Wnt3a Promotes the Vasculogenic Mimicry Formation of Colon Cancer via Wnt/β-Catenin Signaling
Abstract
:1. Introduction
2. Results
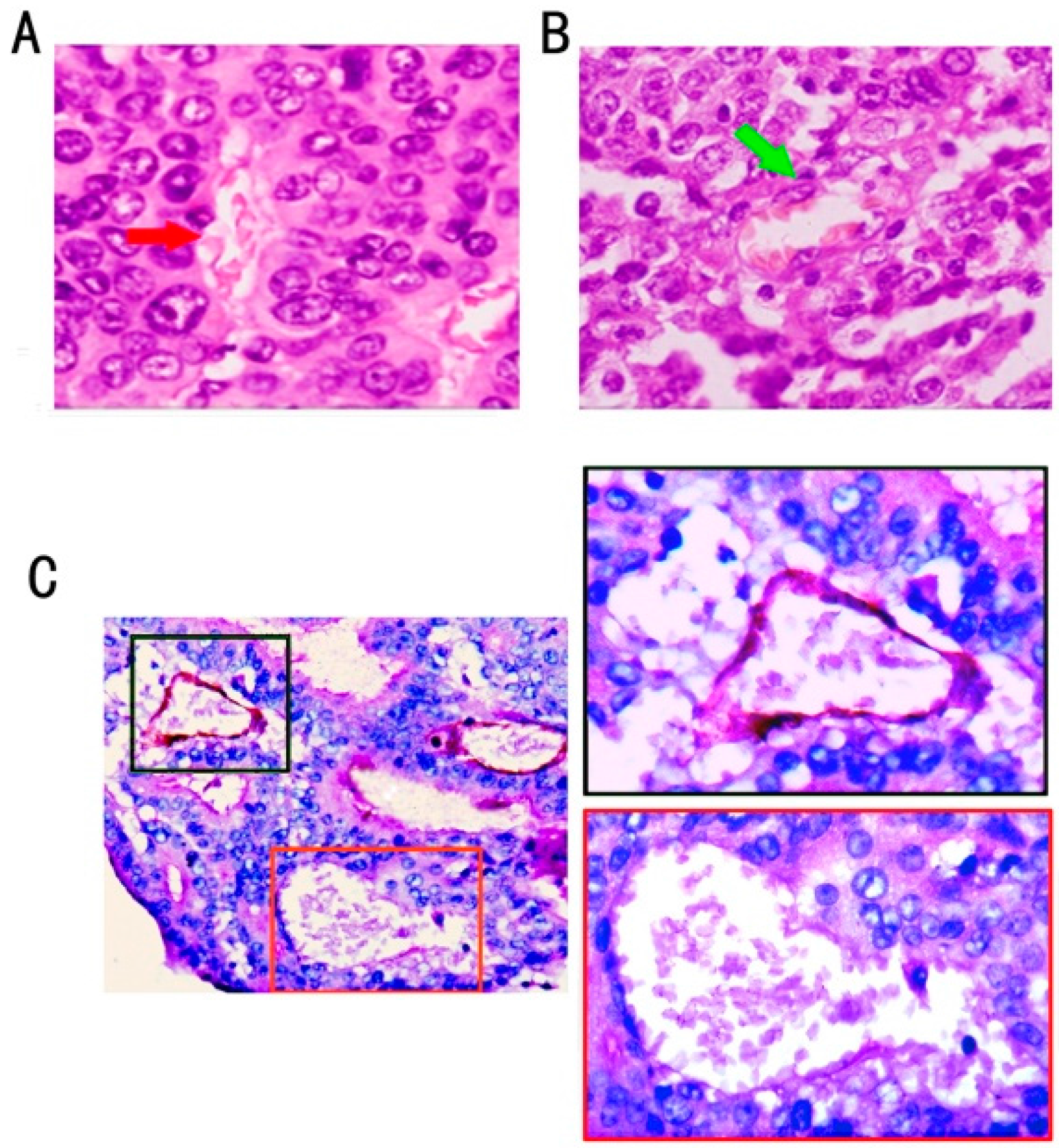
2.1. Association of VM Frequency with Clinicopathological Features of Colon Cancer Cases
| Variable | Total (%) | Tissue Samples | χ2 | p Value | |||||
|---|---|---|---|---|---|---|---|---|---|
| VM (%) | nonVM (%) | ||||||||
| Age | |||||||||
| <45 | 28 (12.9) | 7 (25.0) | 21 (75.0) | 1.077 | 0.298 | ||||
| ≥45 | 189 (87.1) | 32 (16.9) | 157 (83.1) | ||||||
| Sex | |||||||||
| Male | 101 (46.5) | 19 (18.8) | 82 (81.2) | 0.09 | 0.86 | ||||
| Female | 116 (53.5) | 20 (17.2) | 96 (82.8) | ||||||
| Location | |||||||||
| Left hemicolon | 125 (57.6) | 25 (20.0) | 100 (80.0) | 0.475 | 0.822 | ||||
| Right hemicolon | 92 (42.4) | 14 (15.2) | 78 (84.8) | ||||||
| Tumor size(cm) | |||||||||
| ≥10 | 25 (11.5) | 6 (24) | 19 (76) | 0.70 | 0.278 | ||||
| <10 | 192 (88.5) | 33 (17.2) | 159 (82.8) | ||||||
| Histological differentiation | |||||||||
| Well differentiated | 14 (6.4) | 1 (7.1) | 13 (92.9) | 72.11 | <0.001 * | ||||
| Moderately differentiated | 109 (50.2) | 8 (7.3) | 101 (92.7) | ||||||
| Poorly differentiated | 53 (24.4) | 30 (56.6) | 23 (43.4) | ||||||
| Mucinous carcinoma | 41 (18.9) | 0 (0.0) | 41 (100.0) | ||||||
| TNM stage | |||||||||
| TNMⅠ | 10 (4.6) | 0 (0.0) | 10 (100.0) | 23.20 | <0.001 * | ||||
| TNMⅡ | 138 (63.6) | 16 (11.6) | 122 (88.4) | ||||||
| TNMⅢ | 57 (26.3) | 16 (28.1) | 41 (71.9) | ||||||
| TNMⅣ | 12 (5.5) | 7 (58.3) | 5 (41.7) | ||||||
| Metastasis/recurrence | |||||||||
| Present | 77 (35.5) | 24 (31.2) | 53 (68.8) | 14.099 | <0.001 * | ||||
| Abscent | 140 (64.5) | 15 (10.7) | 125 (89.3) | ||||||
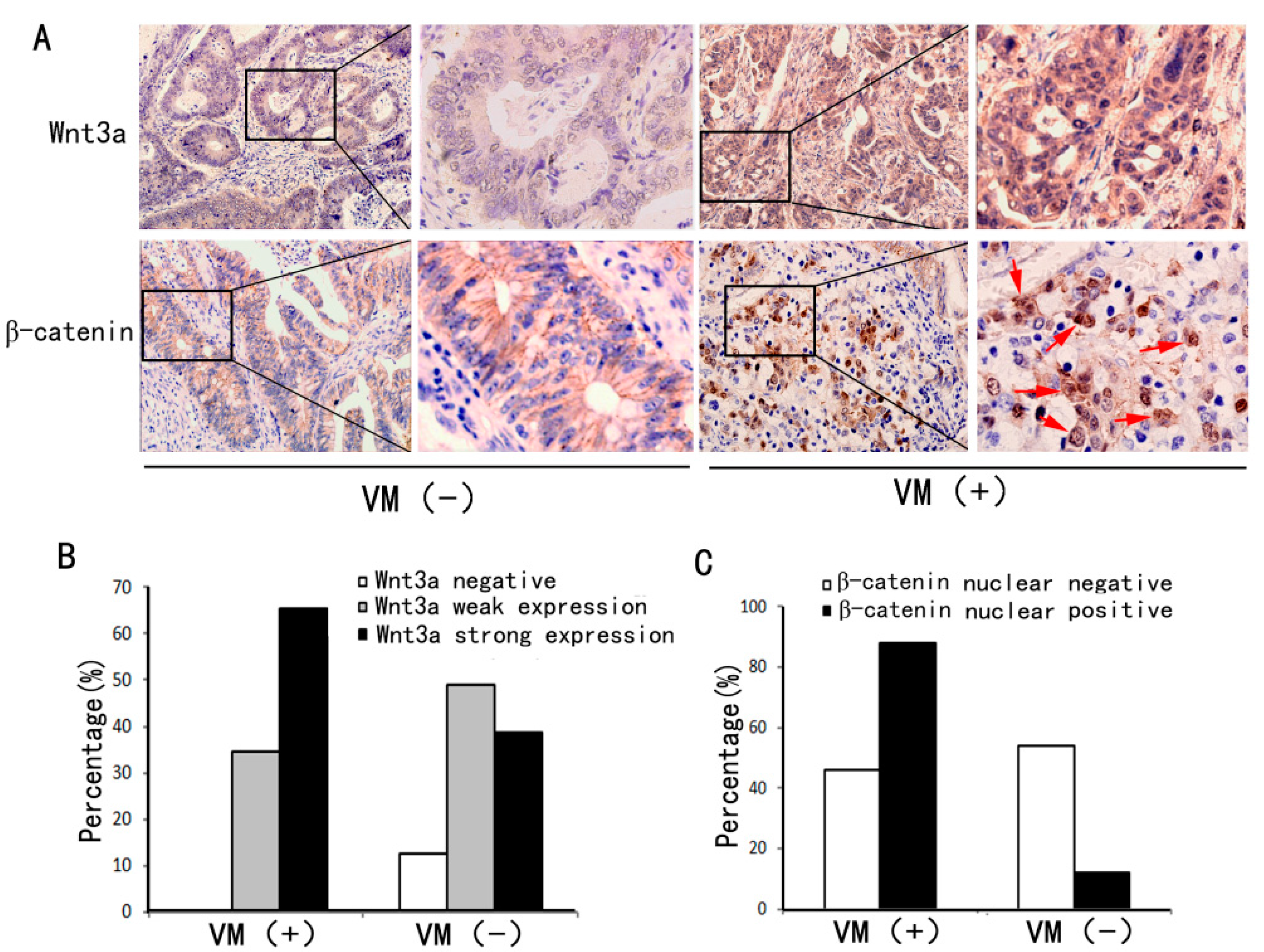
| Wnt3a expression | |||||||||
| Negative | 22(10.1) | 0(0.0) | 22(12.4) | 17.7 | <0.001 * | ||||
| Weak expression | 97(44.7) | 10(34.5) | 87(48.9) | ||||||
| Strong expression | 98(45.2) | 29(65.5) | 69(38.7) | ||||||
| β-catenin expression | |||||||||
| Nuclear negative | 175 (80.6) | 18 (46.2) | 157 (88.2) | 36.2 | <0.001 * | ||||
| Nuclear positive | 42 (19.4) | 21 (53.8) | 21 (11.8) | ||||||
2.2. VM Was Associated with Wnt3a Expression and reCatenin Nuclear Expression
2.3. Wnt3a Overexpression Induced the Activation of Canonical Wnt Signaling in HT29 Cells

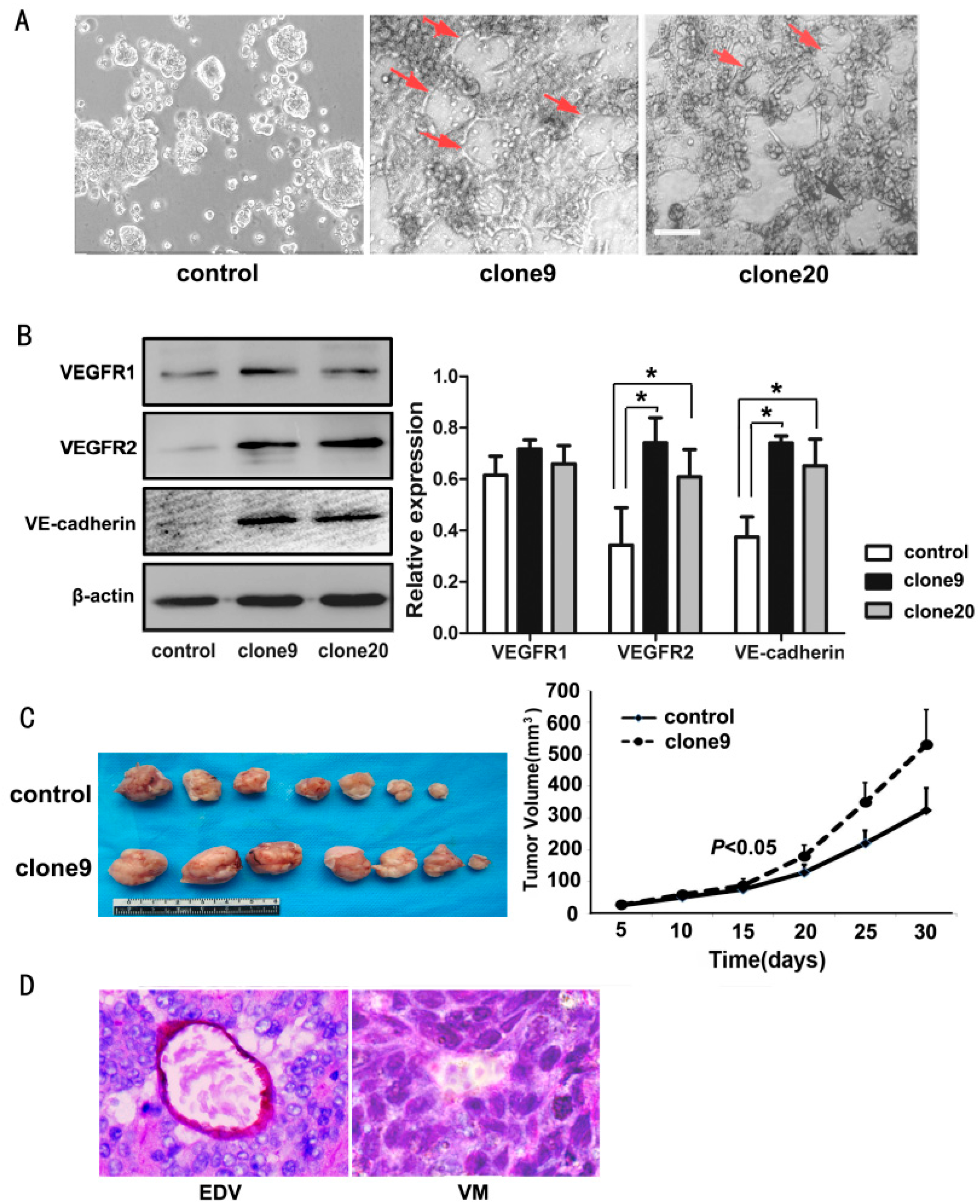
2.4. Wnt3a Overexpression Promoted the Tube-Like Structure Formation of HT29 Cells in Vitro and Promoted in Vivo Tumor Growth and VM Formation in Animal Models
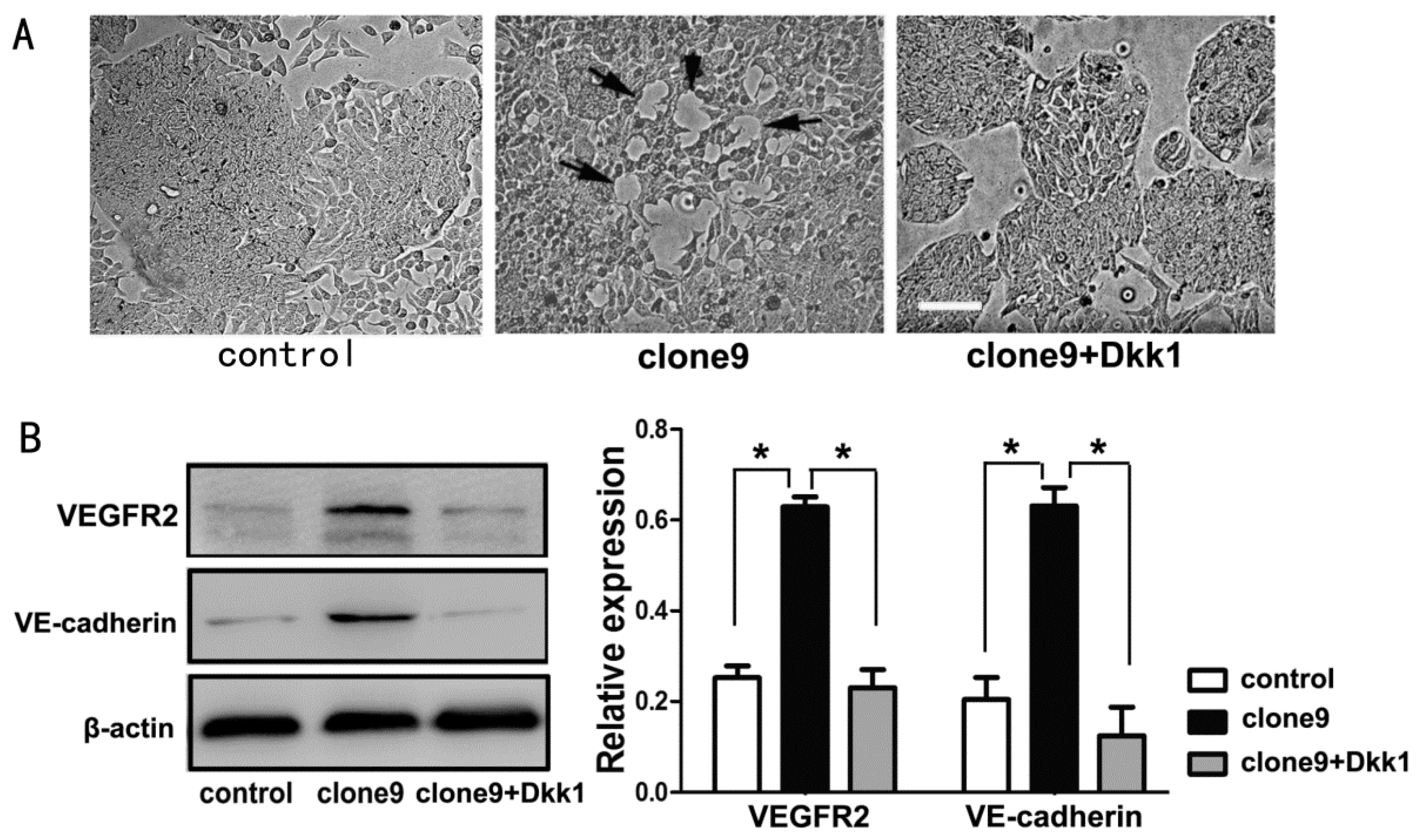
2.5. Dkk1 Inhibited the Tube-Structure Formation in Vitro and Restored the VEGFR2 and VE-Cadherin Expression in Wnt3a-Upregulation HT29 Cells
3. Discussion
4. Materials and Methods
4.1. Tissue Samples
4.2. Cells and Reagents
4.3. Tissue Immunohistochemical Analysis
4.4. CD34/Periodic Acid Schiff Double Staining
4.5. Plasmid Transfection
4.6. Western Blot Analysis
4.7. Immunofluorescence
4.8. In Vitro Three-Dimensional (3-D) Coculture
4.9. Xenograft Mouse Model
4.10. Statistical Analysis
5. Conclusions
Ackonwledgments
Author Contributions
Conflicts of Interest
References
- Moschetta, M.; Mishima, Y.; Sahin, I.; Manier, S.; Glavey, S.; Vacca, A.; Roccaro, A.M.; Ghobrial, I.M. Role of endothelial progenitor cells in cancer progression. Biochim. Biophys. Acta 2014, 1846, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Pezzella, F.; Gatter, K. Non-angiogenic tumours unveil a new chapter in cancer biology. J. Pathol. 2015, 235, 381–383. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.N. Hijacking the vasculature in ccrcc-co-option, remodelling and angiogenesis. Nat. Rev. Urol. 2013, 10, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Maniotis, A.J.; Folberg, R.; Hess, A.; Seftor, E.A.; Gardner, L.M.; Pe’er, J.; Trent, J.M.; Meltzer, P.S.; Hendrix, M.J. Vascular channel formation by human melanoma cells in vivo and in vitro: Vasculogenic mimicry. Am. J. Pathol. 1999, 155, 739–752. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, D.; Wang, Y.; Zhao, W.; Guo, H.; Zhao, X.; Sun, B. Morphologic research of microcirculation patterns in human and animal melanoma. Med. Oncol. 2006, 23, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Frenkel, S.; Barzel, I.; Levy, J.; Lin, A.Y.; Bartsch, D.U.; Majumdar, D.; Folberg, R.; Pe’er, J. Demonstrating circulation in vasculogenic mimicry patterns of uveal melanoma by confocal indocyanine green angiography. Eye 2008, 22, 948–952. [Google Scholar] [CrossRef] [PubMed]
- Ruf, W.; Seftor, E.A.; Petrovan, R.J.; Weiss, R.M.; Gruman, L.M.; Margaryan, N.V.; Seftor, R.E.; Miyagi, Y.; Hendrix, M.J. Differential role of tissue factor pathway inhibitors 1 and 2 in melanoma vasculogenic mimicry. Cancer Res. 2003, 63, 5381–5389. [Google Scholar] [PubMed]
- Zhang, S.; Fu, Z.; Wei, J.; Guo, J.; Liu, M.; Du, K. Peroxiredoxin 2 is involved in vasculogenic mimicry formation by targeting vegfr2 activation in colorectal cancer. Med. Oncol. 2015, 32, 414. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Sun, B.; Zhao, X.; Ma, Y.; Ji, R.; Gu, Q.; Dong, X.; Li, J.; Liu, F.; Jia, X.; et al. Twist1 expression induced by sunitinib accelerates tumor cell vasculogenic mimicry by increasing the population of cd133+ cells in triple-negative breast cancer. Mol. Cancer 2014, 13, 207. [Google Scholar] [CrossRef] [PubMed]
- Kaessmeyer, S.; Bhoola, K.; Baltic, S.; Thompson, P.; Plendl, J. Lung cancer neovascularisation: Cellular and molecular interaction between endothelial and lung cancer cells. Immunobiology 2014, 219, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Sun, B.; Zhao, X.; Gu, Q.; Dong, X.; Mo, J.; Sun, T.; Wang, J.; Sun, R.; Liu, Y. Hypoxia promotes vasculogenic mimicry formation by inducing epithelial-mesenchymal transition in ovarian carcinoma. Gynecol. Oncol. 2014, 133, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, X.; Du, W.; Chen, L.; Wang, G.; Cui, Y.; Liu, Y.; Dou, Z.; Wang, H.; Zhang, P.; et al. Suppressor of fused (sufu) represses gli1 transcription and nuclear accumulation, inhibits glioma cell proliferation, invasion and vasculogenic mimicry, improving glioma chemo-sensitivity and prognosis. Oncotarget 2014, 5, 11681–11694. [Google Scholar] [PubMed]
- Liu, T.; Sun, B.; Zhao, X.; Li, Y.; Gu, Q.; Dong, X.; Liu, F. Oct4 expression and vasculogenic mimicry formation positively correlate with poor prognosis in human breast cancer. Int. J. Mol. Sci. 2014, 15, 19634–19649. [Google Scholar] [CrossRef] [PubMed]
- Soda, Y.; Myskiw, C.; Rommel, A.; Verma, I.M. Mechanisms of neovascularization and resistance to anti-angiogenic therapies in glioblastoma multiforme. J. Mol. Med. 2013, 91, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, Q.; Li, X.Y.; Yang, Q.Y.; Xu, W.W.; Liu, G.L. Short-term anti-vascular endothelial growth factor treatment elicits vasculogenic mimicry formation of tumors to accelerate metastasis. J. Exp. Clin. Cancer Res. 2012, 31, 16. [Google Scholar] [CrossRef] [PubMed]
- Reis, M.; Liebner, S. Wnt signaling in the vasculature. Exp. Cell Res. 2013, 319, 1317–1323. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Park, H.; Lee, H.W.; Kwon, Y.G. The wnt pathway and the roles for its antagonists, dkks, in angiogenesis. IUBMB Life 2012, 64, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Dejana, E. The role of wnt signaling in physiological and pathological angiogenesis. Circ. Res. 2010, 107, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Vincan, E.; Darcy, P.K.; Farrelly, C.A.; Faux, M.C.; Brabletz, T.; Ramsay, R.G. Frizzled-7 dictates three-dimensional organization of colorectal cancer cell carcinoids. Oncogene 2007, 26, 2340–2352. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Sun, B.; Liu, Z.; Cheng, R.; Li, Y.; Zhao, X. Wnt3a expression is associated with epithelial-mesenchymal transition and promotes colon cancer progression. J. Exp. Clin. Cancer Res. 2014, 33, 107. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Sun, B.; Liu, Z.; Li, H.; Gao, J.; Leng, X. Dickkopf-1 inhibits epithelial-mesenchymal transition of colon cancer cells and contributes to colon cancer suppression. Cancer Sci. 2012, 103, 828–835. [Google Scholar] [CrossRef] [PubMed]
- Lavergne, E.; Hendaoui, I.; Coulouarn, C.; Ribault, C.; Leseur, J.; Eliat, P.A.; Mebarki, S.; Corlu, A.; Clement, B.; Musso, O. Blocking wnt signaling by sfrp-like molecules inhibits in vivo cell proliferation and tumor growth in cells carrying active beta-catenin. Oncogene 2011, 30, 423–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, H.; Sun, B.; Zhao, X.; Du, J.; Gu, Q.; Liu, Y.; Cheng, R.; Dong, X. Wnt5a promotes vasculogenic mimicry and epithelial-mesenchymal transition via protein kinase calpha in epithelial ovarian cancer. Oncol. Rep. 2014, 32, 771–779. [Google Scholar] [PubMed]
- Yao, L.; Sun, B.; Zhao, X.; Zhao, X.; Gu, Q.; Dong, X.; Zheng, Y.; Sun, J.; Cheng, R.; Qi, H.; et al. Overexpression of wnt5a promotes angiogenesis in nsclc. BioMed Res. Int. 2014, 2014, 832562. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Sun, B.; Qi, L.; Li, H.; Gao, J.; Leng, X. Zinc finger e-box binding homeobox 1 promotes vasculogenic mimicry in colorectal cancer through induction of epithelial-to-mesenchymal transition. Cancer Sci. 2012, 103, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Corada, M.; Nyqvist, D.; Orsenigo, F.; Caprini, A.; Giampietro, C.; Taketo, M.M.; Iruela-Arispe, M.L.; Adams, R.H.; Dejana, E. The wnt/beta-catenin pathway modulates vascular remodeling and specification by upregulating dll4/notch signaling. Dev. Cell 2010, 18, 938–949. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xiao, Y.; Mou, Y.; Zhao, Y.; Blankesteijn, W.M.; Hall, J.L. A role for the beta-catenin/t-cell factor signaling cascade in vascular remodeling. Circ. Res. 2002, 90, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, A.M.; Kitajewski, J.; D'Amore, P.A. Wnt1 and wnt5a affect endothelial proliferation and capillary length; wnt2 does not. Growth Factors 2007, 25, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Blankesteijn, W.M.; van Gijn, M.E.; Essers-Janssen, Y.P.; Daemen, M.J.; Smits, J.F. Beta-catenin, an inducer of uncontrolled cell proliferation and migration in malignancies, is localized in the cytoplasm of vascular endothelium during neovascularization after myocardial infarction. Am. J. Pathol. 2000, 157, 877–883. [Google Scholar] [CrossRef]
- Masckauchan, T.N.; Shawber, C.J.; Funahashi, Y.; Li, C.M.; Kitajewski, J. Wnt/beta-catenin signaling induces proliferation, survival and interleukin-8 in human endothelial cells. Angiogenesis 2005, 8, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Seftor, E.A.; Meltzer, P.S.; Schatteman, G.C.; Gruman, L.M.; Hess, A.R.; Kirschmann, D.A.; Seftor, R.E.; Hendrix, M.J. Expression of multiple molecular phenotypes by aggressive melanoma tumor cells: Role in vasculogenic mimicry. Crit. Rev. Oncol./Hematol. 2002, 44, 17–27. [Google Scholar] [CrossRef]
- Francescone, R.; Scully, S.; Bentley, B.; Yan, W.; Taylor, S.L.; Oh, D.; Moral, L.; Shao, R. Glioblastoma-derived tumor cells induce vasculogenic mimicry through flk-1 protein activation. J. Biol. Chem. 2012, 287, 24821–24831. [Google Scholar] [CrossRef] [PubMed]
- Hendrix, M.J.; Seftor, E.A.; Meltzer, P.S.; Gardner, L.M.; Hess, A.R.; Kirschmann, D.A.; Schatteman, G.C.; Seftor, R.E. Expression and functional significance of ve-cadherin in aggressive human melanoma cells: Role in vasculogenic mimicry. Proc. Natl. Acad. Sci. USA 2001, 98, 8018–8023. [Google Scholar] [CrossRef] [PubMed]
- Fonar, Y.; Frank, D. Fak and wnt signaling: The meeting of two pathways in cancer and development. Anti-Cancer Agents Med. Chem. 2011, 11, 600–606. [Google Scholar] [CrossRef]
- Van den Bosch, M.H.; Blom, A.B.; van Lent, P.L.; van Beuningen, H.M.; Davidson, E.N.B.; van der Kraan, P.M.; van den Berg, W.B. Canonical wnt signaling skews tgf-beta signaling in chondrocytes towards signaling via alk1 and smad 1/5/8. Cell. Signal. 2014, 26, 951–958. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, L.; Song, W.; Liu, Z.; Zhao, X.; Cao, W.; Sun, B. Wnt3a Promotes the Vasculogenic Mimicry Formation of Colon Cancer via Wnt/β-Catenin Signaling. Int. J. Mol. Sci. 2015, 16, 18564-18579. https://doi.org/10.3390/ijms160818564
Qi L, Song W, Liu Z, Zhao X, Cao W, Sun B. Wnt3a Promotes the Vasculogenic Mimicry Formation of Colon Cancer via Wnt/β-Catenin Signaling. International Journal of Molecular Sciences. 2015; 16(8):18564-18579. https://doi.org/10.3390/ijms160818564
Chicago/Turabian StyleQi, Lisha, Wangzhao Song, Zhiyong Liu, Xiulan Zhao, Wenfeng Cao, and Baocun Sun. 2015. "Wnt3a Promotes the Vasculogenic Mimicry Formation of Colon Cancer via Wnt/β-Catenin Signaling" International Journal of Molecular Sciences 16, no. 8: 18564-18579. https://doi.org/10.3390/ijms160818564






