High Expression of LAMP3 Is a Novel Biomarker of Poor Prognosis in Patients with Esophageal Squamous Cell Carcinoma
Abstract
:1. Introduction
2. Results
2.1. The Expression Level of LAMP3 in ESCC
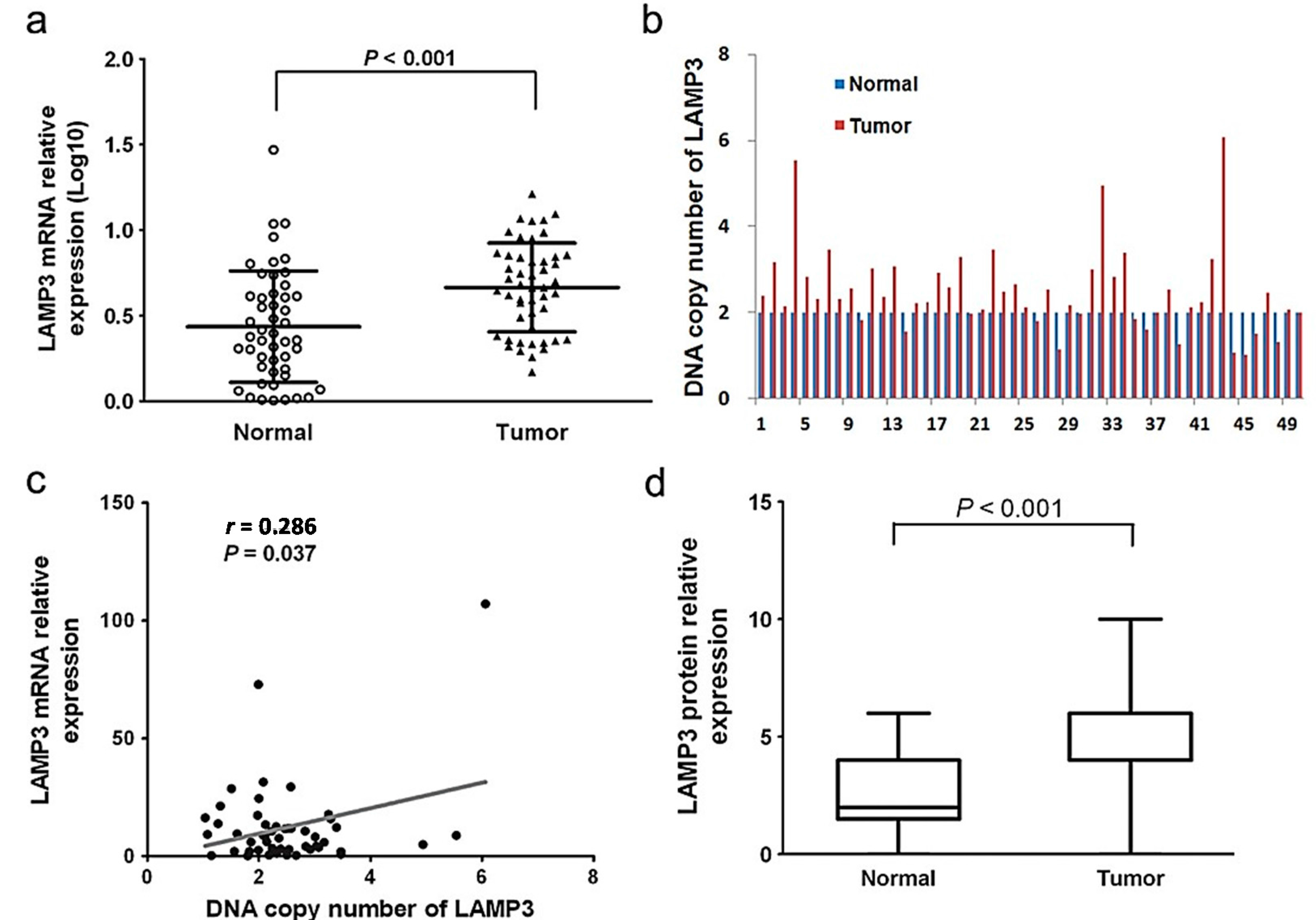

2.3. Association between LAMP3 Expression and Survival
| Variable | Cases | LAMP3 Expression | p Value a | |
|---|---|---|---|---|
| Low (%) | High (%) | |||
| Gender | - | - | - | 0.304 |
| Male | 110 | 51 (46.4) | 59 (53.6) | - |
| Female | 47 | 26 (55.3) | 21 (44.7) | - |
| Age (years) | - | - | - | 0.003 |
| <57 | 73 | 45 (61.6) | 28 (38.4) | - |
| ≥57 | 84 | 32 (38.1) | 52 (61.9) | - |
| Tumor Size (cm) | - | - | - | 0.063 |
| <3.9 | 76 | 32 (42.1) | 44 (57.9) | - |
| ≥3.9 | 77 | 44 (57.1) | 33 (42.9) | - |
| Tumor Location | - | - | - | 0.063 |
| Upper | 12 | 2 (16.7) | 10 (83.3) | - |
| Middle | 102 | 52 (51.0) | 50 (49.0) | - |
| Lower | 43 | 23 (53.5) | 20 (46.5) | - |
| Histologic Grade | - | - | - | 0.344 |
| G1 | 34 | 16 (47.1) | 18 (52.9) | - |
| G2 | 79 | 43 (54.4) | 36 (45.6) | - |
| G3 | 44 | 18 (40.9) | 26 (59.1) | - |
| T Status | - | - | - | 0.749 |
| T1–T2 | 31 | 16 (51.6) | 15 (48.4) | - |
| T3–T4 | 126 | 61 (48.4) | 65 (51.6) | - |
| Lymph Node Metastasis | - | - | - | 0.134 |
| N0 | 68 | 38 (55.9) | 30 (44.1) | - |
| N1 | 89 | 39 (43.8) | 50 (56.2) | - |
| TNM Stage | - | - | - | 0.095 |
| I–IIa | 59 | 34 (57.6) | 25 (42.4) | - |
| IIb–IV | 98 | 43 (43.9) | 55 (56.1) | - |
| Cigarette Smoking | - | - | - | 0.155 |
| Never | 53 | 30 (56.6) | 23 (43.4) | - |
| Smoking | 101 | 45 (44.6) | 56 (55.4) | - |
| Alcohol | - | - | - | 0.018 |
| Never | 92 | 52 (56.5) | 40 (43.5) | - |
| Drinking | 62 | 23 (37.1) | 39 (62.9) | - |

| Risk Factors | Overall Survival | |||
|---|---|---|---|---|
| Univariate | Multivariate | |||
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| LAMP3 Expression (Low/High) | 2.22 (1.39, 3.55) | 0.001 * | 1.90 (1.17, 3.09) | 0.010 * |
| Gender (Male/Female) | 0.97 (0.60, 1.57) | 0.892 | - | - |
| Age (<57/57) | 2.17 (1.35, 3.47) | 0.001 * | 1.66 (1.01, 2.72) | 0.042 * |
| Tumor Size (<3.9/≥3.9 cm) | 0.58 (0.37, 0.92) | 0.022 * | 0.64 (0.40, 1.02) | 0.059 |
| Tumor Location (Upper/Middle/Lower) | 0.84 (0.56, 1.26) | 0.399 | - | - |
| Histologic Grade (G1/G2/G3) | 1.69 (1.23, 2.33) | 0.001 * | 1.40 (0.99, 1.97) | 0.055 |
| T Status (T1–T2/T3–T4) | 1.13 (0.64, 1.99) | 0.671 | - | - |
| Lymph Node Metastasis (N0/N1) | 3.14 (1.90, 5.20) | 0.001 * | 2.66 (1.58, 4.48) | 0.001 * |
| Risk Factors | Disease-Free Survival | |||
|---|---|---|---|---|
| Univariate | Multivariate | |||
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| LAMP3 Expression (Low/High) | 2.00 (1.32, 3.03) | 0.001 * | 1.80 (1.18, 2.74) | 0.006 * |
| Gender (Male/Female) | 0.82 (0.53, 1.28) | 0.387 | - | - |
| Age (<57/57) | 1.69 (1.12, 2.56) | 0.013 * | 1.48 (0.98, 2.26) | 0.065 |
| Tumor Size (<3.9/≥3.9 cm) | 0.76 (0.51, 1.15) | 0.199 | - | - |
| Tumor Location (Upper/Middle/Lower) | 0.81 (0.57, 1.15) | 0.238 | - | - |
| Histologic Grade (G1/G2/G3) | 1.70 (1.27, 2.27) | 0.001 * | 1.42 (1.05, 1.92) | 0.024 * |
| T Status (T1–T2/T3–T4) | 1.26 (0.75, 2.13) | 0.389 | - | - |
| Lymph Node Metastasis (N0/N1) | 2.58 (1.66, 4.01) | 0.001 * | 2.08 (1.31, 3.29) | 0.002 * |
3. Discussion
4. Materials and Methods
4.1. ESCC Patients and Samples
4.2. Quantitative Real-Time PCR (qRT-PCR)
4.3. DNA Copy Number Analysis
4.4. Tissue Microarrays (TMAs) and Immunohistochemistry (IHC)
4.5. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhao, P.; Dai, M.; Chen, W.; Li, N. Cancer trends in China. Jpn. J. Clin. Oncol. 2010, 40, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Siegel, R.; Xu, J.; Ward, E. Cancer statistics, 2010. CA Cancer J. Clin. 2010, 60, 277–300. [Google Scholar] [CrossRef] [PubMed]
- Pennathur, A.; Gibson, M.K.; Jobe, B.A.; Luketich, J.D. Oesophageal carcinoma. Lancet 2013, 381, 400–412. [Google Scholar] [CrossRef]
- Hsia, J.Y.; Chen, C.Y.; Hsu, C.P.; Shai, S.E.; Yang, S.S.; Chuang, C.Y.; Wang, P.Y.; Chen, J.T. Expression of apoptosis-regulating proteins p53, Bcl-2, and Bax in primary resected esophageal squamous cell carcinoma. Neoplasma 2001, 48, 483–488. [Google Scholar] [PubMed]
- Li, Y.; Yang, H.X.; Luo, R.Z.; Zhang, Y.; Li, M.; Wang, X.; Jia, W.H. High expression of p300 has an unfavorable impact on survival in resectable esophageal squamous cell carcinoma. Ann. Thorac. Surg. 2011, 91, 1531–1538. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, K.; Nagata, M.; Suzuki, M.; Fujiwara, T.; Ueda, K.; Miyoshi, Y.; Takahashi, E.; Nakamura, Y. Isolation and characterization of a novel human lung-specific gene homologous to lysosomal membrane glycoproteins 1 and 2: Significantly increased expression in cancers of various tissues. Cancer Res. 1998, 58, 3499–3503. [Google Scholar] [PubMed]
- De Saint-Vis, B.; Vincent, J.; Vandenabeele, S.; Vanbervliet, B.; Pin, J.J.; Ait-Yahia, S.; Patel, S.; Mattei, M.G.; Banchereau, J.; Zurawski, S.; et al. A novel lysosome-associated membrane glycoprotein, DC-LAMP, induced upon DC maturation, is transiently expressed in MHC class II compartment. Immunity 1998, 9, 325–336. [Google Scholar] [CrossRef]
- Fukuda, M. Lysosomal membrane glycoproteins. Structure, biosynthesis, and intracellular trafficking. J. Biol. Chem. 1991, 266, 21327–21330. [Google Scholar] [PubMed]
- Carlsson, S.R.; Fukuda, M. The lysosomal membrane glycoprotein lamp-1 is transported to lysosomes by two alternative pathways. Arch. Biochem. Biophys. 1992, 296, 630–639. [Google Scholar] [CrossRef]
- Saitoh, O.; Wang, W.C.; Lotan, R.; Fukuda, M. Differential glycosylation and cell surface expression of lysosomal membrane glycoproteins in sublines of a human colon cancer exhibiting distinct metastatic potentials. J. Biol. Chem. 1992, 267, 5700–5711. [Google Scholar] [PubMed]
- Nagelkerke, A.; Bussink, J.; Mujcic, H.; Wouters, B.G.; Lehmann, S.; Sweep, F.C.; Span, P.N. Hypoxia stimulates migration of breast cancer cells via the PERK/ATF4/LAMP3-arm of the unfolded protein response. Breast Cancer Res. 2013, 15. [Google Scholar] [CrossRef] [PubMed]
- Mujcic, H.; Nagelkerke, A.; Rouschop, K.M.; Chung, S.; Chaudary, N.; Span, P.N.; Clarke, B.; Milosevic, M.; Sykes, J.; Hill, R.P.; et al. Hypoxic activation of the PERK/eIF2α arm of the unfolded protein response promotes metastasis through induction of LAMP3. Clin. Cancer Res. 2013, 19, 6126–6137. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Wang, X.; Zhu, H.; Mei, H.; Wang, W.; Zhang, S.; Huang, J. Prognostic value of LAMP3 and TP53 overexpression in benign and malignant gastrointestinal tissues. Oncotarget 2014, 5, 12398–12409. [Google Scholar] [PubMed]
- Kanao, H.; Enomoto, T.; Kimura, T.; Fujita, M.; Nakashima, R.; Ueda, Y.; Ueno, Y.; Miyatake, T.; Yoshizaki, T.; Buzard, G.S.; et al. Overexpression of LAMP3/TSC403/DC-LAMP promotes metastasis in uterine cervical cancer. Cancer Res. 2005, 65, 8640–8645. [Google Scholar] [CrossRef] [PubMed]
- Nagelkerke, A.; Bussink, J.; van der Kogel, A.J.; Sweep, F.C.; Span, P.N. The PERK/ATF4/LAMP3-arm of the unfolded protein response affects radioresistance by interfering with the DNA damage response. Radiother. Oncol. 2013, 108, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Nagelkerke, A.; Sieuwerts, A.M.; Bussink, J.; Sweep, F.C.; Look, M.P.; Foekens, J.A.; Martens, J.W.; Span, P.N. LAMP3 is involved in tamoxifen resistance in breast cancer cells through the modulation of autophagy. Endocr. Relat. Cancer 2014, 21, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Racz, A.; Brass, N.; Heckel, D.; Pahl, S.; Remberger, K.; Meese, E. Expression analysis of genes at 3q26-q27 involved in frequent amplification in squamous cell lung carcinoma. Eur. J. Cancer 1999, 35, 641–646. [Google Scholar] [CrossRef]
- Bockmuhl, U.; Schwendel, A.; Dietel, M.; Petersen, I. Distinct patterns of chromosomal alterations in high- and low-grade head and neck squamous cell carcinomas. Cancer Res. 1996, 56, 5325–5329. [Google Scholar] [PubMed]
- Miller, C.T.; Moy, J.R.; Lin, L.; Schipper, M.; Normolle, D.; Brenner, D.E.; Iannettoni, M.D.; Orringer, M.B.; Beer, D.G. Gene amplification in esophageal adenocarcinomas and Barrett’s with high-grade dysplasia. Clin. Cancer Res. 2003, 9, 4819–4825. [Google Scholar] [PubMed]
- Hu, N.; Wang, C.; Ng, D.; Clifford, R.; Yang, H.H.; Tang, Z.Z.; Wang, Q.H.; Han, X.Y.; Giffen, C.; Goldstein, A.M.; et al. Genomic characterization of esophageal squamous cell carcinoma from a high-risk population in China. Cancer Res. 2009, 69, 5908–5917. [Google Scholar] [CrossRef] [PubMed]
- Sakai, N.; Kajiyama, Y.; Iwanuma, Y.; Tomita, N.; Amano, T.; Isayama, F.; Ouchi, K.; Tsurumaru, M. Study of abnormal chromosome regions in esophageal squamous cell carcinoma by comparative genomic hybridization: relationship of lymph node metastasis and distant metastasis to selected abnormal regions. Dis. Esophagus 2010, 23, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fu, L.; Li, J.B.; Qin, Y.; Zeng, T.T.; Zhou, J.; Zeng, Z.L.; Chen, J.; Cao, T.T.; Ban, X.; et al. Increased expression of EIF5A2, via hypoxia or gene amplification, contributes to metastasis and angiogenesis of esophageal squamous cell carcinoma. Gastroenterology 2014, 146, 1701–1713. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Guo, L.; Peiffer, D.A.; Zhou, L.; Chan, O.T.; Bibikova, M.; Wickham-Garcia, E.; Lu, S.H.; Zhan, Q.; Wang-Rodriguez, J.; et al. Genomic profiling of 766 cancer-related genes in archived esophageal normal and carcinoma tissues. Int. J. Cancer 2008, 122, 2249–2254. [Google Scholar] [CrossRef] [PubMed]
- Hunziker, W.; Geuze, H.J. Intracellular trafficking of lysosomal membrane proteins. BioEssays 1996, 18, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Heffernan, M.; Yousefi, S.; Dennis, J.W. Molecular characterization of P2B/LAMP-1, a major protein target of a metastasis-associated oligosaccharide structure. Cancer Res. 1989, 49, 6077–6084. [Google Scholar] [PubMed]
- Laferte, S.; Dennis, J.W. Purification of two glycoproteins expressing β1–6 branched Asn-linked oligosaccharides from metastatic tumour cells. Biochem. J. 1989, 259, 569–576. [Google Scholar] [PubMed]
- Carlsson, S.R.; Roth, J.; Piller, F.; Fukuda, M. Isolation and characterization of human lysosomal membrane glycoproteins, h-lamp-1 and h-lamp-2. Major sialoglycoproteins carrying polylactosaminoglycan. J. Biol. Chem. 1988, 263, 18911–18919. [Google Scholar] [PubMed]
- Viitala, J.; Carlsson, S.R.; Siebert, P.D.; Fukuda, M. Molecular cloning of cDNAs encoding lamp A, a human lysosomal membrane glycoprotein with apparent Mr ≈ 120,000. Proc. Natl. Acad. Sci. USA 1988, 85, 3743–3747. [Google Scholar] [CrossRef] [PubMed]
- Kannan, K.; Stewart, R.M.; Bounds, W.; Carlsson, S.R.; Fukuda, M.; Betzing, K.W.; Holcombe, R.F. Lysosome-associated membrane proteins h-LAMP1 (CD107a) and h-LAMP2 (CD107b) are activation-dependent cell surface glycoproteins in human peripheral blood mononuclear cells which mediate cell adhesion to vascular endothelium. Cell. Immunol. 1996, 171, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lu, G.; Li, Z.; Tang, F.; Liu, Y.; Cui, G. Distinct compartmental distribution of mature and immature dendritic cells in esophageal squamous cell carcinoma. Pathol. Res. Pract. 2010, 206, 602–606. [Google Scholar] [CrossRef] [PubMed]
- Nagelkerke, A.; Mujcic, H.; Bussink, J.; Wouters, B.G.; van Laarhoven, H.W.; Sweep, F.C.; Span, P.N. Hypoxic regulation and prognostic value of LAMP3 expression in breast cancer. Cancer 2011, 117, 3670–3681. [Google Scholar] [CrossRef] [PubMed]
- Treilleux, I.; Blay, J.Y.; Bendriss-Vermare, N.; Ray-Coquard, I.; Bachelot, T.; Guastalla, J.P.; Bremond, A.; Goddard, S.; Pin, J.J.; Barthelemy-Dubois, C.; et al. Dendritic cell infiltration and prognosis of early stage breast cancer. Clin. Cancer Res. 2004, 10, 7466–7474. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.Y.; Wei, S.J.; Lin, Y.C.; Lung, J.C.; Chang, T.C.; Whang-Peng, J.; Liu, J.M.; Yang, D.M.; Yang, W.K.; Shen, C.Y. PIK3CA as an oncogene in cervical cancer. Oncogene 2000, 19, 2739–2744. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Funk, W.D.; Wang, S.S.; Weinrich, S.L.; Avilion, A.A.; Chiu, C.P.; Adams, R.R.; Chang, E.; Allsopp, R.C.; Yu, J.; et al. The RNA component of human telomerase. Science 1995, 269, 1236–1241. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.Y.; Sham, J.S.; Tang, T.C.; Fang, Y.; Huo, K.K.; Yang, J.M. Isolation of a novel candidate oncogene within a frequently amplified region at 3q26 in ovarian cancer. Cancer Res. 2001, 61, 3806–3809. [Google Scholar] [PubMed]
- Xu, F.H.; Xiong, D.; Xu, Y.F.; Cao, S.M.; Xue, W.Q.; Qin, H.D.; Liu, W.S.; Cao, J.Y.; Zhang, Y.; Feng, Q.S.; et al. An epidemiological and molecular study of the relationship between smoking, risk of nasopharyngeal carcinoma, and Epstein-Barr virus activation. J. Natl. Cancer Inst. 2012, 104, 1396–1410. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, X.; Chen, Y.; Liu, D.; Li, F.; Li, X.; Jia, W. High Expression of LAMP3 Is a Novel Biomarker of Poor Prognosis in Patients with Esophageal Squamous Cell Carcinoma. Int. J. Mol. Sci. 2015, 16, 17655-17667. https://doi.org/10.3390/ijms160817655
Liao X, Chen Y, Liu D, Li F, Li X, Jia W. High Expression of LAMP3 Is a Novel Biomarker of Poor Prognosis in Patients with Esophageal Squamous Cell Carcinoma. International Journal of Molecular Sciences. 2015; 16(8):17655-17667. https://doi.org/10.3390/ijms160817655
Chicago/Turabian StyleLiao, Xiaoyu, Yuanbin Chen, Deqing Liu, Fangfang Li, Xizhao Li, and Weihua Jia. 2015. "High Expression of LAMP3 Is a Novel Biomarker of Poor Prognosis in Patients with Esophageal Squamous Cell Carcinoma" International Journal of Molecular Sciences 16, no. 8: 17655-17667. https://doi.org/10.3390/ijms160817655





