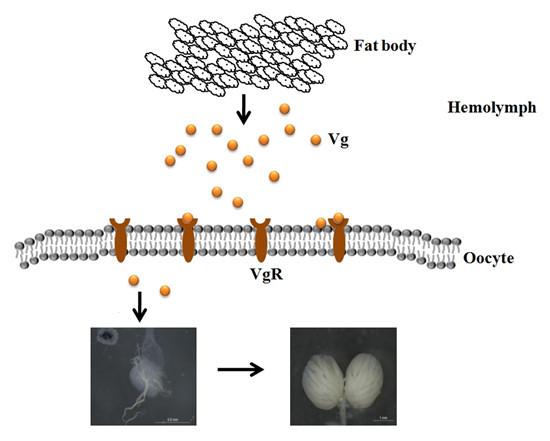
The Essential Role of Vitellogenin Receptor in Ovary Development and Vitellogenin Uptake in Bactrocera dorsalis (Hendel)
Abstract
:1. Introduction
2. Results
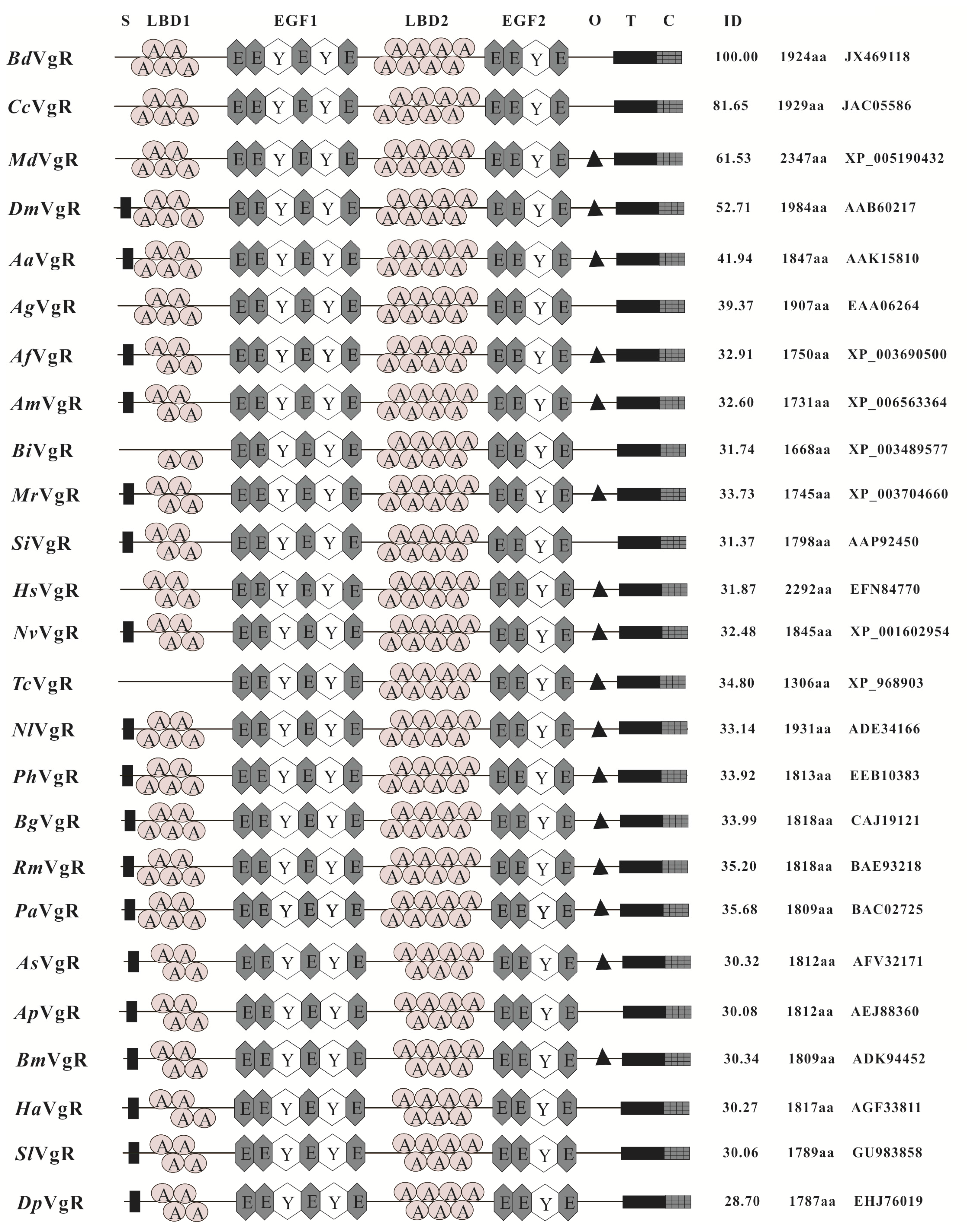
2.1. Sequence and Structural Characteristics of VgR in B. dorsalis
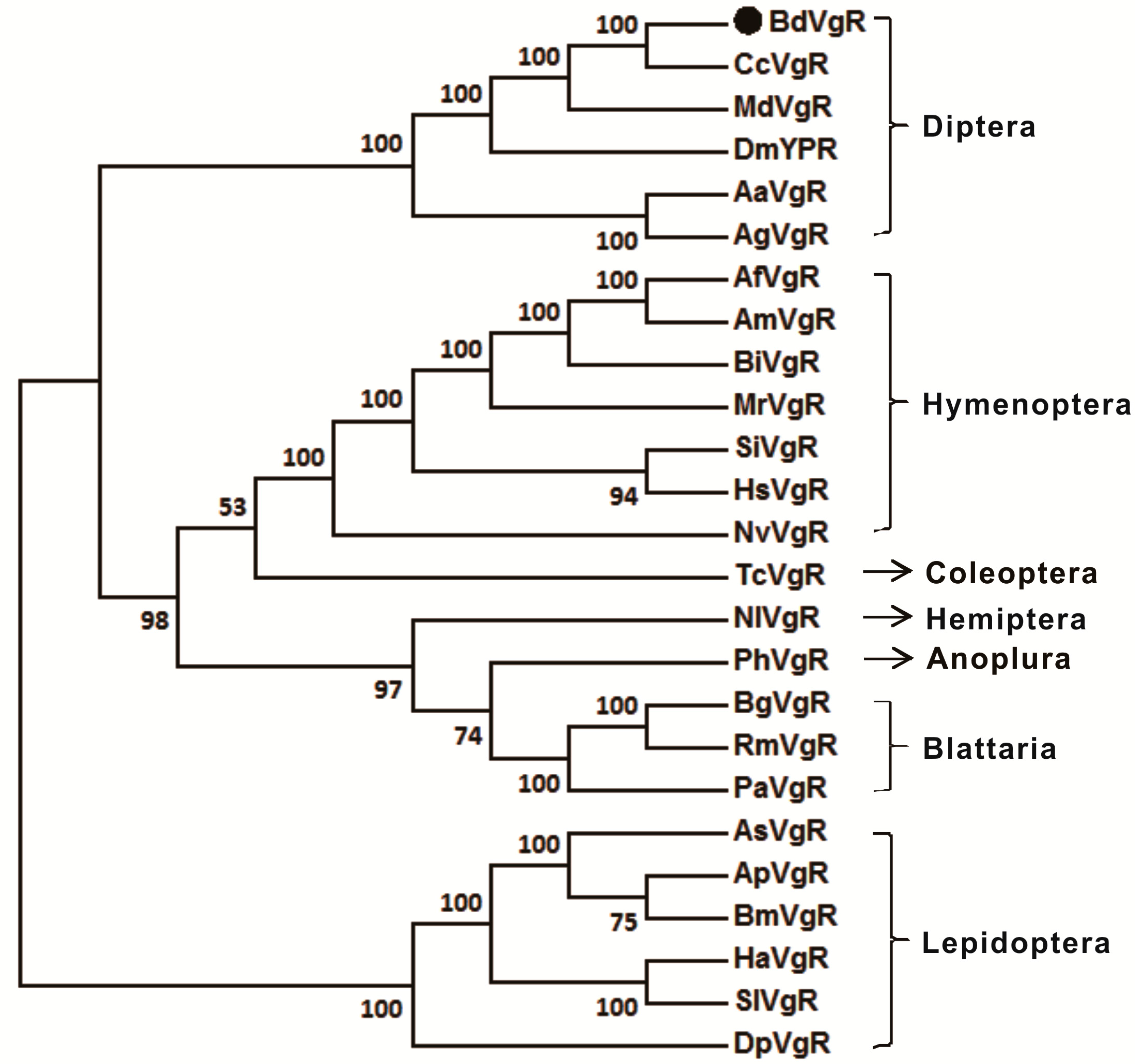
2.2. Sequences Comparison and Phylogenetic Analysis
2.3. Tissue-Specific Expression Pattern of BdVgR

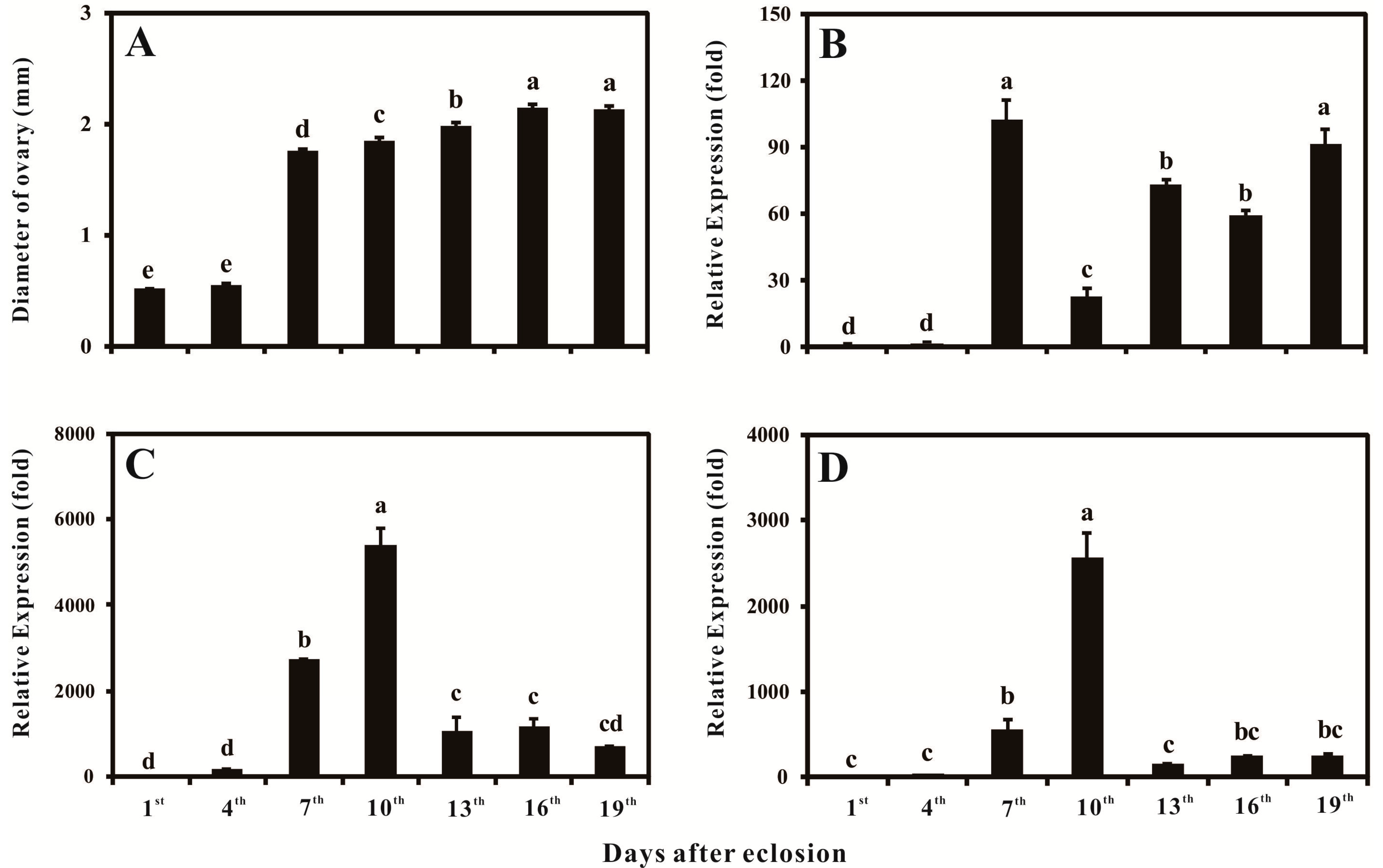
2.4. Ovary Growth, Developmental-Specific Expression Patterns of BdVgR, Bdyp1 and Bdyp2
2.5. Silencing BdVgR Expression by RNA Interference
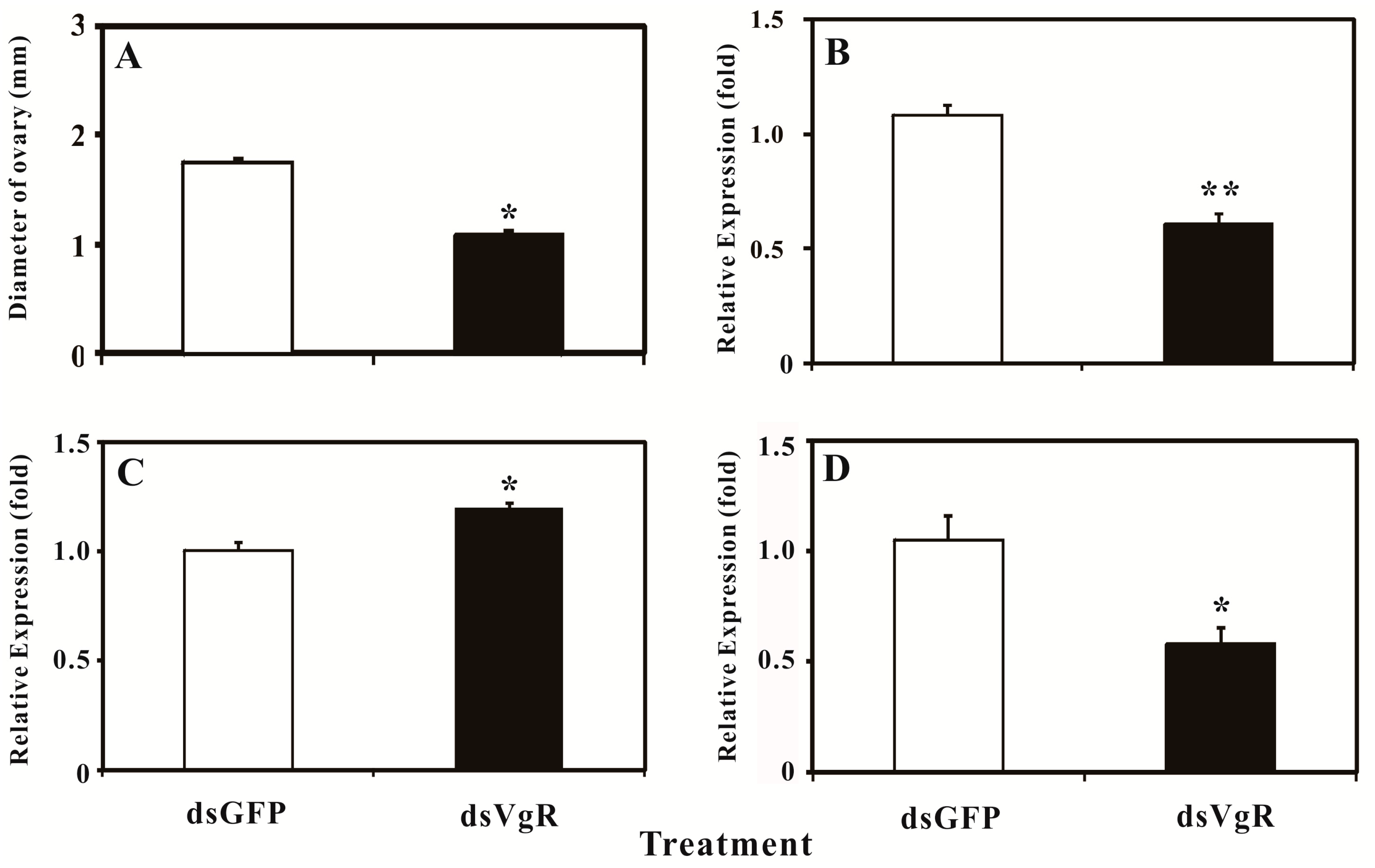
3. Discussion
4. Experimental Section
4.1. Insects Rearing, Sample Preparation and Ovarian Diameter Measurement
4.2. RNA Extraction and Cloning of BdVgR
4.3. Bioinformatics Analysis of BdVgR
4.4. Semi-Quantitative PCR and Quantitative PCR (qPCR)
4.5. RNA Interference
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sappington, T.W.; Raikhel, A.S. Molecular characteristics of insect vitellogenins and vitellogenin receptors. Insect Biochem. Mol. Biol. 1998, 28, 277–300. [Google Scholar]
- Rodenburg, K.W.; Smolenaars, M.M.; van Hoof, D.; van der Horst, D.J. Sequence analysis of the non-recurring C-terminal domains shows that insect lipoprotein receptors constitute a distinct group of LDL receptor family members. Insect Biochem. Mol. Biol. 2006, 36, 250–263. [Google Scholar]
- Tufail, M.; Takeda, M. Insect vitellogenin/lipophorin receptors: Molecular structures, role in oogenesis, and regulatory mechanisms. J. Insect Physiol. 2009, 55, 88–104. [Google Scholar] [CrossRef]
- Zhong, R.; Ding, T.B.; Niu, J.Z.; Xia, W.K.; Liao, C.Y.; Dou, W.; Wang, J.J. Molecular characterization of vitellogenin and its receptor genes from citrus red mite, Panonychus citri (McGregor). Int. J. Mol. Sci. 2015, 16, 4759–4773. [Google Scholar] [CrossRef]
- Smith, A.D.; Kaufman, W.R. Molecular characterization of the vitellogenin receptor from the tick, Amblyomma hebraeum (Acari: Ixodidae). Insect Biochem. Mol. Biol. 2013, 43, 1133–1141. [Google Scholar] [CrossRef]
- Boldbaatar, D.; Battsetseg, B.; Matsuo, T.; Hatta, T.; Umemiya-Shirafuji, R.; Xuan, X.N.; Fujisaki, K. Tick vitellogenin receptor reveals critical role in oocyte development and transovarial transmission of Babesia parasite. Biochem. Cell Biol. 2008, 86, 331–344. [Google Scholar] [CrossRef]
- Mitchell, R.D.; Ross, E.; Osgood, C.; Sonenshine, D.E.; Donohue, K.V.; Khalil, S.M.; Thompson, D.M.; Roe, R.M. Molecular characterization, tissue-specific expression and RNAi knockdown of the first vitellogenin receptor from a tick. Insect Biochem. Mol. Biol. 2007, 37, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, B.K.; Seo, Y.I.; Choi, J.H.; Kang, S.W.; Kang, C.K.; Park, W.G.; Kim, H.W. Four cDNAs encoding lipoprotein receptors from shrimp (Pandalopsis japonica): Structural characterization and expression analysis during maturation. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2014, 169, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Roth, Z.; Khalaila, I. Identification and characterization of the vitellogenin receptor in Macrobrachium rosenbergii and its expression during vitellogenesis. Mol. Reprod. Dev. 2012, 79, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Tiu, S.H.K.; Benzie, J.; Chan, S.M. From hepatopancreas to ovary: molecular characterization of a shrimp vitellogenin receptor involved in the processing of vitellogenin. Biol. Reprod. 2008, 79, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Warrier, S.; Subramoniam, T. Receptor mediated yolk protein uptake in the crab Scylla serrata: Crustacean vitellogenin receptor recognizes related mammalian serum lipoproteins. Mol. Reprod. Dev. 2002, 61, 536–548. [Google Scholar] [CrossRef] [PubMed]
- Grant, B.; Hirsh, D. Receptor-mediated endocytosis in the Caenorhabditis elegans oocyte. Mol. Biol. Cell 1999, 10, 4311–4326. [Google Scholar] [CrossRef] [PubMed]
- Mizuta, H.; Luo, W.S.; Ito, Y.; Mushirobira, Y.; Todo, T.; Hara, A.; Reading, B.J.; Sullivan, C.V.; Hiramatsu, N. Ovarian expression and localization of a vitellogenin receptor with eight ligand binding repeats in the cutthroat trout (Oncorhynchus clarki). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2013, 166, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, G.A.; Quattro, J.M.; Denslow, N.D.; Kroll, K.J.; Prucha, M.S.; Porak, W.F.; Grier, H.J.; Sabo-Attwood, T.L. Identification and transcriptional modulation of the largemouth bass, Micropterus salmoides, vitellogenin receptor during oocyte development by insulin and sex steroids. Biol. Reprod. 2012, 87. [Google Scholar] [CrossRef] [PubMed]
- Pousis, C.; Santamaria, N.; Zupa, R.; de Giorgi, C.; Mylonas, C.C.; Bridges, C.R.; de la Gandara, F.; Vassallo-Agius, R.; Bello, G.; Corriero, A. Expression of vitellogenin receptor gene in the ovary of wild and captive Atlantic bluefin tuna (Thunnus thynnus). Anim. Reprod. Sci. 2012, 132, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Prat, F.; Coward, K.; Sumpter, J.P.; Tyler, C.R. Molecular characterization and expression of two ovarian lipoprotein receptors in the rainbow trout, Oncorhynchus mykiss. Biol. Reprod. 1998, 58, 1146–1153. [Google Scholar] [CrossRef] [PubMed]
- Stifani, S.; Nimpf, J.; Schneider, W.J. Vitellogenesis in Xenopus laevis and chicken: Cognate ligands and oocyte receptors. J. Biol. Chem. 1990, 265, 882–888. [Google Scholar] [PubMed]
- Stifani, S.; Barber, D.L.; Nimpf, J.; Schneider, W.J. A single chicken oocyte plasma membrane protein mediates uptake of very low density lipoprotein and vitellogenin. Proc. Natl. Acad. Sci. USA 1990, 87, 1955–1959. [Google Scholar] [CrossRef] [PubMed]
- Sappington, T.W.; Kokoza, V.A.; Cho, W.L.; Raikhel, A.S. Molecular characterization of the mosquito vitellogenin receptor reveals unexpected high homology to the Drosophila yolk protein receptor. Proc. Natl. Acad. Sci. USA 1996, 93, 8934–8939. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.C.; Raikhel, A.S. Organization and developmental expression of the mosquito vitellogenin receptor gene. Insect Mol. Biol. 2001, 10, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Schonbaum, C.P.; Lee, S.; Mahowald, A.P. The Drosophila yolkless gene encodes a vitellogenin receptor belonging to the low density lipoprotein receptor superfamily. Proc. Natl. Acad. Sci. USA 1995, 92, 1485–1489. [Google Scholar] [CrossRef] [PubMed]
- Clarke, A.R.; Armstrong, K.F.; Carmichael, A.E.; Milne, J.R.; Raghu, S.; Roderick, G.K.; Yeates, D.K. Invasive phytophagous pests arising through a recent tropical evolutionary radiation: The Bactrocera dorsalis complex of fruit flies. Annu. Rev. Entomol. 2005, 50, 293–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, T.; Zeng, L.; Lin, Y.; Lu, Y.; Liang, G. Insecticide resistance of the oriental fruit fly, Bactrocera dorsalis (Hendel) (Diptera: Tephritidae), in mainland China. Pest Manag. Sci. 2011, 67, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.C.; Feng, H.T. Development of resistance to spinosad in oriental fruit fly (Diptera: Tephritidae) in laboratory selection and cross-resistance. J. Econ. Entomol. 2006, 99, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.L.; Lin, C.P.; Lu, K.H. cDNA isolation, expression, and hormonal regulation of yolk protein genes in the oriental fruit fly, Bactrocera dorsalis (Hendel) (Diptera: Tephritidae). J. Insect Physiol. 2012, 58, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Tufail, M.; Takeda, M. Molecular cloning and developmental expression pattern of the vitellogenin receptor from the cockroach, Leucophaea maderae. Insect Biochem. Mol. Biol. 2007, 37, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Ciudad, L.; Piulachs, M.D.; Belles, X. Systemic RNAi of the cockroach vitellogenin receptor results in a phenotype similar to that of the Drosophila yolkless mutant. FEBS J. 2006, 273, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Tufail, M.; Takeda, M. Molecular cloning, characterization and regulation of the cockroach vitellogenin receptor during oogenesis. Insect Mol. Biol. 2005, 14, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Boldbaatar, D.; Umemiya-Shirafuji, R.; Liao, M.; Tanaka, T.; Xuan, X.N.; Fujisaki, K. Multiple vitellogenins from the Haemaphysalis longicornis tick are crucial for ovarian development. J. Insect Physiol. 2010, 56, 1587–1598. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.E.; Lewis, D.K.; Keeley, L.L.; Pietrantonio, P.V. cDNA cloning and transcriptional regulation of the vitellogenin receptor from the imported fire ant, Solenopsis invicta Buren (Hymenoptera: Formicidae). Insect Mol. Biol. 2004, 13, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Meng, Y.; Wang, Y.X.; Luo, J.; Katsuma, S.; Yang, C.W.; Banno, Y.; Kusakabe, T.; Shimada, T.; Xia, Q.Y. Vitellogenin receptor mutation leads to the oogenesis mutant phenotype “scanty vitellin” of the silkworm, Bombyx mori. J. Biol. Chem. 2013, 288, 13345–13355. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.N.; Zhu, B.J.; Liu, C.L.; Wei, G.Q.; Wang, Z.G. Characterization of vitellogenin receptor (VgR) from the Chinese oak silkworm, Antheraea pernyi. Bull. Insectol. 2011, 64, 167–174. [Google Scholar]
- Shu, Y.H.; Wang, J.W.; Lu, K.; Zhou, J.L.; Zhou, Q.; Zhang, G.R. The first vitellogenin receptor from a Lepidopteran insect: Molecular characterization, expression patterns and RNA interference analysis. Insect Mol. Biol. 2011, 20, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Pousis, C.; de Giorgi, C.; Mylonas, C.; Bridges, C.; Zupa, R.; Vassallo-Agius, R.; de la Gándara, F.; Dileo, C.; de Metrio, G.; Corriero, A. Comparative study of liver vitellogenin gene expression and oocyte yolk accumulation in wild and captive Atlantic bluefin tuna (Thunnus thynnus). Anim. Reprod. Sci. 2011, 123, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Davail, B.; Pakdel, F.; Bujo, H.; Perazzolo, L.M.; Waclawek, M.; Schneider, W.J.; Le Menn, F. Evolution of oogenesis: The receptor for vitellogenin from the rainbow trout. J. Lipid Res. 1998, 39, 1929–1937. [Google Scholar] [PubMed]
- Amdam, G.V.; Norberg, K.; Hagen, A.; Omholt, S.W. Social exploitation of vitellogenin. Proc. Natl. Acad. Sci. USA 2003, 100, 1799–1802. [Google Scholar] [CrossRef] [PubMed]
- Guidugli-Lazzarini, K.R.; do Nascimento, A.M.; Tanaka, E.D.; Piulachs, M.D.; Hartfelder, K.; Bitondi, M.G.; Simoes, Z.L.P. Expression analysis of putative vitellogenin and lipophorin receptors in honey bee (Apis mellifera L.) queens and workers. J. Insect Physiol. 2008, 54, 1138–1147. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.K.; Pakkianathan, B.C.; Kumar, M.; Prasad, T.; Kannan, M.; König, S.; Krishnan, M. Vitellogenin from the silkworm, Bombyx mori: An effective anti-bacterial agent. PLoS ONE 2013, 8, e73005. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.N.; Xiao, H.J.; Liang, G.M.; Guo, Y.Y.; Wu, K. Tradeoff between reproduction and resistance evolution to Bt-toxin in Helicoverpa armigera: regulated by vitellogenin gene expression. Bull. Entomol. Res. 2014, 104, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Bujo, H.; Lindstedt, K.A.; Hermann, M.; Dalmau, L.M.; Nimpf, J.; Schneider, W.J. Chicken oocytes and somatic cells express different splice variants of a multifunctional receptor. J. Biol. Chem. 1995, 270, 23546–23551. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.J.; Xu, K.K.; Cong, L.; Wang, J.J. Identification, mRNA expression, and functional analysis of chitin synthase 1 gene and its two alternative splicing variants in oriental fruit fly, Bactrocera dorsalis. Int. J. Biol. Sci. 2013, 9, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Suganya, R.; Chen, S.L.; Lu, K.H. cDNA cloning and characterization of S6 kinase and its effect on yolk protein gene expression in the oriental fruit fly Bactrocera dorsalis (Hendel). Arch. Insect Biochem. Physiol. 2011, 78, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.L.; Dai, S.M.; Lu, K.H.; Chang, C. Female-specific doublesex dsRNA interrupts yolk protein gene expression and reproductive ability in oriental fruit fly, Bactrocera dorsalis (Hendel). Insect Biochem. Mol. Biol. 2008, 38, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.L.; Vinson, S.B.; Pietrantonio, P.V. Oocyte membrane localization of vitellogenin receptor coincides with queen flying age, and receptor silencing by RNAi disrupts egg formation in fire ant virgin queens. FEBS J. 2009, 276, 3110–3123. [Google Scholar] [CrossRef] [PubMed]
- Cong, L.; Yang, W.J.; Shen, G.M.; Dou, W.; Wang, J.J. Molecular characterization of the cDNA encoding ecdysone receptor isoform B1 and its expression in the oriental fruit fly, Bactrocera dorsalis (Diptera: Tephritidae). Fla. Entomol. 2012, 95, 650–658. [Google Scholar] [CrossRef]
- Shen, G.M.; Dou, W.; Niu, J.Z.; Jiang, H.B.; Yang, W.J.; Jia, F.X.; Hu, F.; Cong, L.; Wang, J.J. Transcriptome analysis of the oriental fruit fly (Bactrocera dorsalis). PLoS ONE 2011, 6, e29127. [Google Scholar] [CrossRef] [PubMed]
- Petersen, T.N.; Brunak, S.; Heijne, G.V.; Nielsen, H. SignalP 4.0: Discriminating signal peptides from transmembrane regions. Nat. Methods 2011, 8, 785–786. [Google Scholar] [CrossRef] [PubMed]
- Marchler-Bauer, A.; Derbyshire, M.K.; Gonzales, N.R.; Lu, S.; Chitsaz, F.; Geer, L.Y.; Geer, R.C.; He, J.; Gwadz, M.; Hurwitz, D.I.; et al. CDD: NCBI’s conserved domain database. Nucleic. Acids Res. 2015, 43. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Doerks, T.; Bork, P. SMART: Recent updates, new developments and status in 2015. Nucleic Acids Res. 2015, 43. [Google Scholar] [CrossRef] [PubMed]
- Hamby, S.E.; Hirst, J.D. Prediction of glycosylation sites using random forests. BMC Bioinformatics 2008, 9. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.M.; Jiang, H.B.; Wang, X.N.; Wang, J.J. Evaluation of endogenous references for gene expression profiling in different tissues of the oriental fruit fly Bactrocera dorsalis (Diptera: Tephritidae). BMC Mol. Biol. 2011, 11. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2011, 29, 2002–2007. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cong, L.; Yang, W.-J.; Jiang, X.-Z.; Niu, J.-Z.; Shen, G.-M.; Ran, C.; Wang, J.-J. The Essential Role of Vitellogenin Receptor in Ovary Development and Vitellogenin Uptake in Bactrocera dorsalis (Hendel). Int. J. Mol. Sci. 2015, 16, 18368-18383. https://doi.org/10.3390/ijms160818368
Cong L, Yang W-J, Jiang X-Z, Niu J-Z, Shen G-M, Ran C, Wang J-J. The Essential Role of Vitellogenin Receptor in Ovary Development and Vitellogenin Uptake in Bactrocera dorsalis (Hendel). International Journal of Molecular Sciences. 2015; 16(8):18368-18383. https://doi.org/10.3390/ijms160818368
Chicago/Turabian StyleCong, Lin, Wen-Jia Yang, Xuan-Zhao Jiang, Jin-Zhi Niu, Guang-Mao Shen, Chun Ran, and Jin-Jun Wang. 2015. "The Essential Role of Vitellogenin Receptor in Ovary Development and Vitellogenin Uptake in Bactrocera dorsalis (Hendel)" International Journal of Molecular Sciences 16, no. 8: 18368-18383. https://doi.org/10.3390/ijms160818368