects of salt stress on growth, inorganic ions and proline accumulation in relation to osmotic adjustment in five sugar beet cultivars. Environ. Exp. Bot. 2002, 47, 39–50. [Google Scholar] [CrossRef]
- Amudha, J.; Balasubramani, G. Recent molecular advances to combat abiotic stress tolerance in crop plants. Biotechnol. Mol. Biol. Rev. 2011, 6, 31–58. [Google Scholar]
- Gratao, P.L.; Polle, A.; Lea, P.J.; Azevedo, R.A. Making the life of heavy metal-stressed plants a little easier. Funct. Plant Biol. 2005, 32, 481–494. [Google Scholar] [CrossRef]
- Mhadhbi, H.; Jebara, M.; Limam, F.; Aouani, M.E. Rhizobial strain involvement in plant growth, nodule protein composition and antioxidant enzyme activities of chickpea rhizobia symbioses: Modulation by salt stress. Plant Physiol. Biochem. 2004, 42, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Arab, L.; Hsanpour, A. The effects of ascorbic acid on salt induced alfalfa (Medicago sativa L.) in vitro culture. Biokemistri 2006, 18, 63–69. [Google Scholar] [CrossRef]
- Mahmoudi, H.; Ben Nasri, M.; Baâtour, O.; Huang, J.; Tarchoun, I.; Nasri, N.; Rym, K.; Gruber, M.; Lachaâl, M.; Hannoufa, A.; et al. Differential response to elevated NaCl by antioxidant enzymes and gene transcripts in two contrasting lettuce genotypes. Aust. J. Crop Sci. 2012, 6, 632–640. [Google Scholar]
- Tejera, N.A.; Campos, R.; Sanjuan, J.; Lluch, C. Nitrogenase and antioxidant enzyme activities in Phaseolus vulgaris nodules formed by Rhizobium tropici isogenic strains with varying tolerance to salt stress. J. Plant Physiol. 2004, 161, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Mhadhbi, H.; Fotopoulos, V.; Mylona, P.V.; Jebara, M.; Elarbi Aouani, M.; Polidoros, A.N. Antioxidant gene–enzyme responses in Medicago truncatula genotypes with different degree of sensitivity to salinity. Physiol. Plant. 2011, 141, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Molina, C.; Zaman-Allah, M.; Khan, F.; Fatnassi, N.; Horres, R.; Rotter, B.; Steinhauer, D.; Amenc, L.; Drevon, J.J.; Winter, P.; et al. The salt-responsive transcriptome of chickpea roots and nodules via deep super SAGE. BMC Plant Biol. 2011, 11–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krejsa, C.M.; Schieven, G.L. Impact of oxidative stress on signal transduction control by phosphotyrosine phosphatases. Environ. Health Perspect. 1998, 106, 1179–1184. [Google Scholar] [CrossRef] [PubMed]
- Postnikova, O.A.; Shao, J.; Nemchinov, L.G. Analysis of the alfalfa root transcriptome in response to salinity stress. Plant Cell Physiol. 2013, 54, 1041–1055. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.W.; Thorn, P. Calcium signalling: IP3 rises again and again. Curr. Biol. 2001, 11, 352–355. [Google Scholar] [CrossRef]
- Burnette, R.N.; Gunesekera, B.M.; Gillaspy, G.E. An Arabidopsis inositol 5-phosphatase gain-of-function alters abscisic acid signaling. Plant Physiol. 2003, 132, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, J.M.; Perera, I.Y.; Heilmann, I.; Persson, S.; Boss, W.F. Inositol signaling and plant growth. Trends Plant Sci. 2000, 5, 252–258. [Google Scholar] [CrossRef]
- Goddijn, O.J.; van Dun, K. Trehalose metabolism in plants. Trends Plant Sci. 1999, 4, 315–319. [Google Scholar] [CrossRef]
- Lopez, M.; Herrera-Cervera, J.A.; Lluch, C.; Tejera, N.A. Trehalose metabolism in root nodules of the model legume Lotus japonicas in response to salt stress. Physiol. Plant. 2006, 128, 701–709. [Google Scholar] [CrossRef]
- Streeter, J.G.; Gomez, M.L. Three enzymes for trehalose synthesis in Bradyrhizobium cultured bacteria and in bacteroids from soybean nodules. Appl. Environ. Microbiol. 2006, 72, 4250–4255. [Google Scholar] [CrossRef] [PubMed]
- Salminen, S.O.; Streeter, J.G. Enzymes of a-trehalose metabolism in soybean nodules. Plant Physiol. 1986, 81, 538–541. [Google Scholar] [CrossRef] [PubMed]
- Streeter, J.G. Carbohydrates in soybean nodules. II. Distribution of compounds in seedlings during the onset of nitrogen fixation. Plant Physiol. 1980, 66, 471–476. [Google Scholar] [CrossRef] [PubMed]
- James, E.K.; Sprent, J.I.; Minchin, F.R.; Brewin, N.J. Intercellular location of glycoprotein in soybean nodules: Effect of altered rhizosphere oxygen concentration. Plant Cell Environ. 1991, 14, 467–474. [Google Scholar] [CrossRef]
- Streeter, J.G. Analysis of apoplastic solutes in the cortex of soybean nodules. Plant Physiol. 1992, 85, 768–773. [Google Scholar] [CrossRef]
- Purcell, L.C.; Sinclair, T.R. An osmotic hypothesis for the regulation of oxygen permeability in soybean nodules. Plant Cell Environ. 1994, 17, 837–843. [Google Scholar] [CrossRef]
- Denison, R.F.; Kinraide, T.B. Oxygen membrane depolarization in legume root nodule: Possible evidence for an osmoelectrical mechanism controlling nodule gas permeability. Plant Physiol. 1995, 108, 235–240. [Google Scholar] [PubMed]
- Palma, F.; López-Gómez, M.; Tejera, N.A.; Lluch, C. Salicylic acid improves the salinity tolerance of Medicago sativa in symbiosis with Sinorhizobium meliloti by preventing nitrogen fixation inhibition. Plant Sci. 2013, 208, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.T.; Ma, S.L.; Bai, L.P.; Zhang, L.; Ma, H.; Jia, P.; Liu, J.; Zhong, M.; Guo, Z.F. Signal transduction during cold, salt, and drought stresses in plants. Mol. Biol. Rep. 2012, 39, 969–987. [Google Scholar] [CrossRef] [PubMed]
- Javid, M.G.; Sorooshzadeh, A.; Moradi, F.; Sanavy, S.A.; Allahdadi, I. The role of phytohormones in alleviating salt stress in crop plants. Aust. J. Crop Sci. 2011, 5, 726–734. [Google Scholar]
- Hu, Y.J.; Shi, L.X.; Sun, W.; Guo, J.X. Effects of abscisic acid and brassinolide on photosynthetic characteristics of Leymus chinensis from Songnen Plain grassland in Northeast China. Bot. Stud. 2013, 54, 1–9. [Google Scholar] [CrossRef]
- Khadri, M.; Tejera, N.A.; Lluch, C. Alleviation of salt stress in common bean (Phaseolus vulgaris) by exogenous abscisic acid supply J. Plant Growth Regul. 2006, 25, 110–119. [Google Scholar] [CrossRef]
- Salah, B.; Jelali, N.; Slatni, T.; Gruber, M.; Albacete, A.; Martinez Andujar, C.; Martinez, V.; Perez-Alfocea, F.; Abdelly, C. Involvement of source sink relationship and hormonal control in the response of Medicago ciliaris-Sinorhizobium medicae symbiosis to salt stress. Acta Biol. Hung. 2012, 63, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Saeidi-Sar, S.; Abbaspour, H.; Afshari, H.; Yaghoobi, S.R. Effects of ascorbic acid and gibberellin AG3 on alleviation of salt stress in common bean (Phaseolus vulgaris L.) seedlings. Acta Physiol. Plant. 2012, 35, 667–677. [Google Scholar] [CrossRef]
- Katagiri, F. A global view of defense gene expression regulation—A highly interconnected signaling network. Curr. Opin. Plant Biol. 2004, 7, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Chinnusamy, V.; Schumaker, K.; Zhu, J.K. Molecular genetic perspectives on cross-talk and specificity in abiotic stress signaling in plants. J. Exp. Bot. 2004, 55, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Schumaker, K.S.; Zhu, J.K. Cell signaling during cold, drought and salt stress. Plant Cell 2002, 14, S165–S183. [Google Scholar] [PubMed]
- Jung, J.; Park, C. Auxin modulation of salt stress signaling in Arabidopsis seed germination. Plant Signal. Behav. 2011, 6, 1198–1200. [Google Scholar] [CrossRef] [PubMed]
- Aroca, R.; Ruiz-Lozano, J.M. Induction of plant tolerance to semi-arid environments by beneficial soil microorganisms—A review. Sustain. Agric. Rev. 2009, 2, 121–136. [Google Scholar]
- Smith, S.E.; Smith, F.A. Roles of arbuscular mycorrhizas in plant nutrition and growth: New paradigms from cellular to ecosystem scales. Annu. Rev. Plant Biol. 2011, 62, 227–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, Q.; Yang, R.; Long, L.; Zhu, H. Phosphate application enhances the resistance of arbuscular mycorrhizae in clover plants to cadmium via polyphosphate accumulation in fungal hyphae. Environ. Exp. Bot. 2014, 108, 63–70. [Google Scholar] [CrossRef]
- Li, J.; Bao, S.Q.; Zhang, Y.H.; Ma, X.J.; Mishra-Knyrim, M.; Sun, J.; Sa, G.; Shen, X.; Polle, A.; Chen, S.L. Paxillus involutus strains MAJ and NAU mediate K+/Na+ homeo-stasis in ectomycorrhizal Populus × canescens under sodium chloride stress. Plant Physiol. 2012, 159, 1771–1786. [Google Scholar] [CrossRef] [PubMed]
- De Vallavieille-Pope, C. Management of disease resistance diversity of cultivars of a species in single fields: Controlling epidemics. C. R. Biol. 2004, 327, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, S.-M.; Sun, J.-H.; Zhou, L.-L.; Bao, X.-G.; Zhang, H.-G.; Zhang, F.-S. Diversity enhances agricultural productivity via rhizosphere phosphorus facilitation on phosphorus-deficient soils. Proc. Natl. Acad. Sci. USA 2007, 27, 11192–11196. [Google Scholar] [CrossRef] [PubMed]
- Hauggaard-Nielsen, H.; Ambus, P.; Jensen, E.S. Interspecific competition, N use and interference with weeds in pea–barley intercropping. Field Crops Res. 2001, 70, 101–109. [Google Scholar] [CrossRef]
- Hauggaard-Nielsen, H.; Jørnsgaard, B.; Kinane, J.; Jensen, E.S. Grain legume–cereal intercropping: The practical application of diversity, competition and facilitation in arable and organic cropping systems. Renew. Agric. Food Syst. 2008, 23, 3–12. [Google Scholar] [CrossRef]
- Palmborg, C.; Scherer-Lorenzen, M.; Jumpponen, A.; Carlsson, G.; Huss-Danell, K.; Högberg, P. Inorganic soil nitrogen under grassland plant communities of different species composition and diversity. Oikos 2005, 110, 271–282. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bargaz, A.; Zaman-Allah, M.; Farissi, M.; Lazali, M.; Drevon, J.-J.; Maougal, R.T.; Georg, C. Physiological and Molecular Aspects of Tolerance to Environmental Constraints in Grain and Forage Legumes. Int. J. Mol. Sci. 2015, 16, 18976-19008. https://doi.org/10.3390/ijms160818976
Bargaz A, Zaman-Allah M, Farissi M, Lazali M, Drevon J-J, Maougal RT, Georg C. Physiological and Molecular Aspects of Tolerance to Environmental Constraints in Grain and Forage Legumes. International Journal of Molecular Sciences. 2015; 16(8):18976-19008. https://doi.org/10.3390/ijms160818976
Chicago/Turabian StyleBargaz, Adnane, Mainassara Zaman-Allah, Mohamed Farissi, Mohamed Lazali, Jean-Jacques Drevon, Rim T. Maougal, and Carlsson Georg. 2015. "Physiological and Molecular Aspects of Tolerance to Environmental Constraints in Grain and Forage Legumes" International Journal of Molecular Sciences 16, no. 8: 18976-19008. https://doi.org/10.3390/ijms160818976






