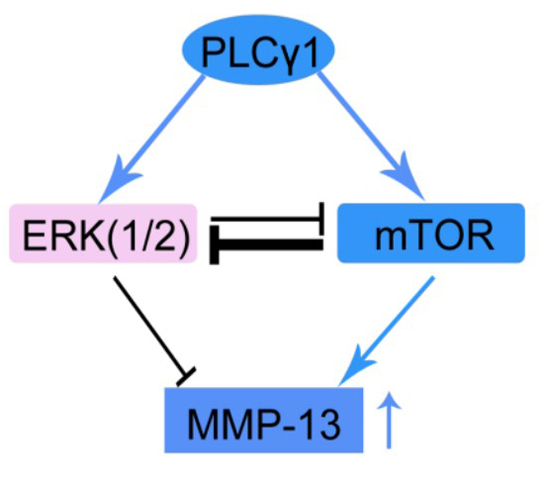
The Involvement of Mutual Inhibition of ERK and mTOR in PLCγ1-Mediated MMP-13 Expression in Human Osteoarthritis Chondrocytes
Abstract
:1. Introduction
2. Results
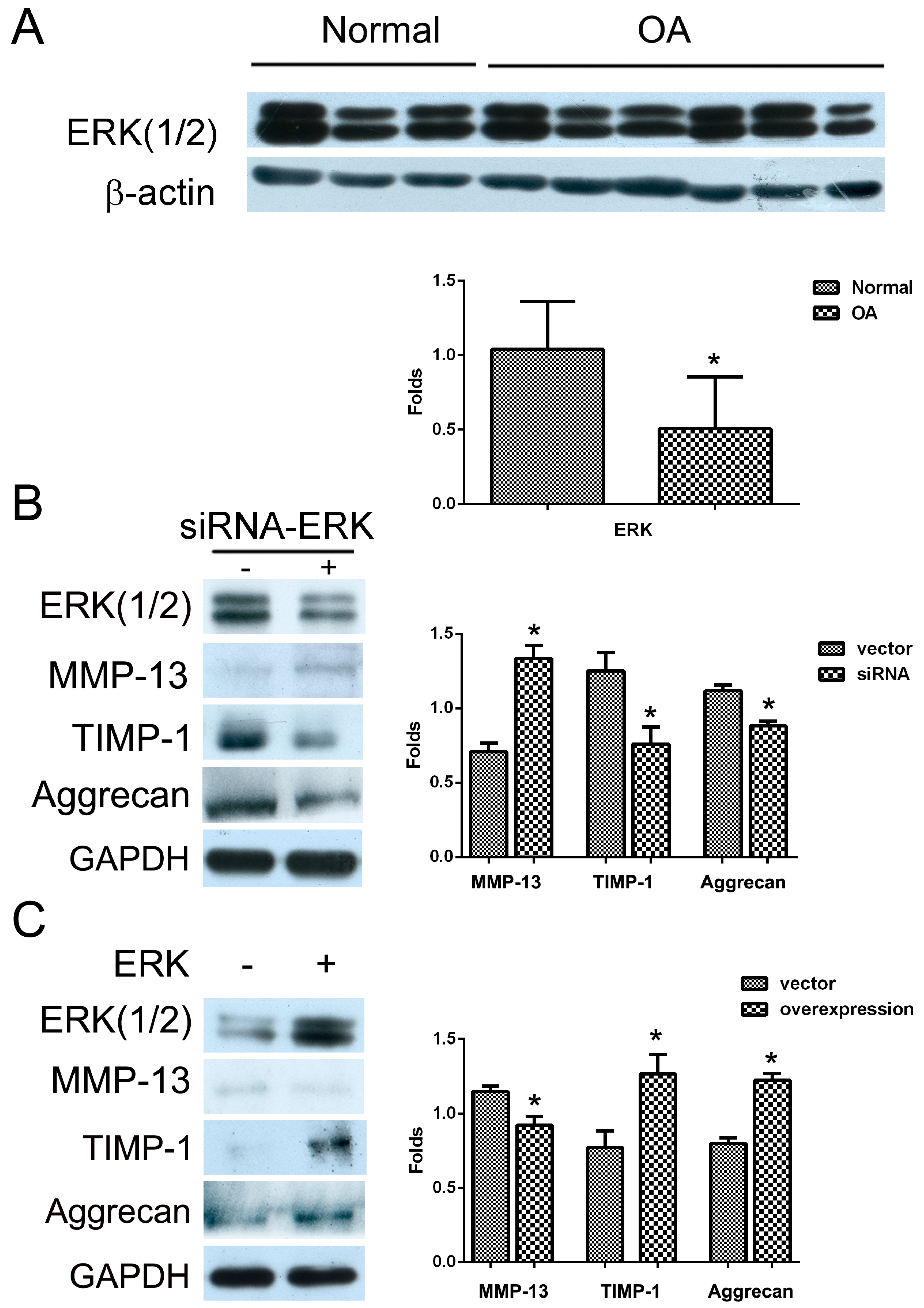
2.1. The Effect of ERK on Matrix Synthesis in Human OA Chondrocytes
2.2. The Effect of PLCγ1 on the Activation of ERK in Human OA Chondrocytes
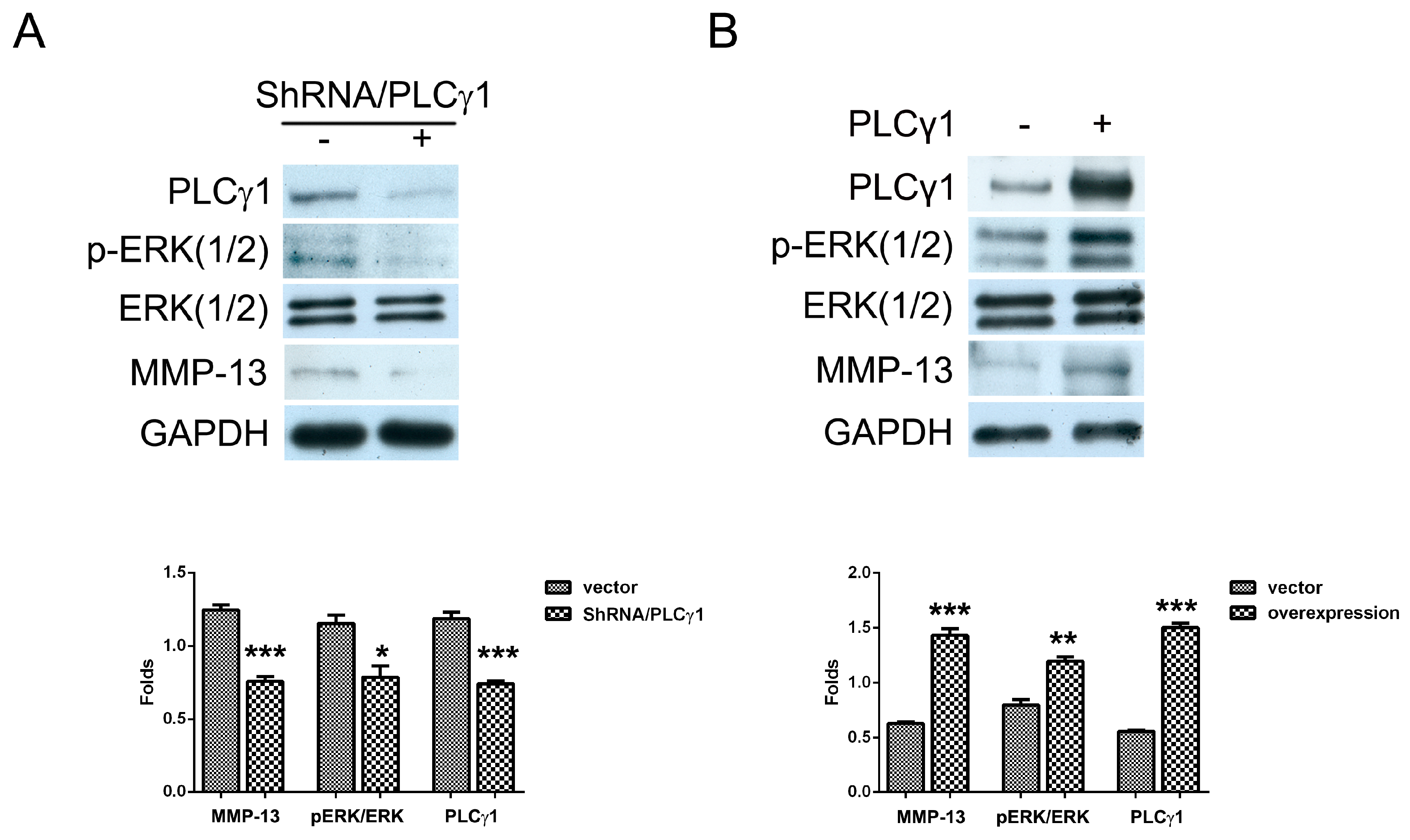
2.3. Mutual Inhibition of ERK and mTOR Signaling in Human OA Chondrocytes

2.4. The PLCγ1/mTOR Axis Was a Dominating Pathway in Human OA Chondrocytes, Compared to the PLCγ1/ERK Axis

3. Discussion
4. Experimental Section
4.1. Reagents and Antibodies
4.2. Human Normal and OA Chondrocyte Isolation and Culture
| Age (year) | Case | Sex | Duration of OA (year) | * K.L. Image Criterion | Pro-Treatment | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | ≤3 | >3 | I | II | III | IV | |||
| 60- | 23 | 1 | 22 | 7 | 16 | 0 | 2 | 12 | 9 | 0 |
| 65- | 27 | 6 | 21 | 7 | 20 | 1 | 3 | 10 | 13 | 0 |
4.3. Plasmid Construction and Transient Transfection
4.4. Protein Extraction and Western Blotting Analysis
4.5. Statistical Analysis
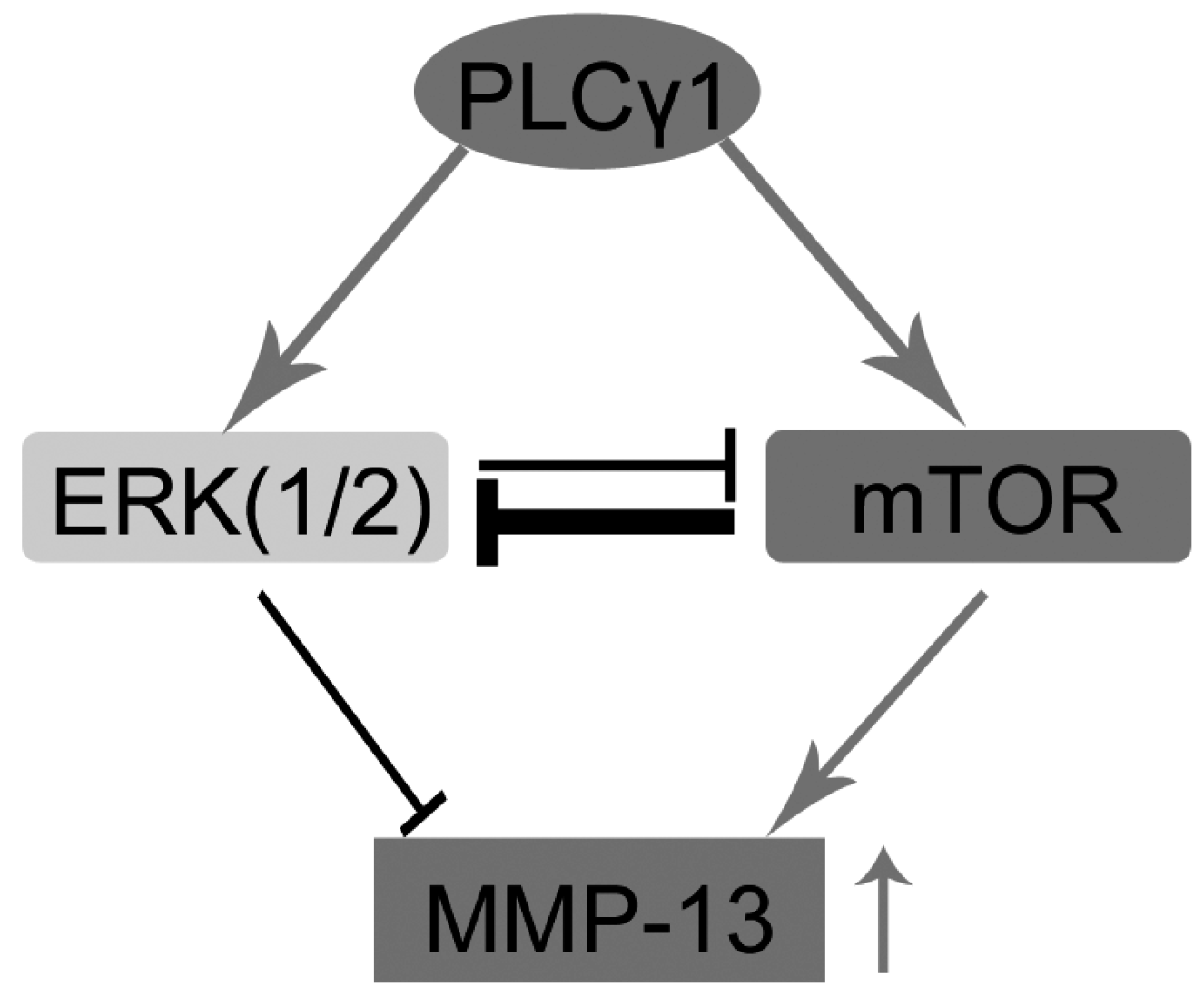
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Maldonado, M.; Nam, J. The role of changes in extracellular matrix of cartilage in the presence of inflammation on the pathology of osteoarthritis. Biomed. Res. Int. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Peng, H.; Wu, D.; Hu, K.; Goldring, M.B.; Olsen, B.R.; Li, Y. Activation of the discoidin domain receptor 2 induces expression of matrix metalloproteinase 13 associated with osteoarthritis in mice. J. Biol. Chem. 2005, 280, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Otero, M.; Plumb, D.A.; Tsuchimochi, K.; Dragomir, C.L.; Hashimoto, K.; Peng, H.; Olivotto, E.; Bevilacqua, M.; Tan, L.; Yang, Z.; et al. E74-like factor 3 (ELF3) impacts on matrix metalloproteinase 13 (MMP13) transcriptional control in articular chondrocytes under proinflammatory stress. J. Biol. Chem. 2012, 287, 3559–3572. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.P.; Liu, B.; Tian, Q.Y.; Wei, J.L.; Richbourgh, B.; Liu, C.J. Progranulin protects against osteoarthritis through interacting with TNF-α and β-Catenin signalling. Ann. Rheum. Dis. 2014. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Chen, D.; Hawse, J.R.; van Wijnen, A.J.; Im, H.J. Bovine lactoferricin induces TIMP-3 via the ERK1/2-Sp1 axis in human articular chondrocytes. Gene 2013, 517, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Hay, N.; Sonenberg, N. Upstream and downstream of mTOR. Genes Dev. 2004, 18, 1926–1945. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Vasheghani, F.; Li, Y.H.; Blati, M.; Simeone, K.; Fahmi, H.; Lussier, B.; Roughley, P.; Lagares, D.; Pelletier, J.P.; et al. Cartilage-specific deletion of mTOR upregulates autophagy and protects mice from osteoarthritis. Ann. Rheum. Dis. 2015, 74, 1432–1440. [Google Scholar] [CrossRef] [PubMed]
- Carracedo, A.; Ma, L.; Teruya-Feldstein, J.; Rojo, F.; Salmena, L.; Alimonti, A.; Egia, A.; Sasaki, A.T.; Thomas, G.; Kozma, S.C.; et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J. Clin. Investig. 2008, 118, 3065–3074. [Google Scholar] [PubMed]
- Rhee, S.G. Regulation of phosphoinositide-specific phospholipase C. Annu. Rev. Biochem. 2001, 70, 281–312. [Google Scholar] [CrossRef] [PubMed]
- Bunney, T.D.; Katan, M. PLC regulation: Emerging pictures for molecular mechanisms. Trends Biochem. Sci. 2011, 36, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Elbaradie, K.B.; Wang, Y.; Boyan, B.D.; Schwartz, Z. Sex-specific response of rat costochondral cartilage growth plate chondrocytes to 17β-estradiol involves differential regulation of plasma membrane associated estrogen receptors. Biochim. Biophys. Acta 2013, 1833, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.; Cui, X.; Liu, Z.; Zhao, H.; Zheng, X.; Zhang, B.; Xia, C. Disruption of phosphoinositide-specific phospholipases Cγ1 contributes to extracellular matrix synthesis of human osteoarthritis chondrocytes. Int. J. Mol. Sci. 2014, 15, 13236–13246. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Siminovitch, K.A.; Downey, G.P.; McCulloch, C.A. Ras-guanine-nucleotide-releasing factors 1 and 2 interact with PLCγ at focal adhesions to enable IL-1-induced Ca2+ signalling, ERK activation and MMP-3 expression. Biochem. J. 2013, 449, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Söder, S.; Oehler, S.; Fundel, K.; Aigner, T. Activation of interleukin-1 signaling cascades in normal and osteoarthritic articular cartilage. Am. J. Pathol. 2007, 171, 938–946. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Zeng, G.; Liu, Z.; Zhang, B.; Cui, X.; Zhao, H.; Zheng, X.; Song, G.; Kang, J.; Xia, C. Protein kinase B and extracellular signal-regulated kinase contribute to the chondroprotective effect of morroniside on osteoarthritis chondrocytes. J. Cell. Mol. Med. 2015, 19, 1877–1886. [Google Scholar] [CrossRef] [PubMed]
- Starkman, B.G.; Cravero, J.D.; Delcarlo, M.; Loeser, R.F. IGF-I stimulation of proteoglycan synthesis bychondrocytes requires activation of the PI 3-kinase pathway but not ERK MAPK. Biochem. J. 2005, 389, 723–729. [Google Scholar]
- Pelletier, J.P.; Fernandes, J.C.; Brunet, J.; Moldovan, F.; Schrier, D.; Flory, C.; Martel-Pelletier, J. In vivo selective inhibition of mitogen-activated protein kinase kinase 1/2 in rabbit experimental osteoarthritis is associated with a reduction in the development of structural changes. Arthritis. Rheum. 2003, 48, 1582–1593. [Google Scholar] [CrossRef] [PubMed]
- Boileau, C.; Martel-Pelletier, J.; Fahmi, H.; Mineau, F.; Boily, M.; Pelletier, J.P. The peroxisome proliferator-activated receptor gamma agonist pioglitazone reduces the development of cartilage lesions in an experimental dog model of osteoarthritis: in vivo protective effects mediated through the inhibition of key signaling and catabolic pathways. Arthritis. Rheum. 2007, 56, 2288–2298. [Google Scholar] [PubMed]
- Suri, S.; Walsh, D.A. Osteochondral alterations in osteoarthritis. Bone 2012, 51, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Sledge, C.B.; Reddi, A.H.; Walsh, D.A.; Blake, D.R. Biology of the normal joint. In Kelley’s Textbook of Rheumatology; Ruddy, S., Harris, E.D.J., Sledge, C.B., Eds.; WB Saunders: Philadelphia, PA, USA, 2001; pp. 1–26. [Google Scholar]
- Gusenbauer, S.; Zanucco, E.; Knyazev, P.; Ullrich, A. Erk2 but not Erk1 regulates crosstalk between Met and EGFR in squamous cell carcinoma cell lines. Mol. Cancer 2015, 14, 54. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.K.; Hong, S.K.; Yoon, S.H.; Park, J.I. Active ERK2 is sufficient to mediate growth arrest and differentiation signaling. FEBS J. 2015, 282, 1017–1030. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Xu, Y.; Yin, Z.; Yang, X.; Jiang, Y.; Gui, J. Chondrocyte migration affects tissue-engineered cartilage integration by activating the signal transduction pathways involving Src, PLCγ1, and ERK1/2. Tissue Eng. Part A 2013, 19, 2506–2516. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Cigliano, A.; Delogu, S.; Armbruster, J.; Dombrowski, F.; Evert, M.; Chen, X.; Calvisi, D. Functional crosstalk between AKT/mTOR and Ras/MAPK pathways in hepatocarcinogenesis: Implications for the treatment of human liver cancer. Cell Cycle 2013, 12, 1999–2010. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Bercury, K.K.; Macklin, W.B. Interaction of mTOR and Erk1/2 signaling to regulate oligodendrocyte differentiation. Glia 2014, 62, 2096–2109. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Lu, X.; Zhang, S.; Zhou, Q.; Zhang, L. mTOR and Erk1/2 signaling in the cerebrospinal fluid-contacting nucleus is involved in neuropathic pain. Neurochem. Res. 2015, 40, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Attur, M.G.; Dave, M.; Cipolletta, C.; Kang, P.; Goldring, M.B.; Patel, I.R.; Abramson, S.B.; Amin, A.R. Reversal of autocrine and paracrine effects of interleukin 1 (IL-1) in human arthritis by type II IL-1 decoy receptor. Potential for pharmacological intervention. J. Biol. Chem. 2000, 275, 40307–40315. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.G.; Xia, C.; Zheng, X.P.; Yi, T.T.; Wang, X.Y.; Song, G.; Zhang, B. 17β-Estradiol promotes cell proliferation in rat osteoarthritis model chondrocytes via PI3K/Akt pathway. Cell. Mol. Biol. Lett. 2011, 16, 564–575. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Cai, H.; Zheng, X.; Zhang, B.; Xia, C. The Involvement of Mutual Inhibition of ERK and mTOR in PLCγ1-Mediated MMP-13 Expression in Human Osteoarthritis Chondrocytes. Int. J. Mol. Sci. 2015, 16, 17857-17869. https://doi.org/10.3390/ijms160817857
Liu Z, Cai H, Zheng X, Zhang B, Xia C. The Involvement of Mutual Inhibition of ERK and mTOR in PLCγ1-Mediated MMP-13 Expression in Human Osteoarthritis Chondrocytes. International Journal of Molecular Sciences. 2015; 16(8):17857-17869. https://doi.org/10.3390/ijms160817857
Chicago/Turabian StyleLiu, Zejun, Heguo Cai, Xinpeng Zheng, Bing Zhang, and Chun Xia. 2015. "The Involvement of Mutual Inhibition of ERK and mTOR in PLCγ1-Mediated MMP-13 Expression in Human Osteoarthritis Chondrocytes" International Journal of Molecular Sciences 16, no. 8: 17857-17869. https://doi.org/10.3390/ijms160817857