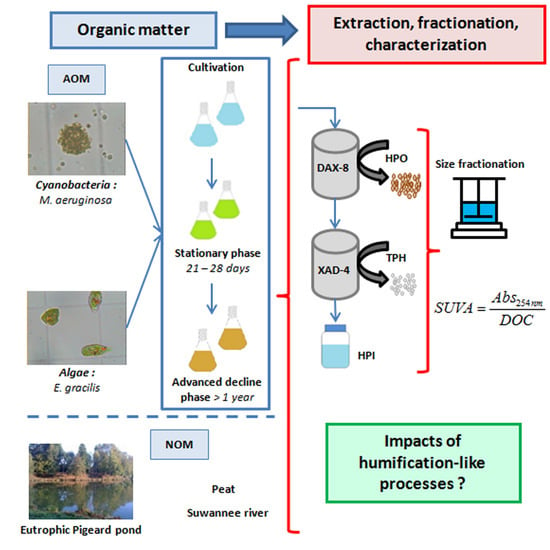
Assessing Transformations of Algal Organic Matter in the Long-Term: Impacts of Humification-Like Processes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Evolution of the Hydrophobic and Aromatic Characteristics
| Stationary Phase 1 | Decline Phase 1 | Advanced Decline Phase | |||||
|---|---|---|---|---|---|---|---|
| Origin | Fractions | % of DOC | SUVA 2 | % of DOC | SUVA 2 | % of DOC | SUVA 2 |
| Euglena gracilis | Total | – | 13.9 ± 0.1 | – | 11.2 ± 0.1 | – | 12.1 ± 0.1 |
| HPO 3 | 18 | 19.5 ± 1.6 | 24 | 26.4 ± 3.9 | 32 | 33.1 ± 0.9 | |
| TPH 4 | 13 | 17.1 ± 1.4 | 15 | 13.5 ± 1.4 | 20 | 19.8 ± 1.1 | |
| HPI 5 | 69 | 8.4 ± 0.6 | 61 | 6.9 ± 0.1 | 48 | 9.9 ± 0.1 | |
| Microcystis aeruginosa | Total | – | 10.7 ± 1.3 | – | 10.4 ± 0.8 | – | 10.4 ± 0.1 |
| HPO 3 | 20 | 12.0 ± 0.4 | 24 | 19.0 ± 2.7 | 24 | 20.8 ± 0.4 | |
| TPH 4 | 19 | 7.3 ± 0.6 | 29 | 4.0 ± 1.0 | 28 | 15.3 ± 0.3 | |
| HPI 5 | 61 | 11.9 ± 0.5 | 47 | 11.6 ± 0.4 | 48 | 9.2 ± 0.8 | |
| Origin | Fractions | % of DOC | SUVA 2 | ||||
| Pigeard pond | Total | – | 36.5 ± 0.3 | ||||
| HPO 3 | 43 | 52.8 ± 0.8 | |||||
| TPH 4 | 21 | 27.2 ± 0.9 | |||||
| HPI 5 | 36 | 14.1 ± 0.1 | |||||
| Suwannee River | HA | – | 67.9 ± 0.5 | ||||
| FA | – | 47.4 ± 0.5 | |||||
| Peat | HA | – | 101.0 ± 0.5 | ||||
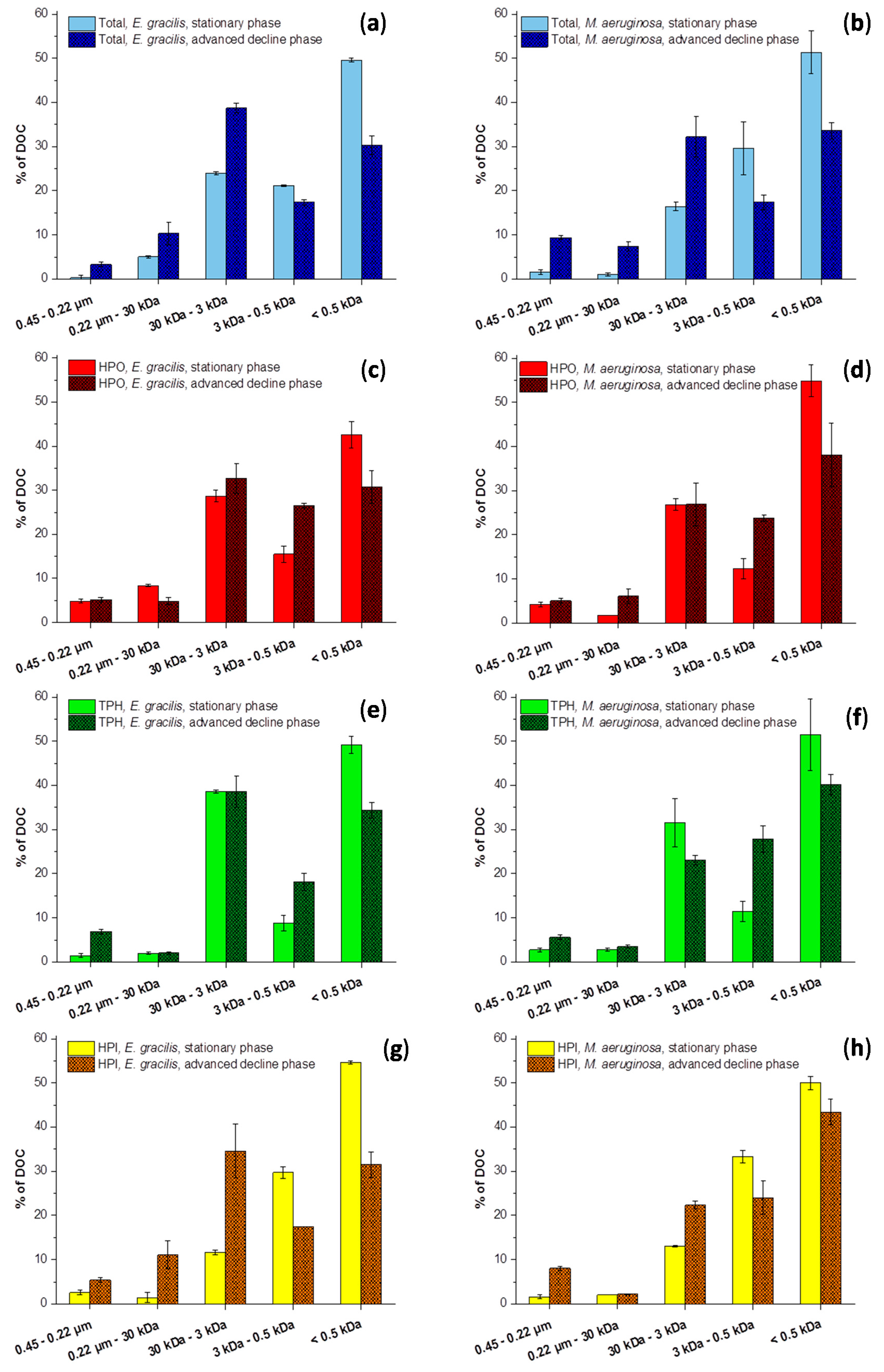
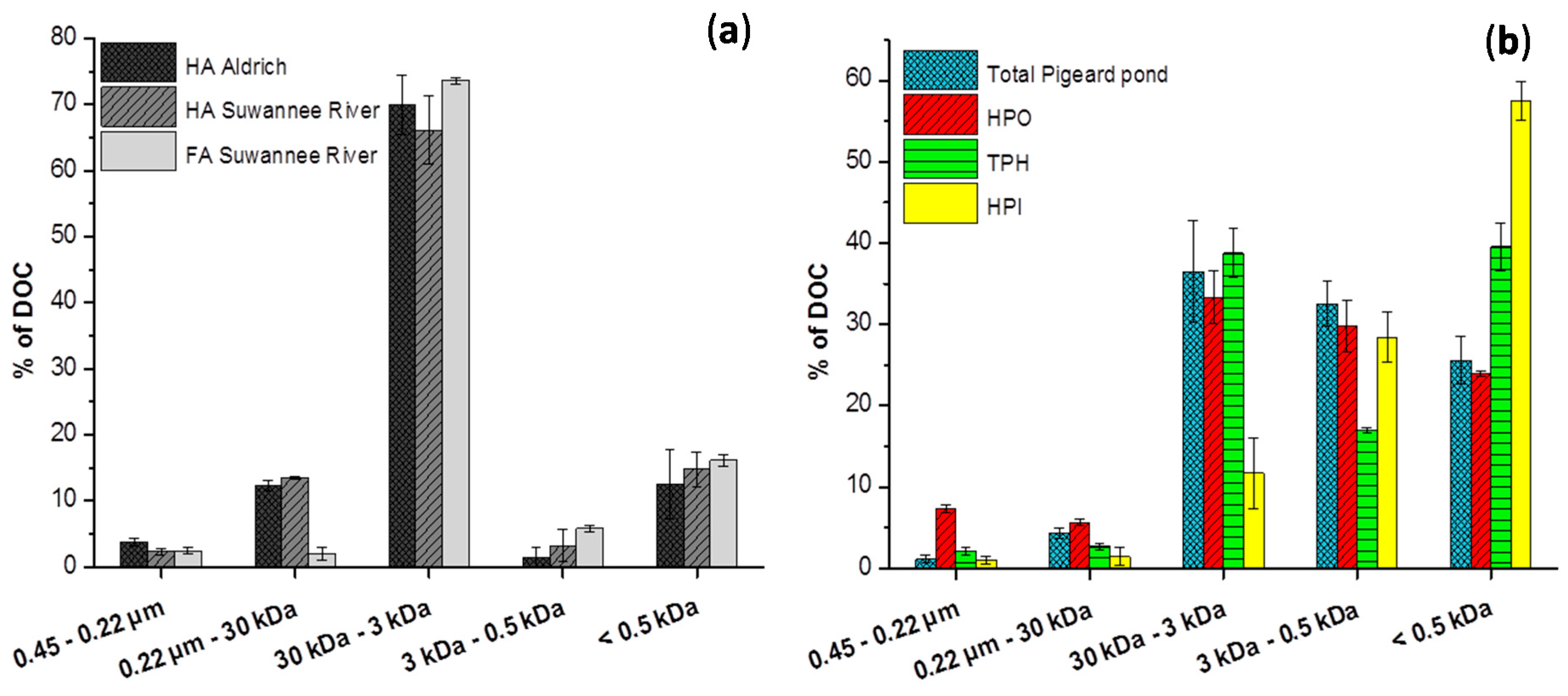
2.2. Size Fractionation of Organic Matters in Different Stages
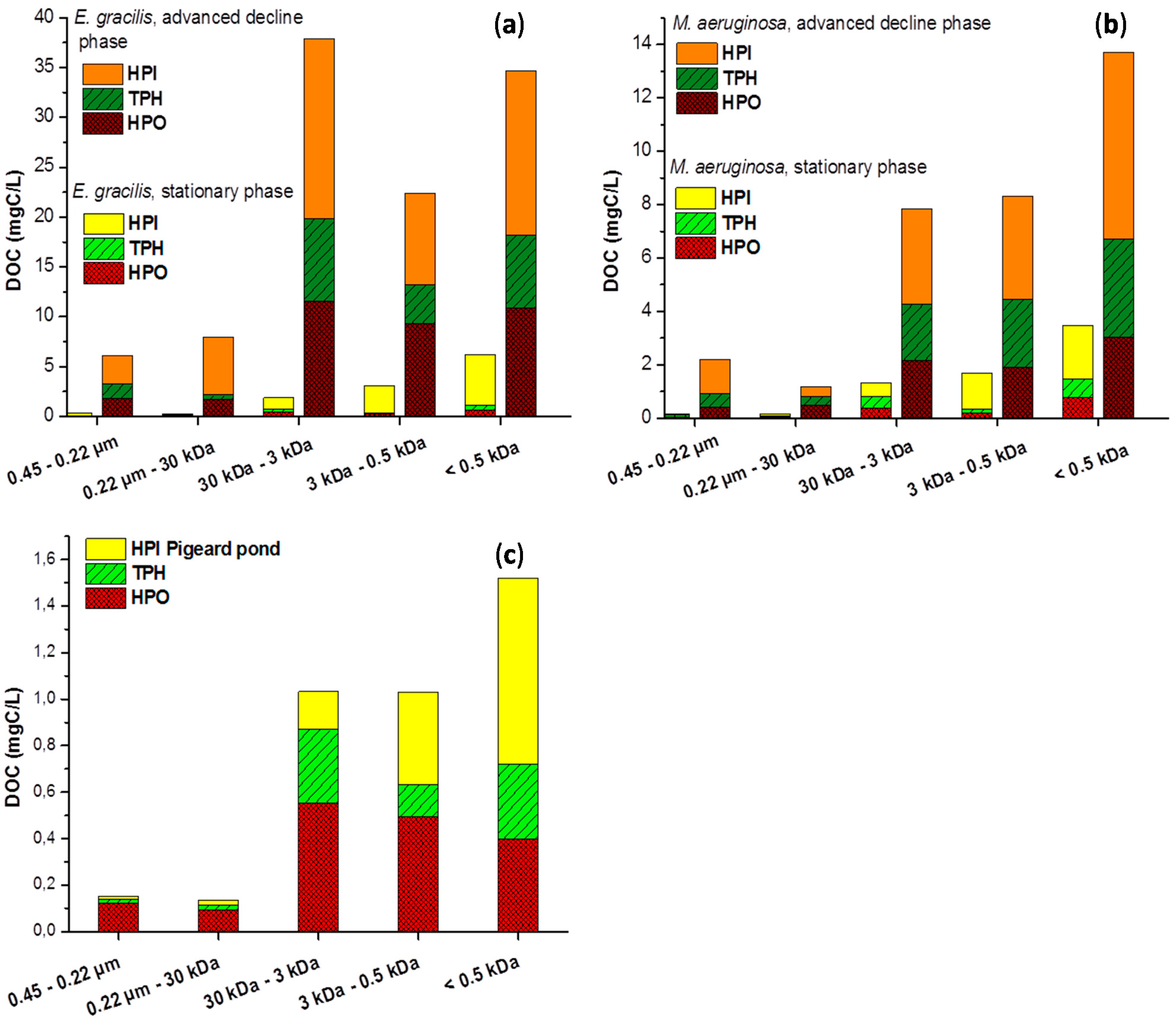
3. Experimental Section
3.1. Samples Preparation
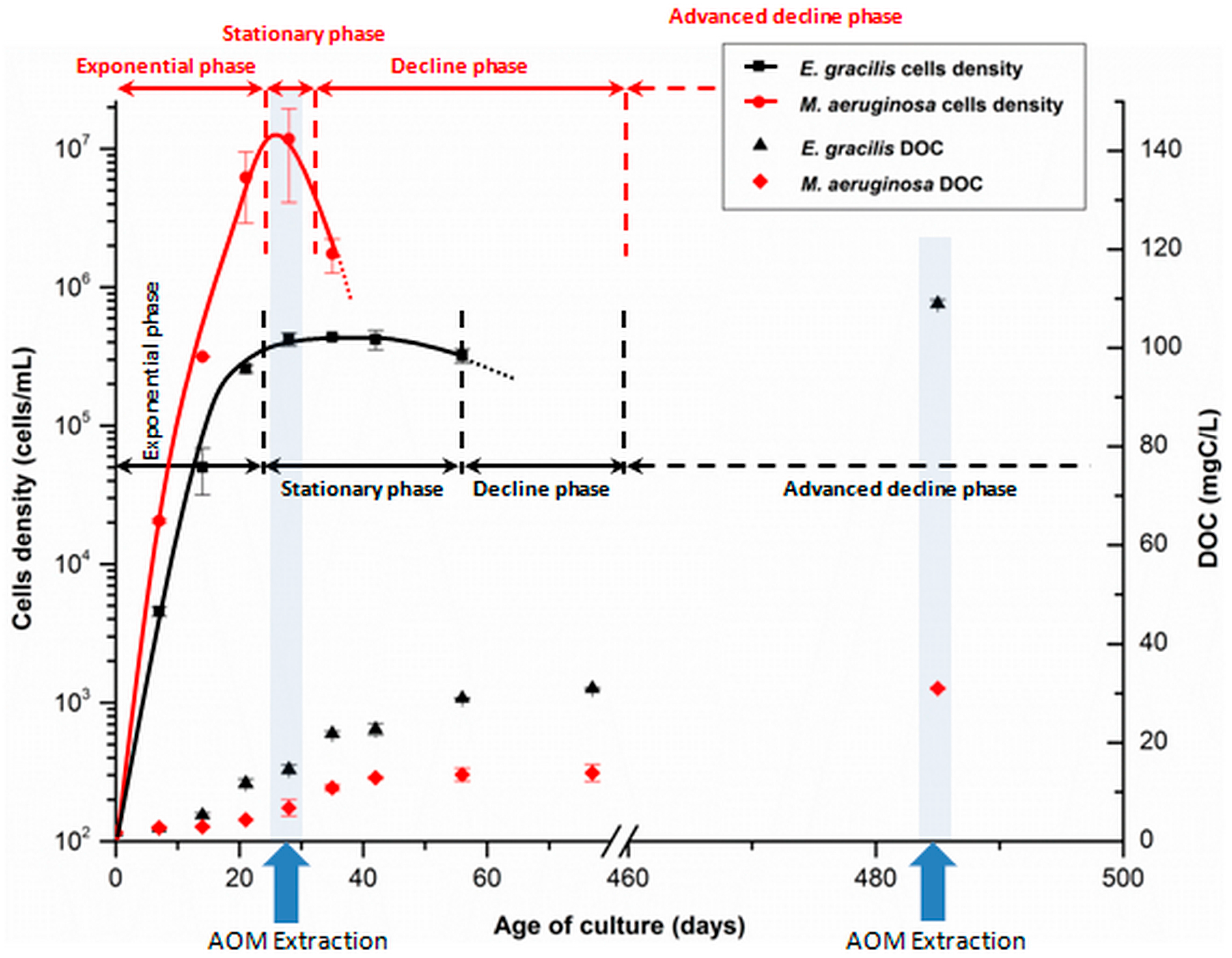
3.1.1. Algae and Cyanobacteria Cultivation
3.1.2. Organic Matter Extraction Procedure
3.2. Organic Matter Characterization
3.2.1. Size Fractionation
I ionic strength in mmol·L−1 and χ conductivity in mS·cm−1
3.2.2. Dissolved Organic Carbon Measurements
3.2.3. Specific UV Absorbance Index
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Thurman, E.M. Organic Geochemistry of Natural Waters; Springer Science & Business: Dordrecht, The Netherlands, 1985. [Google Scholar]
- Semenov, V.M.; Tulina, A.S.; Semenova, N.A.; Ivannikova, L.A. Humification and nonhumification pathways of the organic matter stabilization in soil: A review. Eurasian Soil Sci. 2013, 46, 355–368. [Google Scholar] [CrossRef]
- Stevenson, F.J. Humus Chemistry: Genesis, Composition, Reactions; John Wiley & Sons: New York, NY, USA, 1982. [Google Scholar]
- Wershaw, R.L. Evaluation of Conceptual Models of Natural Organic Matter (Humus) from a Consideration of the Chemical and Biochemical Processes of Humification; Scientific Investigations Report 2004-5121: Denver, CO, USA, 2004. [Google Scholar]
- Stevenson, F.J. Humus Chemistry: Genesis, Composition, Reactions,, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1994. [Google Scholar]
- Cheshire, M.V.; Russell, J.D.; Fraser, A.R.; Bracewell, J.M.; Robertsons, G.W.; Benzing-Purdie, L.M.; Ratcliffe, C.I.; Ripmeester, J.A.; Goodman, B.A. Nature of soil carbohydrate and its association with soil humic substances. J. Soil Sci. 1992, 43, 359–373. [Google Scholar] [CrossRef]
- Zech, W.; Ziegler, F.; Kogel-Knabner, I.; Haumaier, L. Humic substances distribution and transformation in forest soils. Sci. Total Environ. 1992, 117–118, 155–174. [Google Scholar] [CrossRef]
- Piccolo, A. The supramolecular structure of humic substances. Soil Sci. 2001, 166, 810–832. [Google Scholar] [CrossRef]
- Sutton, R.; Sposito, G. Molecular structure in soil humic substances: The new view. Environ. Sci. Technol. 2005, 39, 9009–9015. [Google Scholar] [CrossRef] [PubMed]
- Chin, Y.-P.; Aiken, G.; O’Loughlin, E. Molecular weight, polydispersity, and spectroscopic properties of aquatic humic substances. Environ. Sci. Technol. 1994, 28, 1853–1858. [Google Scholar] [CrossRef] [PubMed]
- Peuravuori, J.; Pihlaja, K. Molecular size distribution and spectroscopic properties of aquatic humic substances. Anal. Chim. Acta 1997, 337, 133–149. [Google Scholar] [CrossRef]
- Perminova, I.V.; Frimmel, F.H.; Kudryavtsev, A.V.; Kulikova, N.A.; Abbt-Braun, G.; Hesse, S.; Petrosyan, V.S. Molecular weight characteristics of humic substances from different environments as determined by size exclusion chromatography and their statistical evaluation. Environ. Sci. Technol. 2003, 37, 2477–2485. [Google Scholar] [CrossRef] [PubMed]
- Zech, W.; Haumaier, L.; Kögel-Knabner, I. Changes in aromaticity and carbon distribution of soil organic matter due to pedogenesis. Sci. Total Environ. 1989, 81–82, 179–186. [Google Scholar] [CrossRef]
- Merritt, K.A.; Erich, M.S. Influence of organic matter decomposition on soluble carbon and its copper-binding capacity. J. Environ. Qual. 2003, 32, 2122. [Google Scholar] [CrossRef] [PubMed]
- McKnight, D.M.; Boyer, E.W.; Westerhoff, P.K.; Doran, P.T.; Kulbe, T.; Andersen, D.T. Spectrofluorometric characterization of dissolved organic matter for indication of precursor organic material and aromaticity. Limnol. Oceanogr. 2001, 46, 38–48. [Google Scholar] [CrossRef]
- Wang, L.; Wu, F.; Zhang, R.; Li, W.; Liao, H. Characterization of dissolved organic matter fractions from Lake Hongfeng, Southwestern China Plateau. J. Environ. Sci. 2009, 21, 581–588. [Google Scholar] [CrossRef]
- Her, N.; Amy, G.; Park, H.-R.; Song, M. Characterizing algogenic organic matter (AOM) and evaluating associated NF membrane fouling. Water Res. 2004, 38, 1427–1438. [Google Scholar] [CrossRef] [PubMed]
- Henderson, R.K.; Baker, A.; Parsons, S.A.; Jefferson, B. Characterisation of algogenic organic matter extracted from cyanobacteria, green algae and diatoms. Water Res. 2008, 42, 3435–3445. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tian, J.; Nan, J.; Gao, S.; Liang, H.; Wang, M.; Li, G. Effect of PAC addition on immersed ultrafiltration for the treatment of algal-rich water. J. Hazard. Mater. 2011, 186, 1415–1424. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Gao, N.; Deng, Y.; Yao, J.; Zhang, K. Characterization of intracellular & extracellular algae organic matters (AOM) of Microcystic aeruginosa and formation of AOM-associated disinfection byproducts and odor & taste compounds. Water Res. 2012, 46, 1233–1240. [Google Scholar] [PubMed]
- Leloup, M.; Nicolau, R.; Pallier, V.; Yéprémian, C.; Feuillade-Cathalifaud, G. Organic matter produced by algae and cyanobacteria: Quantitative and qualitative characterization. J. Environ. Sci. 2013, 25, 1089–1097. [Google Scholar] [CrossRef]
- Kassim, G.; Martin, J.P.; Haider, K. Incorporation of a wide variety of organic substrate carbons into soil biomass as estimated by the fumigation procedure. Soil Sci. Soc. Am. J. 1981, 45, 1106–1112. [Google Scholar] [CrossRef]
- Pivokonsky, M.; Safarikova, J.; Baresova, M.; Pivokonska, L.; Kopecka, I. A comparison of the character of algal extracellular versus cellular organic matter produced by cyanobacterium, diatom and green alga. Water Res. 2014, 51, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Yang, X.; Ma, J.; Shang, C.; Zhao, Q. Characterization of algal organic matter and formation of DBPs from chlor(am)ination. Water Res. 2010, 44, 5897–5906. [Google Scholar] [CrossRef] [PubMed]
- Labanowski, J.; Feuillade, G. Combination of biodegradable organic matter quantification and XAD-fractionation as effective working parameter for the study of biodegradability in environmental and anthropic samples. Chemosphere 2009, 74, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Weishaar, J.L.; Aiken, G.R.; Bergamaschi, B.A.; Fram, M.S.; Fujii, R.; Mopper, K. Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon. Environ. Sci. Technol. 2003, 37, 4702–4708. [Google Scholar] [CrossRef] [PubMed]
- Qu, F.; Liang, H.; He, J.; Ma, J.; Wang, Z.; Yu, H.; Li, G. Characterization of dissolved extracellular organic matter (dEOM) and bound extracellular organic matter (bEOM) of Microcystis aeruginosa and their impacts on UF membrane fouling. Water Res. 2012, 46, 2881–2890. [Google Scholar] [CrossRef] [PubMed]
- Nicolau, R.; Leloup, M.; Lachassagne, D.; Pinault, E.; Feuillade-Cathalifaud, G. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI–TOF–MS) coupled to XAD fractionation: Method to algal organic matter characterization. Talanta 2015, 136, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T. Lignin biochemistry: Biosynthesis and biodegradation. Wood Sci. Technol. 1990, 24, 23–63. [Google Scholar] [CrossRef]
- Juttner, F. Physiology and biochemistry of odorous compounds from freshwater cyanobacteria and algae. Water Sci. Technol. 1995, 31, 69–78. [Google Scholar] [CrossRef]
- American Water Works Association. M57-Algae: Source to Treatment; American Water Works Association: Denver, CO, USA, 2010. [Google Scholar]
- Pivokonsky, M.; Kloucek, O.; Pivokonska, L. Evaluation of the production, composition and aluminum and iron complexation of algogenic organic matter. Water Res. 2006, 40, 3045–3052. [Google Scholar] [CrossRef] [PubMed]
- López, C.V.G.; García, M.D.C.C.; Fernández, F.G.A.; Bustos, C.S.; Chisti, Y.; Sevilla, J.M.F. Protein measurements of microalgal and cyanobacterial biomass. Bioresour. Technol. 2010, 101, 7587–7591. [Google Scholar] [CrossRef] [PubMed]
- Cardozo, K.H.M.; Guaratini, T.; Barros, M.P.; Falcão, V.R.; Tonon, A.P.; Lopes, N.P.; Campos, S.; Torres, M.A.; Souza, A.O.; Colepicolo, P.; et al. Metabolites from algae with economical impact. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2007, 146, 60–78. [Google Scholar] [CrossRef] [PubMed]
- Jokic, A.; Frenkel, A.I.; Vairavamurthy, M.A.; Huang, P.M. Birnessite catalysis of the maillard reaction: Its significance in natural humification. Geophys. Res. Lett. 2001, 28, 3899–3902. [Google Scholar] [CrossRef]
- Qi, G.; Yue, D.; Fukushima, M.; Fukuchi, S.; Nishimoto, R.; Nie, Y. Enhanced humification by carbonated basic oxygen furnace steel slag–II. Process characterization and the role of inorganic components in the formation of humic-like substances. Bioresour. Technol. 2012, 114, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Malcolm, R.L.; MacCarthy, P. Quantitative evaluation of XAD-8 and XAD-4 resins used in tandem for removing organic solutes from water. Environ. Int. 1992, 18, 597–607. [Google Scholar] [CrossRef]
- Croué, J.-P. Isolation of humic and non-humic NOM fractions: Structural characterizations. Environ. Monit. Assess. 2004, 92, 193–207. [Google Scholar] [CrossRef] [PubMed]
- Sposito, G. The Chemistry of Solids; Oxford Univerity Press: New York, NY, USA, 1989. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leloup, M.; Pallier, V.; Nicolau, R.; Feuillade-Cathalifaud, G. Assessing Transformations of Algal Organic Matter in the Long-Term: Impacts of Humification-Like Processes. Int. J. Mol. Sci. 2015, 16, 18096-18110. https://doi.org/10.3390/ijms160818096
Leloup M, Pallier V, Nicolau R, Feuillade-Cathalifaud G. Assessing Transformations of Algal Organic Matter in the Long-Term: Impacts of Humification-Like Processes. International Journal of Molecular Sciences. 2015; 16(8):18096-18110. https://doi.org/10.3390/ijms160818096
Chicago/Turabian StyleLeloup, Maud, Virginie Pallier, Rudy Nicolau, and Geneviève Feuillade-Cathalifaud. 2015. "Assessing Transformations of Algal Organic Matter in the Long-Term: Impacts of Humification-Like Processes" International Journal of Molecular Sciences 16, no. 8: 18096-18110. https://doi.org/10.3390/ijms160818096