Identification of Hydroxyanthraquinones as Novel Inhibitors of Hepatitis C Virus NS3 Helicase
Abstract
:1. Introduction
2. Results and Discussion
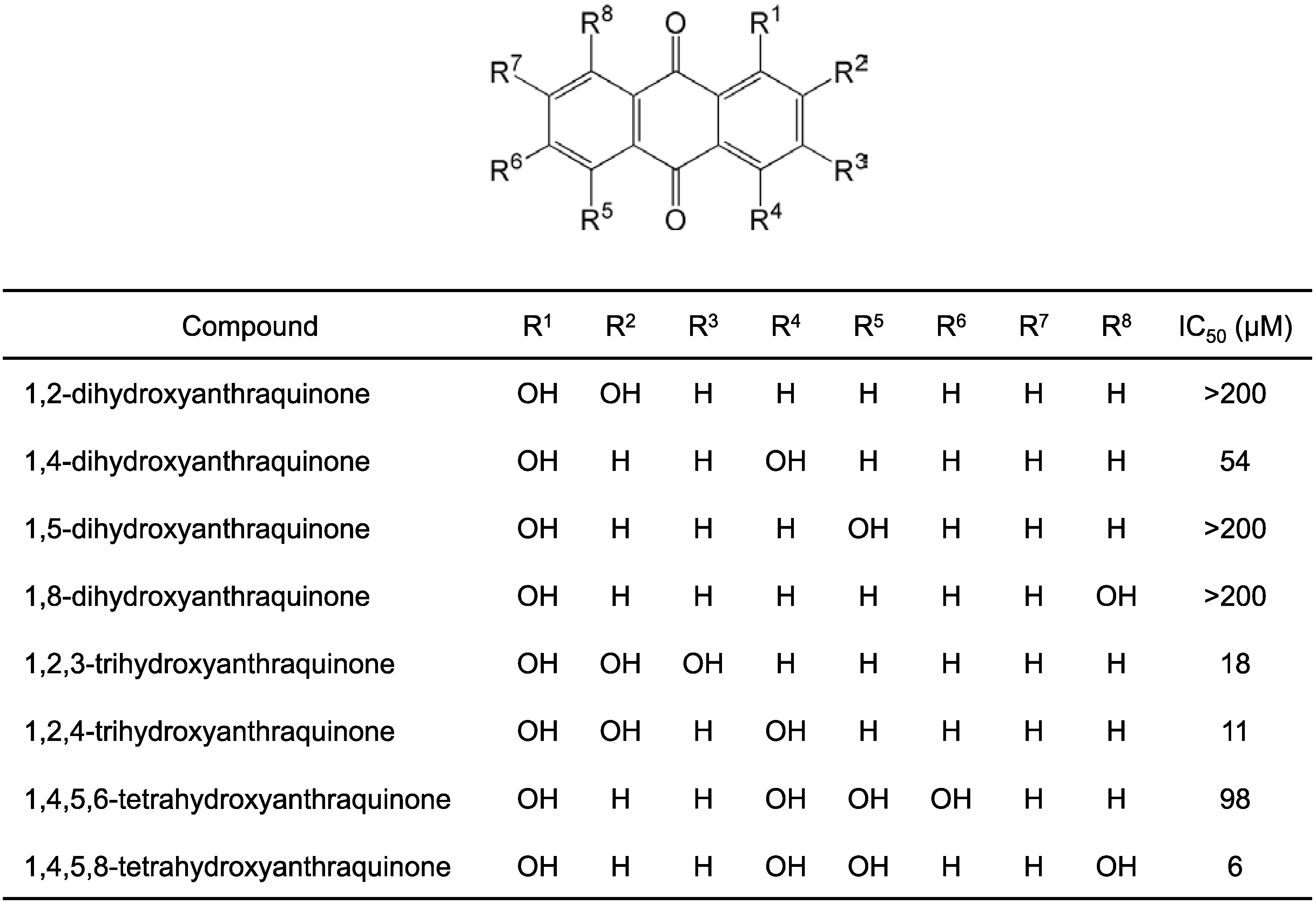
2.1. Structure–Activity Relationship Study on Hydroxyanthraquinones
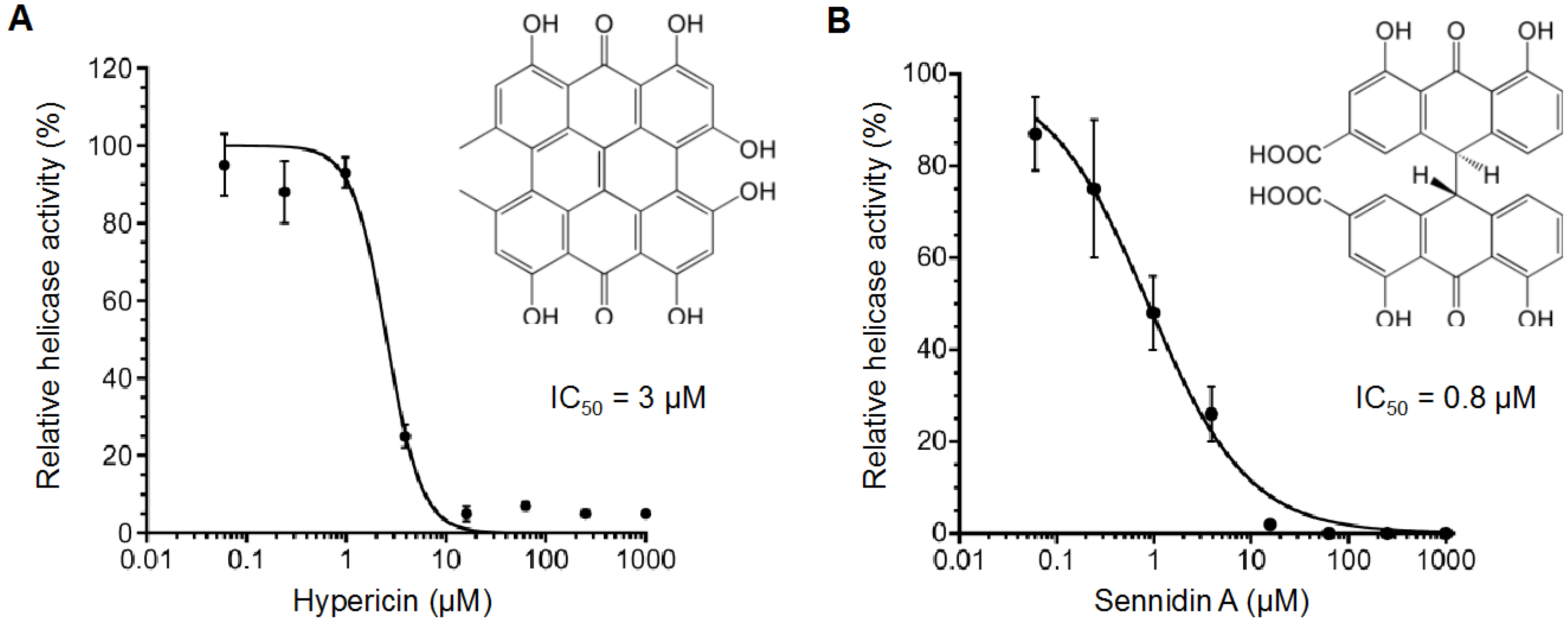
2.2. Inhibitory Activity of Hypericin and Sennidin A
2.3. Effect of Hypericin and Sennidin A on HCV Replication and Cytotoxicity
| Compound | EC50 (µM) | CC50 (µM) | SI |
|---|---|---|---|
| Hypericin | 3.5 ± 0.2 | 41.1 ± 9.5 | 11.7 |
| Sennidin A | >80 | >80 | n.d. |
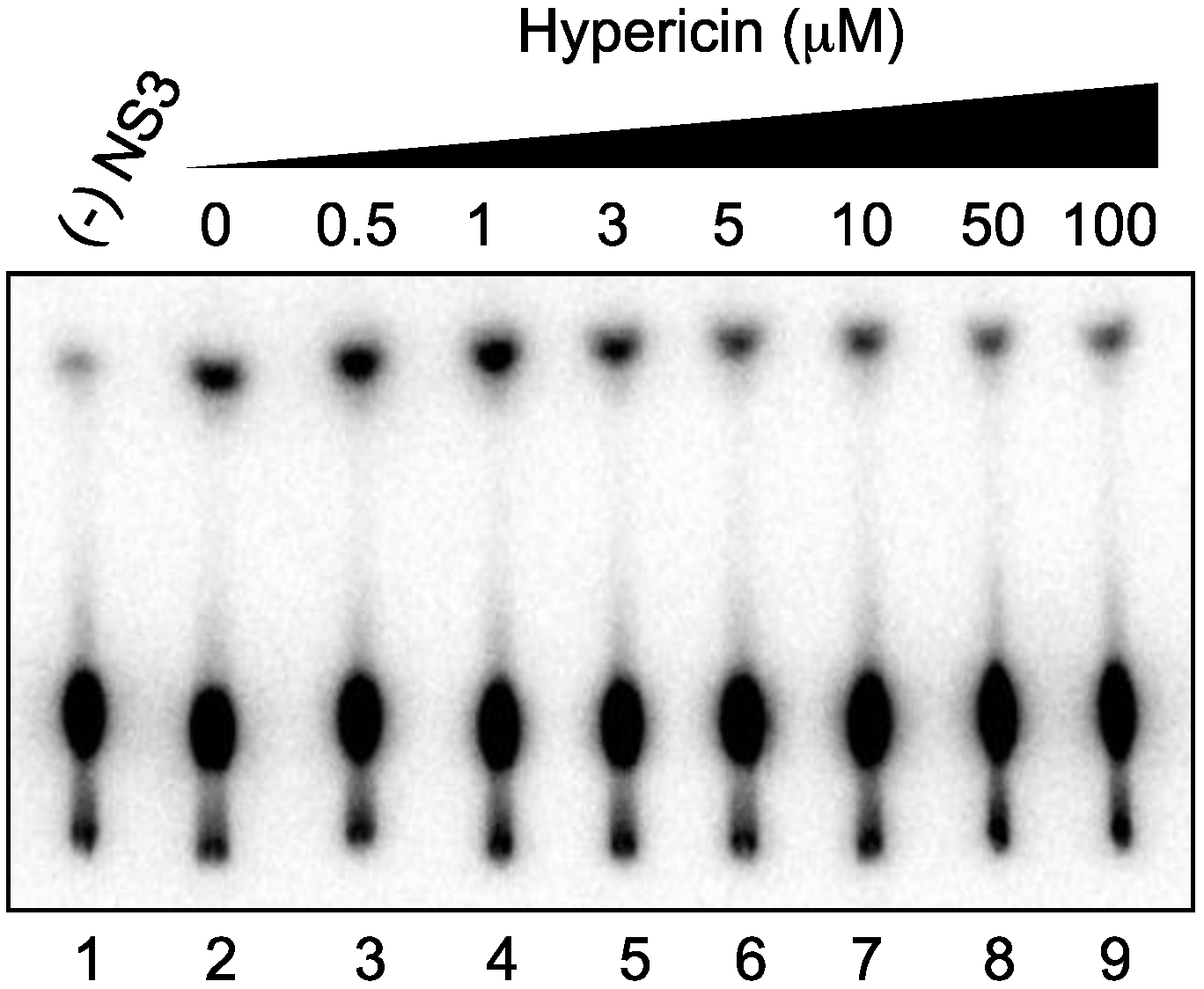
2.4. Effect of Hypericin on NS3 ATPase Activity
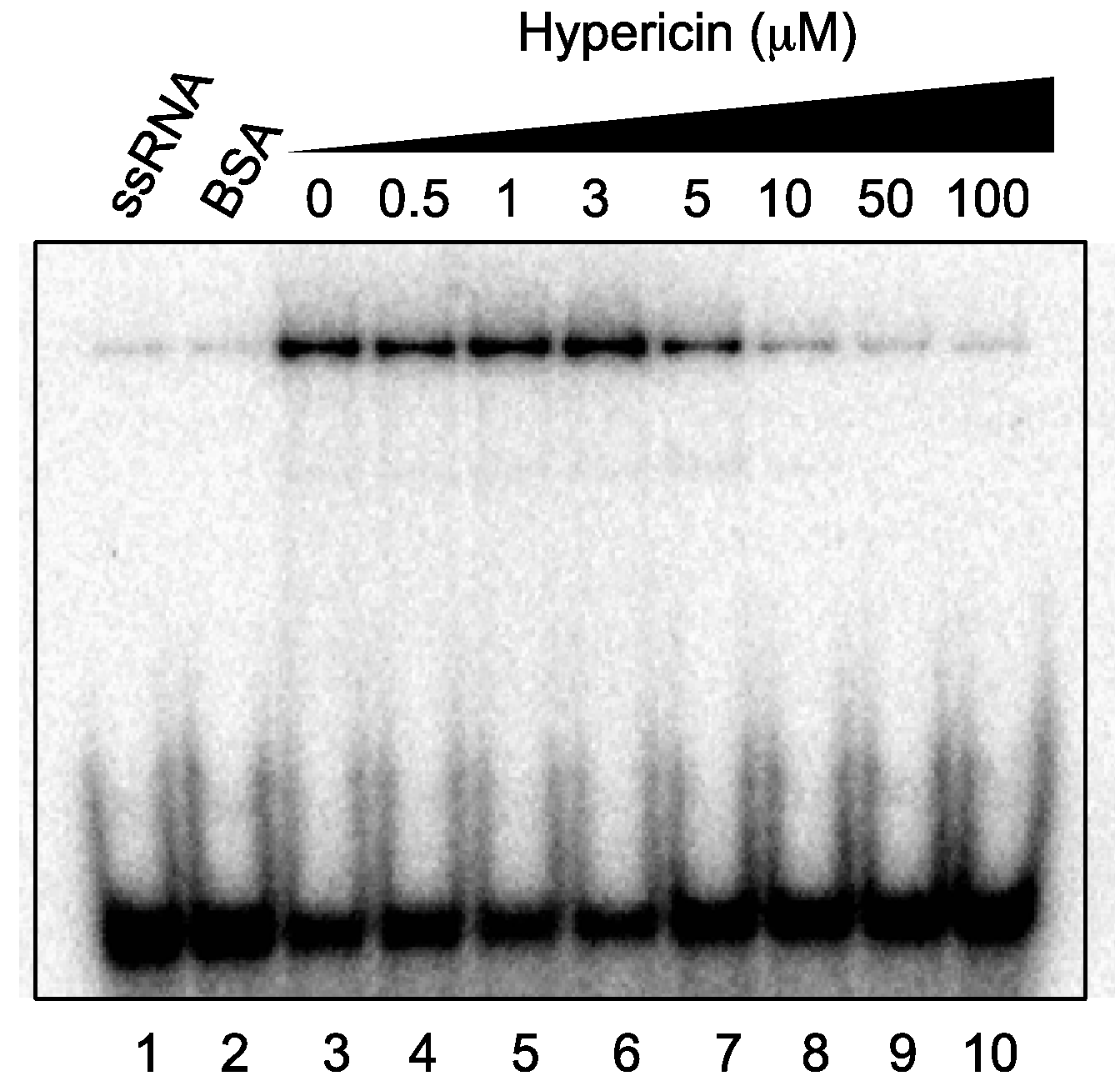
2.5. Effect of Hypericin on NS3 RNA-Binding Activity
3. Experimental Section
3.1. Chemicals
3.2. FRET-Based Fluorescence Helicase Assay
3.3. Gel-Based Helicase Assay
3.4. HCV Replicon Assay
3.5. Cytotoxicity Assay
3.6. ATPase Assay
3.7. RNA-Binding Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Marcellin, P.; Asselah, T.; Boyer, N. Fibrosis and disease progression in hepatitis C. Hepatology 2002, 36, S47–S56. [Google Scholar] [PubMed]
- Ghany, M.G.; Strader, D.B.; Thomas, D.L.; Seeff, L.B. Diagnosis, management, and treatment of hepatitis C: An update. Hepatology 2009, 49, 1335–1374. [Google Scholar] [PubMed]
- Manns, M.P.; McHutchison, J.G.; Gordon, S.C.; Rustgi, V.K.; Shiffman, M.; Reindollar, R.; Goodman, Z.D.; Koury, K.; Ling, M.; Albrecht, J.K. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: A randomised trial. Lancet 2001, 358, 958–965. [Google Scholar] [PubMed]
- Fried, M.W.; Shiffman, M.L.; Reddy, K.R.; Smith, C.; Marinos, G.; Gonçales, F.L.; Häussinger, D.; Diago, M.; Carosi, G.; Dhumeaux, D.; et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N. Engl. J. Med. 2002, 347, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Bartenschlager, R.; Lohmann, V.; Penin, F. The molecular and structural basis of advanced antiviral therapy for hepatitis C virus infection. Nat. Rev. Microbiol. 2013, 11, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Poordad, F.; McCone, J.; Bacon, B.R.; Bruno, S.; Manns, M.P.; Sulkowski, M.S.; Jacobson, I.M.; Reddy, K.R.; Goodman, Z.D.; Boparai, N.; et al. Boceprevir for untreated chronic HCV genotype 1 infection. N. Engl. J. Med. 2011, 364, 1195–1206. [Google Scholar] [CrossRef] [PubMed]
- Hézode, C.; Forestier, N.; Dusheiko, G.; Ferenci, P.; Pol, S.; Goeser, T.; Bronowicki, J.-P.; Bourlière, M.; Gharakhanian, S.; Bengtsson, L.; et al. Telaprevir and peginterferon with or without ribavirin for chronic HCV infection. N. Engl. J. Med. 2009, 360, 1839–1850. [Google Scholar] [CrossRef] [PubMed]
- Ghany, M.G.; Nelson, D.R.; Strader, D.B.; Thomas, D.L.; Seeff, L.B. An update on treatment of genotype 1 chronic hepatitis C virus infection: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology 2011, 54, 1433–1444. [Google Scholar] [CrossRef] [PubMed]
- Lawitz, E.; Poordad, F.F.; Pang, P.S.; Hyland, R.H.; Ding, X.; Mo, H.; Symonds, W.T.; McHutchison, J.G.; Membreno, F.E. Sofosbuvir and ledipasvir fixed-dose combination with and without ribavirin in treatment-naive and previously treated patients with genotype 1 hepatitis C virus infection (LONESTAR): An open-label, randomised, phase 2 trial. Lancet 2014, 383, 515–523. [Google Scholar] [CrossRef]
- Afdhal, N.; Zeuzem, S.; Kwo, P.; Chojkier, M.; Gitlin, N.; Puoti, M.; Romero-Gomez, M.; Zarski, J.-P.; Agarwal, K.; Buggisch, P.; et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N. Engl. J. Med. 2014, 370, 1889–1898. [Google Scholar] [CrossRef] [PubMed]
- Kowdley, K.V.; Gordon, S.C.; Reddy, K.R.; Rossaro, L.; Bernstein, D.E.; Lawitz, E.; Shiffman, M.L.; Schiff, E.; Ghalib, R.; Ryan, M.; et al. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N. Engl. J. Med. 2014, 370, 1879–1888. [Google Scholar] [CrossRef] [PubMed]
- Afdhal, N.; Reddy, K.R.; Nelson, D.R.; Lawitz, E.; Gordon, S.C.; Schiff, E.; Nahass, R.; Ghalib, R.; Gitlin, N.; Herring, R.; et al. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N. Engl. J. Med. 2014, 370, 1483–1493. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.M.; Lee, S.W.; Han, S.Y.; Baek, Y.H.; Kim, S.Y.; Kim, W.J.; Ahn, J.H.; Lee, J.Y. Retreatment with peginterferon and ribavirin in chronic hepatitis C. World J. Gastroenterol. 2015, 21, 1994–1999. [Google Scholar] [CrossRef] [PubMed]
- Choo, Q.L.; Kuo, G.; Weiner, A.J.; Overby, L.R.; Bradley, D.W.; Houghton, M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science 1989, 244, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Bartenschlager, R.; Penin, F.; Lohmann, V.; André, P. Assembly of infectious hepatitis C virus particles. Trends Microbiol. 2011, 19, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Gallinari, P.; Brennan, D.; Nardi, C.; Brunetti, M.; Tomei, L.; Steinkühler, C.; de Francesco, R. Multiple enzymatic activities associated with recombinant NS3 protein of hepatitis C virus. J. Virol. 1998, 72, 6758–6769. [Google Scholar] [PubMed]
- Kim, D.W.; Gwack, Y.; Han, J.H.; Choe, J. C-terminal domain of the hepatitis C virus NS3 protein contains an RNA helicase activity. Biochem. Biophys. Res. Commun. 1995, 215, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Tai, C.L.; Chi, W.K.; Chen, D.S.; Hwang, L.H. The helicase activity associated with hepatitis C virus nonstructural protein 3 (NS3). J. Virol. 1996, 70, 8477–8484. [Google Scholar] [PubMed]
- Gwack, Y.; Kim, D.W.; Han, J.H.; Choe, J. Characterization of RNA binding activity and RNA helicase activity of the hepatitis C virus NS3 protein. Biochem. Biophys. Res. Commun. 1996, 225, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Gwack, Y.; Kim, D.W.; Han, J.H.; Choe, J. DNA helicase activity of the hepatitis C virus nonstructural protein 3. Eur. J. Biochem. 1997, 250, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Lam, A.M.I.; Frick, D.N. Hepatitis C virus subgenomic replicon requires an active NS3 RNA helicase. J. Virol. 2006, 80, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Kolykhalov, A.A.; Mihalik, K.; Feinstone, S.M.; Rice, C.M. Hepatitis C virus-encoded enzymatic activities and conserved RNA elements in the 3′ nontranslated region are essential for virus replication in vivo. J. Virol. 2000, 74, 2046–2051. [Google Scholar] [CrossRef] [PubMed]
- Kwong, A.D.; Rao, B.G.; Jeang, K.-T. Viral and cellular RNA helicases as antiviral targets. Nat. Rev. Drug Discov. 2005, 4, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Borowski, P.; Schalinski, S.; Schmitz, H. Nucleotide triphosphatase/helicase of hepatitis C virus as a target for antiviral therapy. Antivir. Res. 2002, 55, 397–412. [Google Scholar] [CrossRef]
- Krawczyk, M.; Wasowska-Lukawska, M.; Oszczapowicz, I.; Boguszewska-Chachulska, A.M. Amidinoanthracyclines—A new group of potential anti-hepatitis C virus compounds. Biol. Chem. 2009, 390, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Tani, H.; Fujita, O.; Furuta, A.; Matsuda, Y.; Miyata, R.; Akimitsu, N.; Tanaka, J.; Tsuneda, S.; Sekiguchi, Y.; Noda, N. Real-time monitoring of RNA helicase activity using fluorescence resonance energy transfer in vitro. Biochem. Biophys. Res. Commun. 2010, 393, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Furuta, A.; Salam, K.A.; Tani, H.; Tsuneda, S.; Sekiguchi, Y.; Akimitsu, N.; Noda, N. A fluorescence-based screening assay for identification of hepatitis C virus NS3 helicase inhibitors and characterization of their inhibitory mechanism. Methods Mol. Biol. 2015, 1259, 211–228. [Google Scholar] [PubMed]
- El Ezaby, M.S.; Salem, T.M.; Zewail, A.H.; Issa, R. Spectral studies of some hydroxy-derivatives of anthraquinones. J. Chem. Soc. B 1970, 7, 1293–1296. [Google Scholar] [CrossRef]
- Furuta, A.; Salam, K.A.; Hermawan, I.; Akimitsu, N.; Tanaka, J.; Tani, H.; Yamashita, A.; Moriishi, K.; Nakakoshi, M.; Tsubuki, M.; et al. Identification and biochemical characterization of halisulfate 3 and suvanine as novel inhibitors of hepatitis C virus NS3 helicase from a marine sponge. Mar. Drugs 2014, 12, 462–476. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.H.; Chung, T.D.; Oldenburg, K.R. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J. Biomol. Screen. 1999, 4, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Fain, V.Y.; Zaitsev, B.E.; Ryabov, M.A. A quantum-chemical and correlation study of ionization of α,α′-dihydroxyanthraquinones. Russ. J. Gen. Chem. 2003, 73, 1925–1931. [Google Scholar] [CrossRef]
- Fain, V.Y.; Zaitsev, B.E.; Ryabov, M.A. Metal complexes with 1-hydroxyanthraquinone and its derivatives: Electronic absorption spectra and ligand structures. Russ. J. Coord. Chem. 2006, 32, 610–613. [Google Scholar] [CrossRef]
- Hilgert, I.; Cudlín, J.; Steinerová, N.; Vanĕk, Z. Antitumour and immunosuppressive activity of hydroxyanthraquinones and their glucosides. Folia Biol. (Praha) 1977, 23, 99–109. [Google Scholar] [PubMed]
- Tikkanen, L.; Matsushima, T.; Natori, S. Mutagenicity of anthraquinones in the Salmonella preincubation test. Mutat. Res. 1983, 116, 297–304. [Google Scholar] [CrossRef]
- Kawai, K.; Mori, H.; Sugie, S.; Yoshimi, N.; Inoue, T.; Nakamaru, T.; Nozawa, Y.; Matsushima, T. Genotoxicity in the hepatocyte/DNA repair test and toxicity to liver mitochondria of 1-hydroxyanthraquinone and several dihydroxyanthraquinones. Cell Biol. Toxicol. 1986, 2, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Yoshimi, N.; Iwata, H.; Mori, Y.; Hara, A.; Tanaka, T.; Kawai, K. Carcinogenicity of naturally occurring 1-hydroxyanthraquinone in rats: Induction of large bowel, liver and stomach neoplasms. Carcinogenesis 1990, 11, 799–802. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Yoshimi, N.; Iwata, H.; Tanaka, T.; Mori, H. The synergistic effect of 1-hydroxyanthraquinone on methylazoxymethanol acetate-induced carcinogenesis in rats. Carcinogenesis 1991, 12, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Kojima, T.; Yoshimi, N.; Sugie, S.; Mori, H. Inhibitory effect of the non-steroidal anti-inflammatory drug, indomethacin on the naturally occurring carcinogen, 1-hydroxyanthraquinone in male ACI/N rats. Carcinogenesis 1991, 12, 1949–1952. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Ohnishi, M.; Kawamori, T.; Sugie, S.; Tanaka, T.; Ino, N.; Kawai, K. Toxicity and tumorigenicity of purpurin, a natural hydroxanthraquinone in rats: Induction of bladder neoplasms. Cancer Lett. 1996, 102, 193–198. [Google Scholar] [CrossRef]
- Kuo, D.H.; Kuo, S.C.; Cheng, J.T. Structure-activity relationships of anthraquinones in the decrease of intestinal motility. J. Pharm. Pharmacol. 2000, 52, 839–841. [Google Scholar] [CrossRef] [PubMed]
- Fotia, C.; Avnet, S.; Granchi, D.; Baldini, N. The natural compound Alizarin as an osteotropic drug for the treatment of bone tumors. J. Orthop. Res. 2012, 30, 1486–1492. [Google Scholar] [CrossRef] [PubMed]
- Shadrick, W.R.; Mukherjee, S.; Hanson, A.M.; Sweeney, N.L.; Frick, D.N. Aurintricarboxylic acid modulates the affinity of hepatitis C virus NS3 helicase for both nucleic acid and ATP. Biochemistry 2013, 52, 6151–6159. [Google Scholar] [CrossRef] [PubMed]
- Karioti, A.; Bilia, A.R. Hypericins as potential leads for new therapeutics. Int. J. Mol. Sci. 2010, 11, 562–594. [Google Scholar] [CrossRef] [PubMed]
- Abe, D.; Saito, T.; Sekiya, K. Sennidin stimulates glucose incorporation in rat adipocytes. Life Sci. 2006, 79, 1027–1033. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Jiang, C.; Zhang, X.; Liu, W.; Gao, M.; Li, Y.; Wang, J.; Wang, Q.; Sun, Z.; Jiang, X.; et al. Necrosis targeted combinational theragnostic approach using radioiodinated Sennidin A in rodent tumor models. Oncotarget 2014, 5, 2934–2946. [Google Scholar] [PubMed]
- Belon, C.A.; Frick, D.N. Helicase inhibitors as specifically targeted antiviral therapy for hepatitis C. Future Virol. 2009, 4, 277–293. [Google Scholar] [CrossRef] [PubMed]
- Norvez, S. Liquid crystalline triptycene derivatives. J. Org. Chem. 1993, 58, 2414–2418. [Google Scholar] [CrossRef]
- Tani, H.; Akimitsu, N.; Fujita, O.; Matsuda, Y.; Miyata, R.; Tsuneda, S.; Igarashi, M.; Sekiguchi, Y.; Noda, N. High-throughput screening assay of hepatitis C virus helicase inhibitors using fluorescence-quenching phenomenon. Biochem. Biophys. Res. Commun. 2009, 379, 1054–1059. [Google Scholar] [CrossRef] [PubMed]
- Copeland, R.A. Lead optimization and structure–activity relationships for reversible inhibitors. In Evaluation of Enzyme Inhibitors in Drug Discovery: A Guide for Medicinal Chemists and Pharmacologists; Copeland, R.A., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005; pp. 111–140. [Google Scholar]
- Furuta, A.; Salam, K.A.; Akimitsu, N.; Tanaka, J.; Tani, H.; Yamashita, A.; Moriishi, K.; Nakakoshi, M.; Tsubuki, M.; Sekiguchi, Y.; et al. Cholesterol sulfate as a potential inhibitor of hepatitis C virus NS3 helicase. J. Enzym. Inhib. Med. Chem. 2014, 29, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Yokota, T.; Sakamoto, N.; Enomoto, N.; Tanabe, Y.; Miyagishi, M.; Maekawa, S.; Yi, L.; Kurosaki, M.; Taira, K.; Watanabe, M.; et al. Inhibition of intracellular hepatitis C virus replication by synthetic and vector-derived small interfering RNAs. EMBO Rep. 2003, 4, 602–608. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furuta, A.; Tsubuki, M.; Endoh, M.; Miyamoto, T.; Tanaka, J.; Salam, K.A.; Akimitsu, N.; Tani, H.; Yamashita, A.; Moriishi, K.; et al. Identification of Hydroxyanthraquinones as Novel Inhibitors of Hepatitis C Virus NS3 Helicase. Int. J. Mol. Sci. 2015, 16, 18439-18453. https://doi.org/10.3390/ijms160818439
Furuta A, Tsubuki M, Endoh M, Miyamoto T, Tanaka J, Salam KA, Akimitsu N, Tani H, Yamashita A, Moriishi K, et al. Identification of Hydroxyanthraquinones as Novel Inhibitors of Hepatitis C Virus NS3 Helicase. International Journal of Molecular Sciences. 2015; 16(8):18439-18453. https://doi.org/10.3390/ijms160818439
Chicago/Turabian StyleFuruta, Atsushi, Masayoshi Tsubuki, Miduki Endoh, Tatsuki Miyamoto, Junichi Tanaka, Kazi Abdus Salam, Nobuyoshi Akimitsu, Hidenori Tani, Atsuya Yamashita, Kohji Moriishi, and et al. 2015. "Identification of Hydroxyanthraquinones as Novel Inhibitors of Hepatitis C Virus NS3 Helicase" International Journal of Molecular Sciences 16, no. 8: 18439-18453. https://doi.org/10.3390/ijms160818439






