RGD Peptides-Conjugated Pluronic Triblock Copolymers Encapsulated with AP-2α Expression Plasmid for Targeting Gastric Cancer Therapy in Vitro and in Vivo
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of RGD@P123@AP-2α Nanocomposites
| Group | Original Size | Stored at 30 Days | Stored at 90 Days |
|---|---|---|---|
| P123 | 19.3 ± 1.5 nm | 20.1 ± 2.1 nm | 21.4 ± 2.3 nm |
| P123@AP-2α | 21.2 ± 2.0 nm | 22.5 ± 3.1 nm | 23.3 ± 2.5 nm |
| RGD@P123@AP-2α | 23.5 ± 2.1 nm | 23.8 ± 2.3 nm | 25.3 ± 3.2 nm |
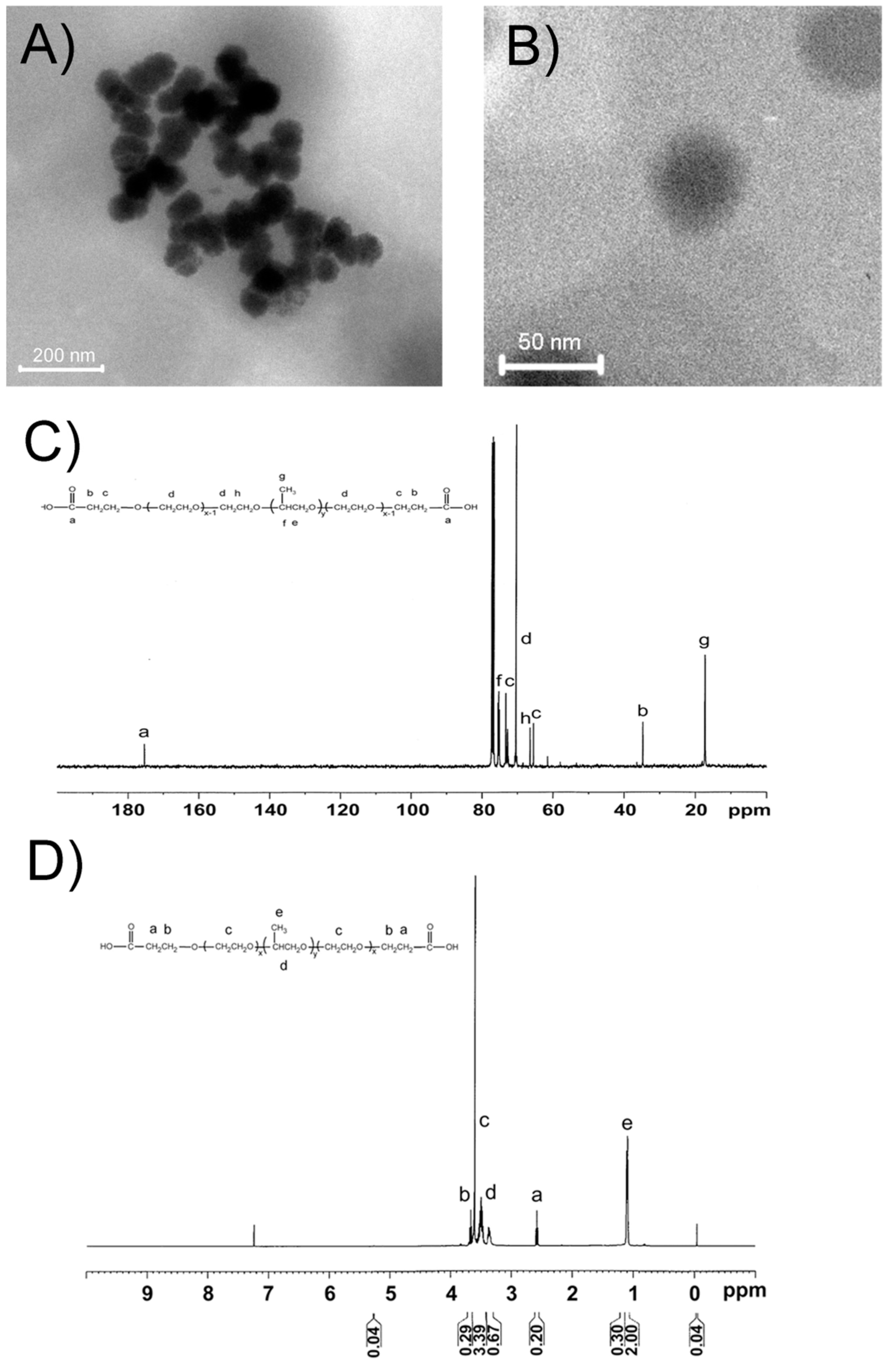
2.2. In Vitro Release of AP-2α Expression Plasmids from RGD@P123 Nano composites

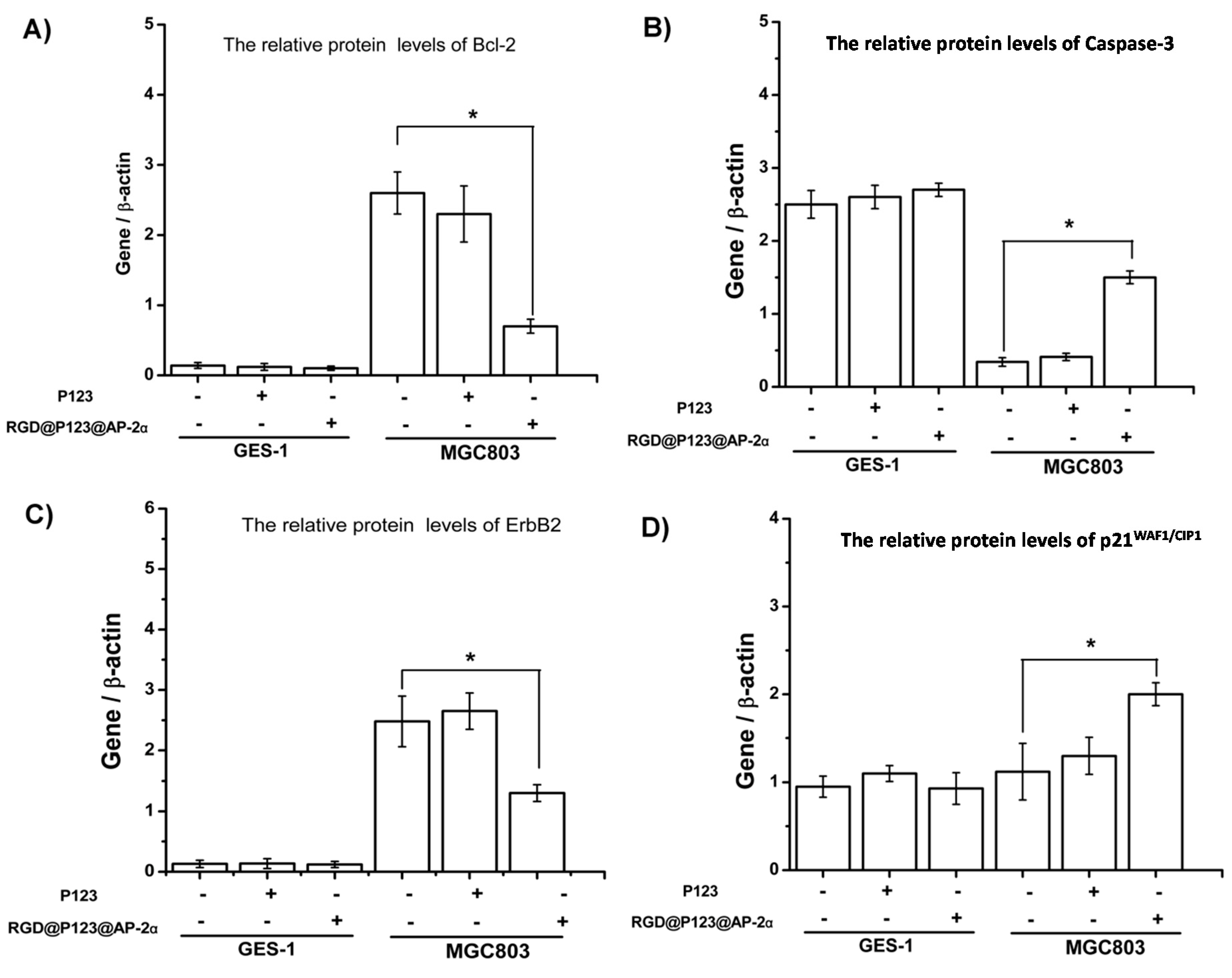
2.3. The Biocompatibility and Antitumor Activity of RGD@P123@AP-2α Nano composites
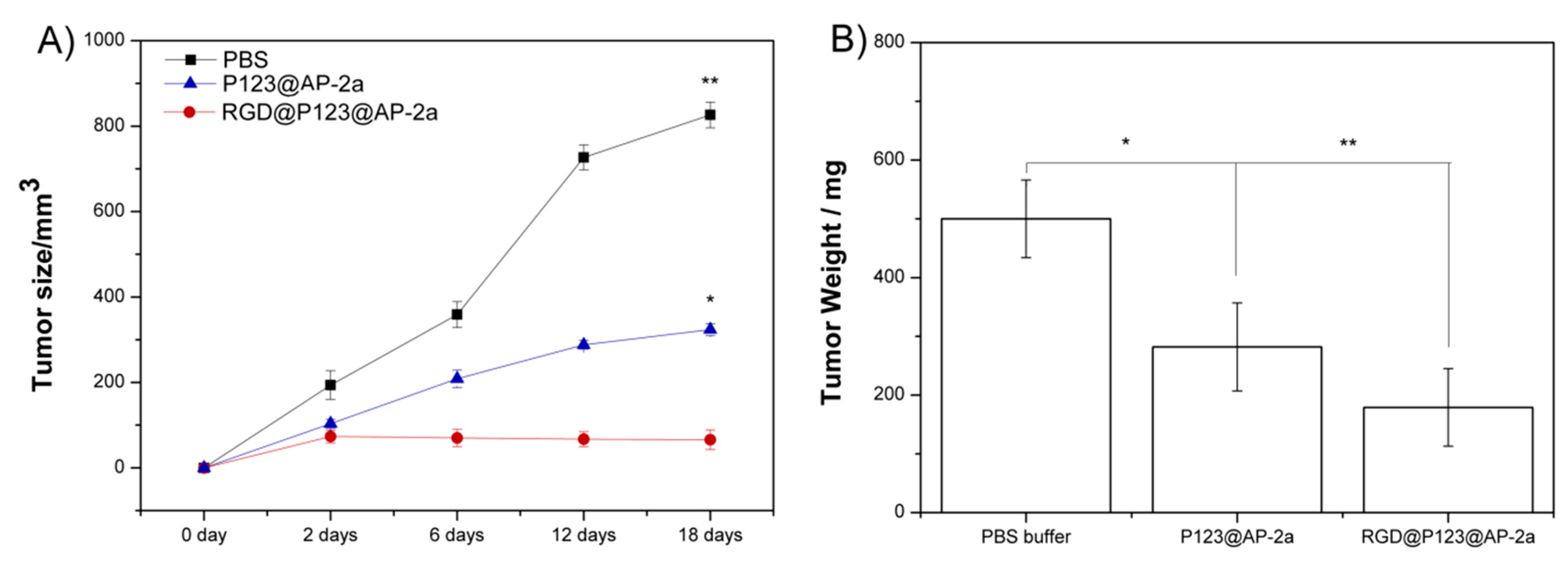
2.4. AP-2α Expression Attenuated Gastric Tumor Growth in Nude Mice
3. Experimental Section
3.1. The Preparation of Carboxyl-Functionalized Pluronic P123 Copolymer Filled with AP-2α Expression Plasmid
3.2. Cells and Cell Culture
3.3. In Vitro Release of AP-2α Protein Expression Plasmids from Nanoclusters
3.4. MTT Analysis
3.5. In Vitro Evaluation of RGD@P123@ AP-2α Nanocomposites
3.6. Establishment of Animal Models
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jemal, A.; Siegel, R.; Ward, E.; Hao, Y.; Xu, J.; Thun, M.J. Cancer statistics. CA Cancer J. Clin. 2009, 59, 225–249. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2009, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Parkin, D.M. International variation. Oncogene 2004, 23, 6340–6340. [Google Scholar] [CrossRef]
- Ginn, S.L.; Alexander, I.E.; Edelstein, M.L.; Abedi, M.R.; Wixon, J. Gene therapy clinical trials worldwide to 2012—An update. J. Gene Med. 2013, 15, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Wilson, W.R.; Hay, M.P. Targeting hypoxia in cancer therapy. Nat. Rev. Cancer 2011, 11, 393–410. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, W.; Zhang, T.; Ji, J.; Qian, Q.; Lu, L.; Fu, H.; Jin, W.; Cui, D. Differential expression of phospholipase C epsilon 1 is associated with chronic atrophic gastritis and gastric cancer. PLoS ONE 2012, 7, e47563. [Google Scholar] [CrossRef] [PubMed]
- Abnet, C.C.; Freedman, N.D.; Hu, N.; Wang, Z.; Yu, K.; Shu, X.-O.; Yuan, J.-M.; Zheng, W.; Dawsey, S.M.; Dong, L.M.; et al. A shared susceptibility locus in PLCE1 at 10q23 for gastric adenocarcinoma and esophageal squamous cell carcinoma. Nat. Genet. 2010, 42, 764–767. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Hu, Z.; Wu, C.; Dai, J.; Li, H.; Dong, J.; Wang, M.; Miao, X.; Zhou, Y.; Lu, F.; et al. A genome-wide association study identifies new susceptibility loci for non-cardia gastric cancer at 3q13.31 and 5p13.1. Nat. Genet. 2011, 43, 1215–1218. [Google Scholar] [CrossRef] [PubMed]
- Gee, M.S.; Sarkisian, C.J.; El-Deiry, W.S. Identification of a novel AP-2 consensus DNA binding site. Biochem. Biophys. Res. Commun. 1998, 243, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Schorle, H.; Meier, P.; Buchert, M.; Jaenisch, R.; Mitchell, P.J. Transcription factor AP-2 essential for cranial closure and craniofacial development. Nature 1996, 381, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hagopian-Donaldson, S.; Serbedzija, G.; Elsemore, J.; Plehn-Dujowich, D.; McMahon, A.P.; Flavell, R.A.; Williams, T. Neural tube, skeletal and body wall defects in mice lacking transcription factor AP-2. Nature 1996, 381, 238–241. [Google Scholar] [CrossRef] [PubMed]
- Orso, F.; Penna, E.; Cimino, D.; Astanina, E.; Maione, F.; Valdembri, D.; Giraudo, E.; Serini, G.; Sismondi, P.; de Bortoli, M.; et al. AP-2α and AP-2γ regulate tumor progression via specific genetic programs. FASEB J. 2008, 22, 2702–2714. [Google Scholar] [CrossRef] [PubMed]
- Wajapeyee, N.; Britto, R.; Ravishankar, H.M.; Somasundaram, K. Apoptosis induction by activator protein 2α involves transcriptional repression of Bcl-2. J. Biol. Chem. 2006, 281, 16207–16219. [Google Scholar] [CrossRef] [PubMed]
- Ebert, M.P.A.; Tänzer, M.; Balluff, B.; Burgermeister, E.; Kretzschmar, A.K.; Hughes, D.J.; Tetzner, R.; Lofton-Day, C.; Rosenberg, R.; Reinacher-Schick, A.C.; et al. TFAP2E–DKK4 and chemoresistance in colorectal cancer. N. Engl. J. Med. 2012, 366, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Jonckheere, N.; Fauquette, V.; Stechly, L.; Saint-Laurent, N.; Aubert, S.; Susini, C.; Huet, G.; Porchet, N.; van Seuningen, I.; Pigny, P. Tumour growth and resistance to gemcitabine of pancreatic cancer cells are decreased by AP-2α over-expression. Br. J. Cance 2009, 101, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lv, L.; Pan, K.; Zhang, Y.; Zhao, J.-J.; Chen, J.-G.; Chen, Y.-B.; Li, Y.-Q.; Wang, Q.-J.; He, J.; et al. Reduced expression of transcription factor AP-2α is associated with gastric adenocarcinoma prognosis. PLoS ONE 2011, 6, e24897. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, J. Functions of the AP-2α Gene in Activating Apoptosis and Inhibiting Proliferation of Gastric Cancer Cells both in Vivo and in Vitro. Arch. Med. Sci. Revision.
- Dull, T. A third-generation lentivirus vector with a conditional packaging system. J. Virol. 1998, 72, 8463–8471. [Google Scholar] [PubMed]
- Jiang, G.-B.; Geng, B.-W.; Fang, Y.-S.; Gao, Y.-F. Polymeric micelles as new drug carriers. Mod. Chem. Ind. 2007, 27, 69. [Google Scholar]
- Zou, J.; Zhang, F.; Zhang, S.; Pollack, S.F.; Elsabahy, M.; Fan, J.; Wooley, K.L. Poly(ethylene oxide)-block-polyphosphoester-graft-Paclitaxel conjugates with acid-labile linkages as a pH-sensitive and functional nanoscopic platform for Paclitaxel delivery. Adv. Healthc. Mater. 2014, 3, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Jeong, B.; Bae, Y.H.; Lee, D.S.; Kim, S.W. Biodegradable block copolymers as injectable drug-delivery systems. Nature 1997, 388, 860–862. [Google Scholar] [PubMed]
- Maranski, K.; Andreev, Y.G.; Bruce, P.G. Synthesis of poly(ethylene oxide) approaching monodispersity. Angew. Chem. Int. Ed. 2014, 53, 6411–6413. [Google Scholar] [CrossRef] [PubMed]
- Batrakova, E.V.; Kabanov, A.V. Pluronic block copolymers: Evolution of drug delivery concept from inert nanocarriers to biological response modifiers. J. Control. Release 2008, 130, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; He, R.; Wang, K.; Ruan, J.; Bao, C.; Li, N.; Ji, J.; Cui, D. Anti-HIF-1α antibody-conjugated pluronic triblock copolymers encapsulated with Paclitaxel for tumor targeting therapy. Biomaterials 2010, 31, 2302–2312. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Zhang, L.; Chen, J.; Lanza, G.M.; Wickline, S.A. Diffusional mechanisms augment the fluorine MR relaxation in paramagnetic perfluorocarbon nanoparticles that provides a “relaxation switch” for detecting cellular endosomal activation. J. Magn. Reson. Imaging 2011, 34, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Tao, K.; Cheng, T.; Zhu, J.; Zhang, X. Efficient Inhibition of wear debris-induced inflammation by locally delivered siRNA. Biochem. Biophys. Res. Commun. 2008, 377, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Zhang, X.; Zeng, B. Locally administered lentivirus-mediated siRNA inhibits wear debris-induced inflammation in murine air pouch model. Biotechnol. Lett. 2008, 30, 1923–1929. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Liu, Z.; Sun, P.; Fang, C.; Fang, H.; Wang, Y.; Ji, J.; Chen, J. RGD Peptides-Conjugated Pluronic Triblock Copolymers Encapsulated with AP-2α Expression Plasmid for Targeting Gastric Cancer Therapy in Vitro and in Vivo. Int. J. Mol. Sci. 2015, 16, 16263-16274. https://doi.org/10.3390/ijms160716263
Wang W, Liu Z, Sun P, Fang C, Fang H, Wang Y, Ji J, Chen J. RGD Peptides-Conjugated Pluronic Triblock Copolymers Encapsulated with AP-2α Expression Plasmid for Targeting Gastric Cancer Therapy in Vitro and in Vivo. International Journal of Molecular Sciences. 2015; 16(7):16263-16274. https://doi.org/10.3390/ijms160716263
Chicago/Turabian StyleWang, Wei, Zhimin Liu, Peng Sun, Cheng Fang, Hongwei Fang, Yueming Wang, Jiajia Ji, and Jun Chen. 2015. "RGD Peptides-Conjugated Pluronic Triblock Copolymers Encapsulated with AP-2α Expression Plasmid for Targeting Gastric Cancer Therapy in Vitro and in Vivo" International Journal of Molecular Sciences 16, no. 7: 16263-16274. https://doi.org/10.3390/ijms160716263






