The Photoprotective Effect of S-Methylmethionine Sulfonium in Skin
Abstract
:1. Introduction
2. Results and Discussion
2.1. Photoprotective Effect of S-Methylmethionine Sulfonium (SMMS) in Keratinocyte Progenitor Cells (KPCs)


2.2. Photoprotective Effect of SMMS in Human Dermal Fibroblasts (hDFs)
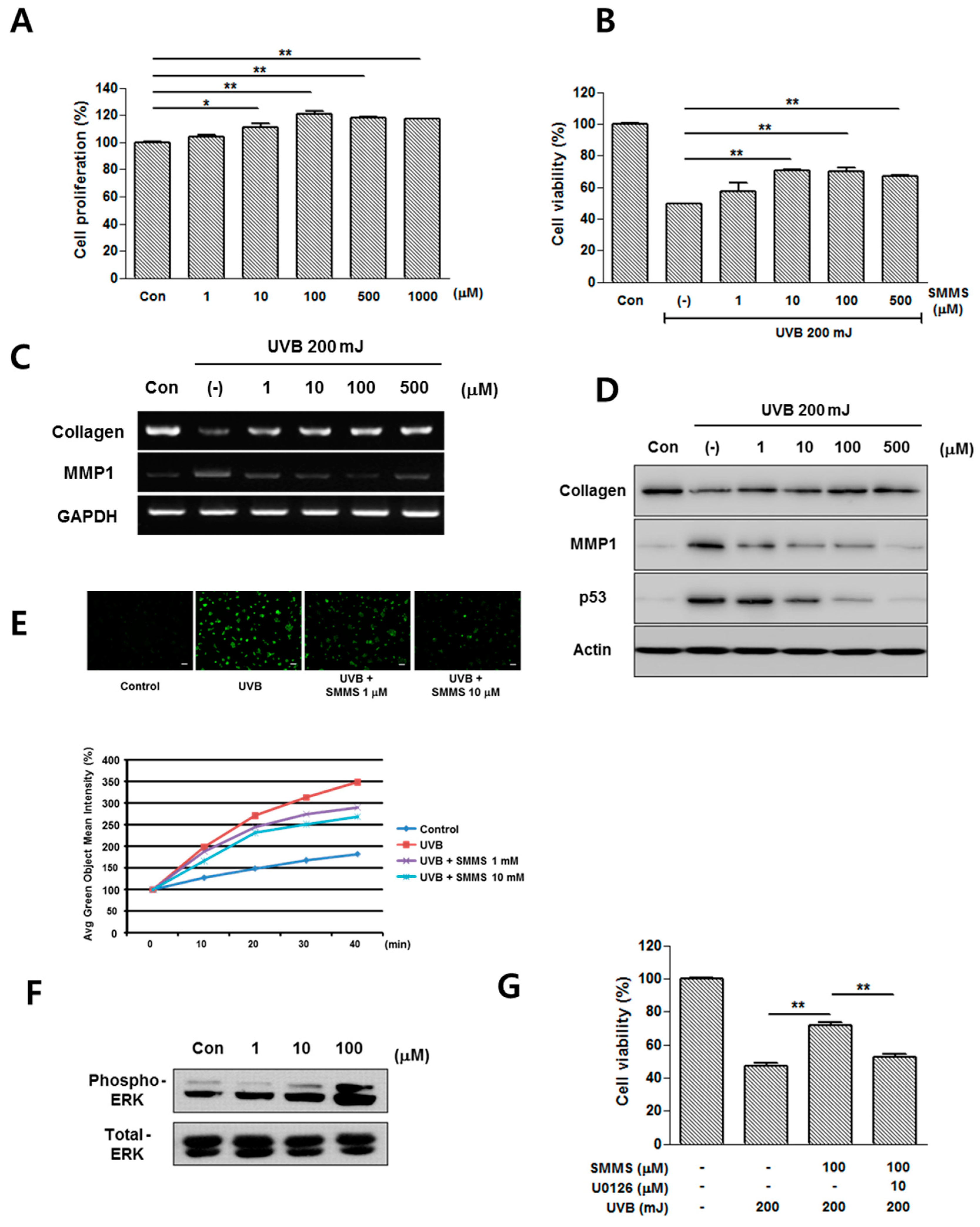
2.3. Protective Effect of SMMS in Ultraviolet B-Induced Erythema

2.4. Protective Effect of SMMS in UVB-Induced Depletion of Epidermal Langerhans Cells (LCs)
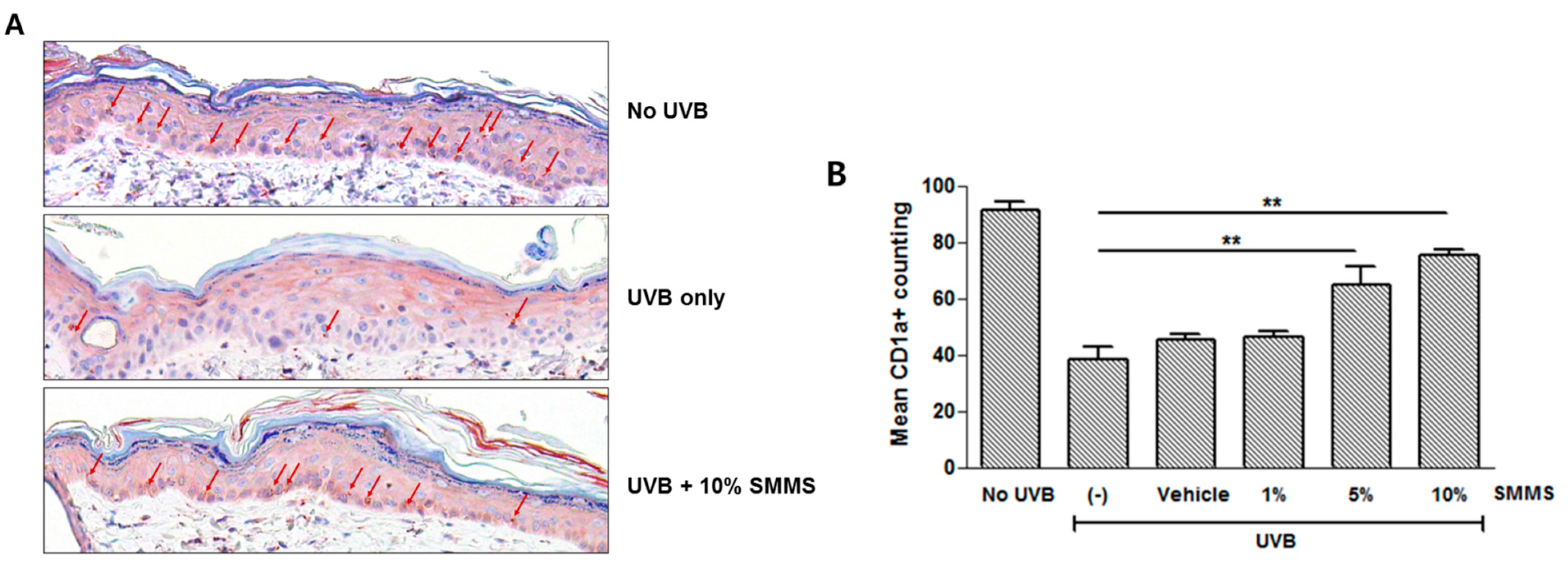
2.5. Discussion
3. Experimental Section
3.1. Cell Culture
3.2. Proliferation and Viability Assay
3.3. Immunocytochemistry
3.4. Cellular Reactive Oxygen Species (ROS) Generation Assay
3.5. Western Blot Analysis
3.6. Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
3.7. Animal Study
3.8. UV Source and Irradiation Protocol
3.9. Spectrophotometric Measurements
- Ig: reflected intensity at 620 ± 30 nm; Ir: reflected intensity at 550 ± 30 nm.
- Then, the erythema index (EI) can be calculated as describe previously [22]: 100 × log(Ir/Ig).
3.10. Immunohistochemical Analysis
3.11. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Drouin, R.; Therrien, J.P. UVB-induced cyclobutane pyrimidine dimer frequency correlates with skin cancer mutational hotspots in p53. Photochem. Photobiol. 1997, 66, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Nomura, T.; Nakajima, H.; Hongyo, T.; Taniguchi, E.; Fukuda, K.; Li, L.Y.; Kurooka, M.; Sutoh, K.; Hande, P.M.; Kawaguchi, T.; et al. Induction of cancer, actinic keratosis, and specific p53 mutations by UVB light in human skin maintained in severe combined immunodeficient mice. Cancer Res. 1997, 57, 2081–2084. [Google Scholar] [PubMed]
- Ramachandran, S.; Rajendra Prasad, N.; Karthikeyan, S. Sesamol inhibits UVB-induced ROS generation and subsequent oxidative damage in cultured human skin dermal fibroblasts. Arch. Dermatol. Res. 2010, 302, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Wu, S. ROS and p53 in regulation of UVB-induced HDM2 alternative splicing. Photochem. Photobiol. 2015, 91, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Ikehata, H.; Ono, T. Mutation induction with UVB in mouse skin epidermis is suppressed in acute high-dose exposure. Mutat. Res. 2002, 508, 41–47. [Google Scholar] [CrossRef]
- Taguchi, K.; Fukunaga, A.; Ogura, K.; Nishigori, C. The role of epidermal Langerhans cells in NB-UVB-induced immunosuppression. Kobe J. Med. Sci. 2013, 59, E1–E9. [Google Scholar] [PubMed]
- Salim, A.S. Administration of sulfhydryls to stimulate the healing of ischemia-induced acute gastric mucosal injury in the rat. J. Pharm. Sci. 1991, 80, 539–541. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Yang, Y.J.; Min, H.G.; Song, M.G.; Lee, J.S.; Park, K.Y.; Kim, J.J.; Sung, J.H.; Choi, J.S.; Cha, H.J. Accelerated wound healing by S-methylmethionine sulfonium: Evidence of dermal fibroblast activation via the ERK1/2 pathway. Pharmacology 2010, 85, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, T.; Ito, Y.; Saegusa, Y.; Iwai, T.; Goso, Y.; Ikezawa, T.; Ishihara, K. Effects of combination treatment with famotidine and methylmethionine sulfonium chloride on the mucus barrier of rat gastric mucosa. J. Gastroenterol. Hepatol. 2009, 24, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Maxis, G. Gastritis and its treatment with methylmethionine sulfonium bromide. Die Therapiewoche 1965, 15, 736–739. [Google Scholar] [PubMed]
- Nakamura, N.; Uzawa, H.; Kanazawa, K.; Tamai, Y.; Tashiro, Y.; Koide, M. Hypolipidemic effect of l-form S-methylmethionine sulfonium chloride in man. Arzneimittel-Forschung 1981, 31, 725–729. [Google Scholar] [PubMed]
- Urazaeva, L.G. Anti-inflammatory effect of methylmethionine sulfonium chloride (vitamin U). Farmakol. Toksikol. 1976, 39, 316–319. [Google Scholar] [PubMed]
- Cao, C.; Lu, S.; Kivlin, R.; Wallin, B.; Card, E.; Bagdasarian, A.; Tamakloe, T.; Wang, W.J.; Song, X.; Chu, W.M.; et al. SIRT1 confers protection against UVB- and H2O2-induced cell death via modulation of p53 and JNK in cultured skin keratinocytes. J. Cell. Mol. Med. 2009, 13, 3632–3643. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.W.; Choi, Y.J.; Park, M.H.; Jang, E.J.; Kim, D.H.; Park, B.H.; Yu, B.P.; Chung, H.Y. Molecular insights into SIRT1 protection against UVB-induced skin fibroblast senescence by suppression of oxidative stress and p53 acetylation. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Strozyk, E.; Kulms, D. The role of AKT/mTOR pathway in stress response to UV-irradiation: Implication in skin carcinogenesis by regulation of apoptosis, autophagy and senescence. Int. J. Mol. Sci. 2013, 14, 15260–15285. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, L.E.; Belgi, G.; Parslew, R.; McLoughlin, L.; Clough, G.F.; Friedmann, P.S. Ultraviolet-B-induced erythema is mediated by nitric oxide and prostaglandin E2 in combination. J. Investig. Dermatol. 2001, 117, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Diffey, B.L.; Farr, P.M.; Oakley, A.M. Quantitative studies on UVA-induced erythema in human skin. Br. J. Dermatol. 1987, 117, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Granstein, R.D.; Matsui, M.S. UV radiation-induced immunosuppression and skin cancer. Cutis 2004, 74, 4–9. [Google Scholar] [PubMed]
- Shreedhar, V.K.; Pride, M.W.; Sun, Y.; Kripke, M.L.; Strickland, F.M. Origin and characteristics of ultraviolet-B radiation-induced suppressor T lymphocytes. J. Immunol. 1998, 161, 1327–1335. [Google Scholar] [PubMed]
- Won, C.H.; Jeong, Y.M.; Kang, S.; Koo, T.S.; Park, S.H.; Park, K.Y.; Sung, Y.K.; Sung, J.H. Hair-growth-promoting effect of conditioned medium of high integrin α6 and low CD 71 (α6bri/CD71dim) positive keratinocyte cells. Int. J. Mol. Sci. 2015, 16, 4379–4391. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Park, B.S.; Park, S.H.; Kim, H.K.; Sung, J.H. Antiwrinkle effect of adipose-derived stem cell: Activation of dermal fibroblast by secretory factors. J. Dermatol. Sci. 2009, 53, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Diffey, B.L.; Oliver, R.J.; Farr, P.M. A portable instrument for quantifying erythema induced by ultraviolet radiation. Br. J. Dermatol. 1984, 111, 663–672. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, W.-S.; Seo, H.-M.; Kim, W.-K.; Choi, J.-S.; Kim, I.; Sung, J.-H. The Photoprotective Effect of S-Methylmethionine Sulfonium in Skin. Int. J. Mol. Sci. 2015, 16, 17088-17100. https://doi.org/10.3390/ijms160817088
Kim W-S, Seo H-M, Kim W-K, Choi J-S, Kim I, Sung J-H. The Photoprotective Effect of S-Methylmethionine Sulfonium in Skin. International Journal of Molecular Sciences. 2015; 16(8):17088-17100. https://doi.org/10.3390/ijms160817088
Chicago/Turabian StyleKim, Won-Serk, Hyun-Min Seo, Wang-Kyun Kim, Joon-Seok Choi, Ikyon Kim, and Jong-Hyuk Sung. 2015. "The Photoprotective Effect of S-Methylmethionine Sulfonium in Skin" International Journal of Molecular Sciences 16, no. 8: 17088-17100. https://doi.org/10.3390/ijms160817088




