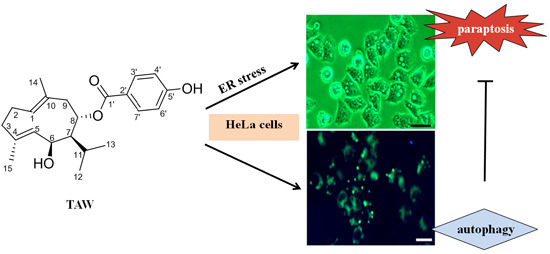
8-p-Hdroxybenzoyl Tovarol Induces Paraptosis Like Cell Death and Protective Autophagy in Human Cervical Cancer HeLa Cells
Abstract
:1. Introduction
2. Results
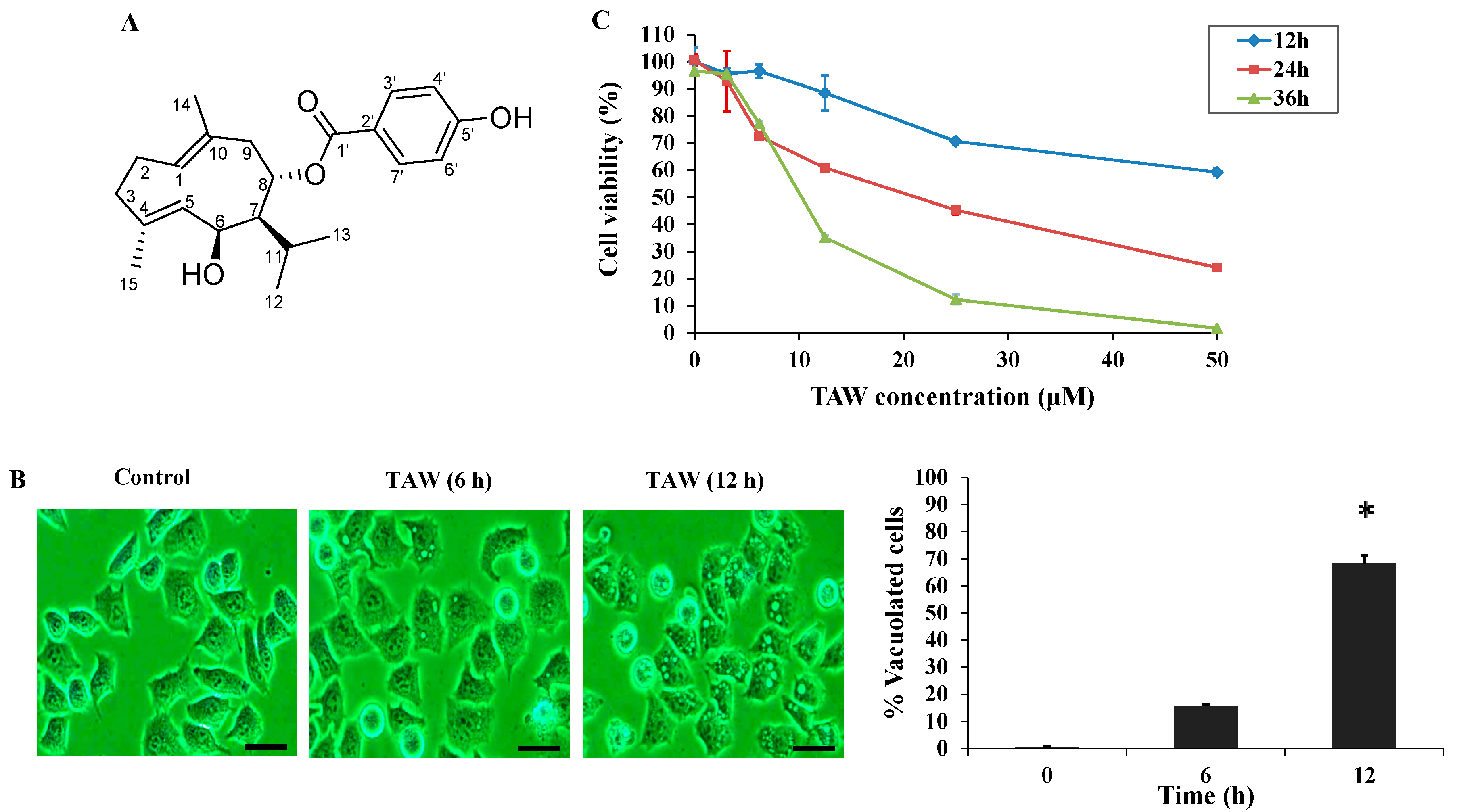
2.1. 8-p-Hdroxybenzoyl Tovarol (TAW) Induces Cytoplasmic Vacuolization and Cell Death in HeLa Cells
2.2. 8-p-Hdroxybenzoyl Tovarol (TAW) Induces Paraptosis Like Cell Death in HeLa Cells
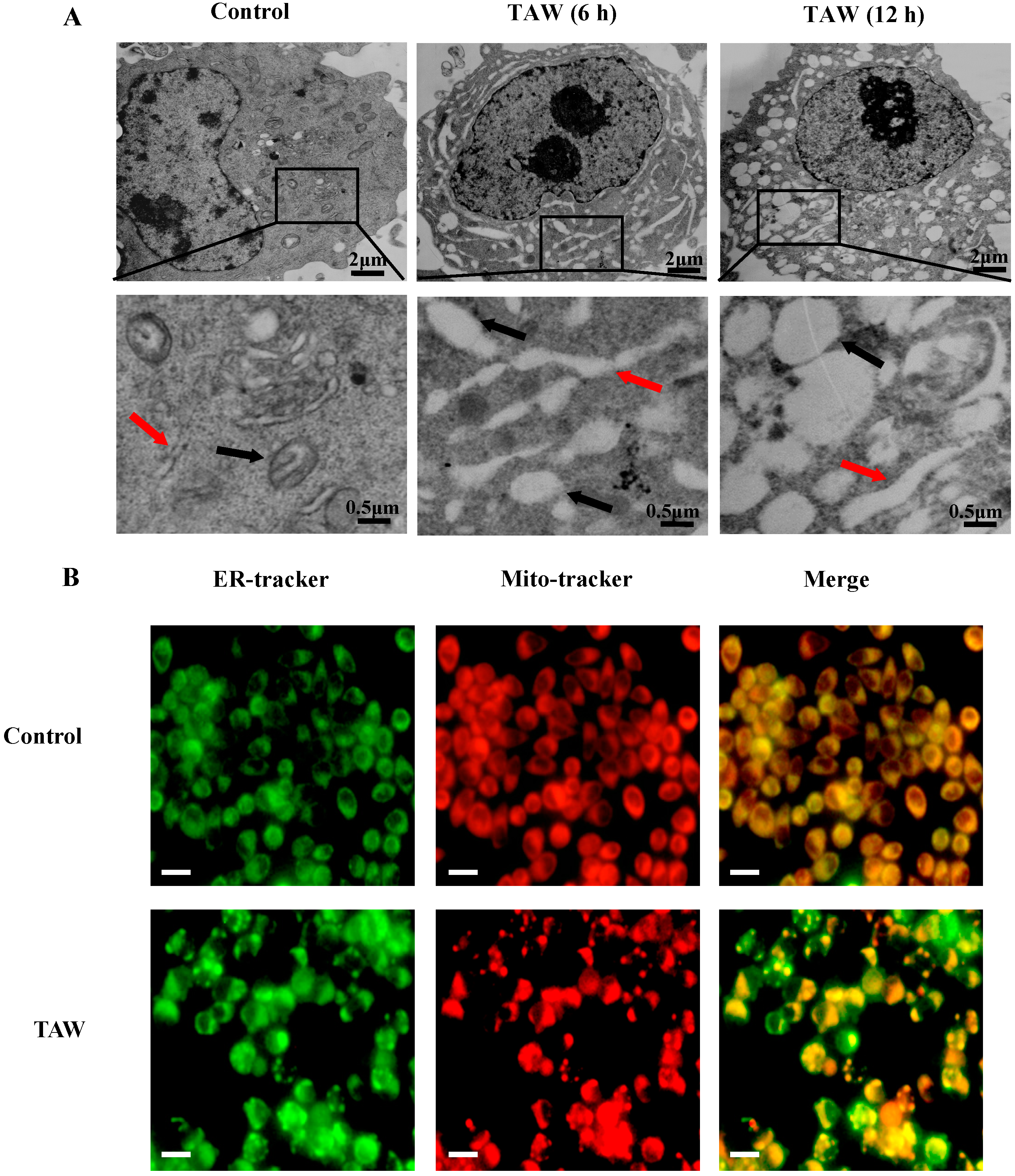
2.3. 8-p-Hdroxybenzoyl Tovarol (TAW) Treatment Induces Depletion of Mitochondrial Membrane Potential (MMP)
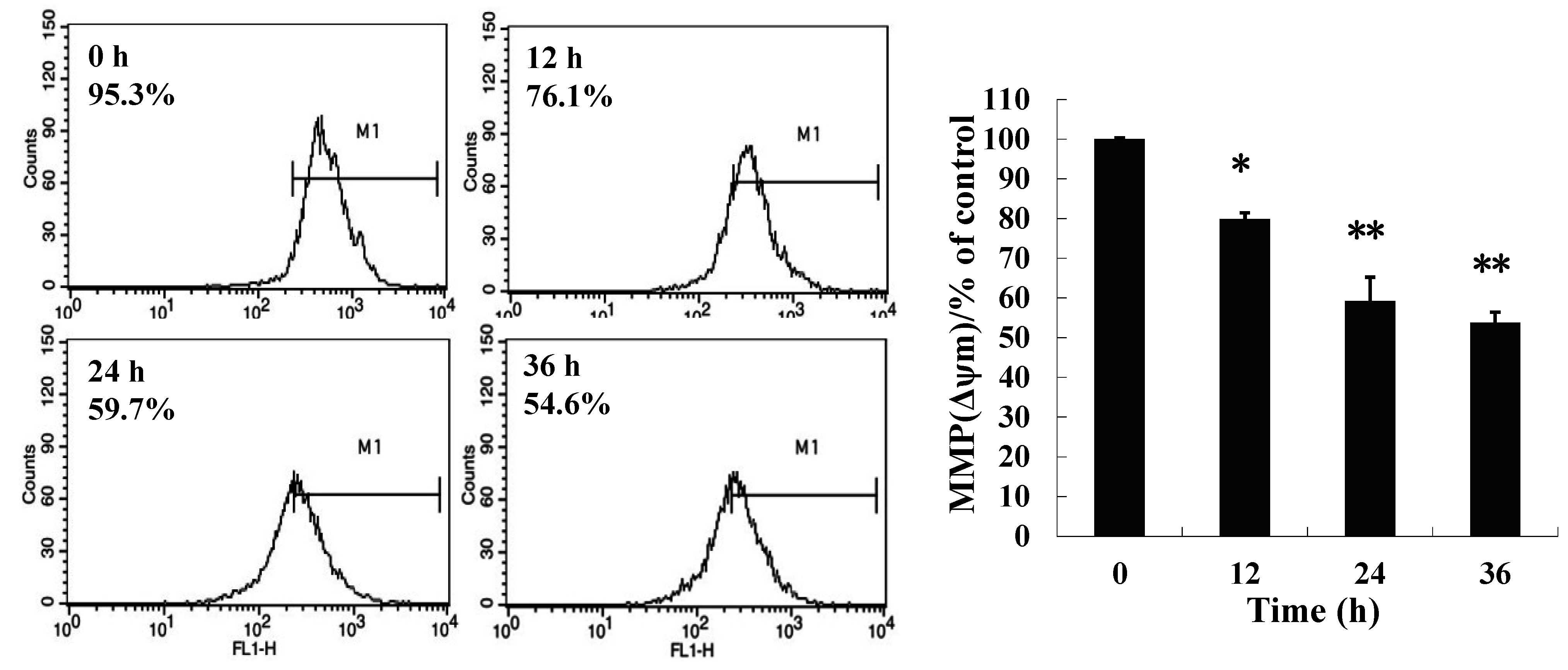
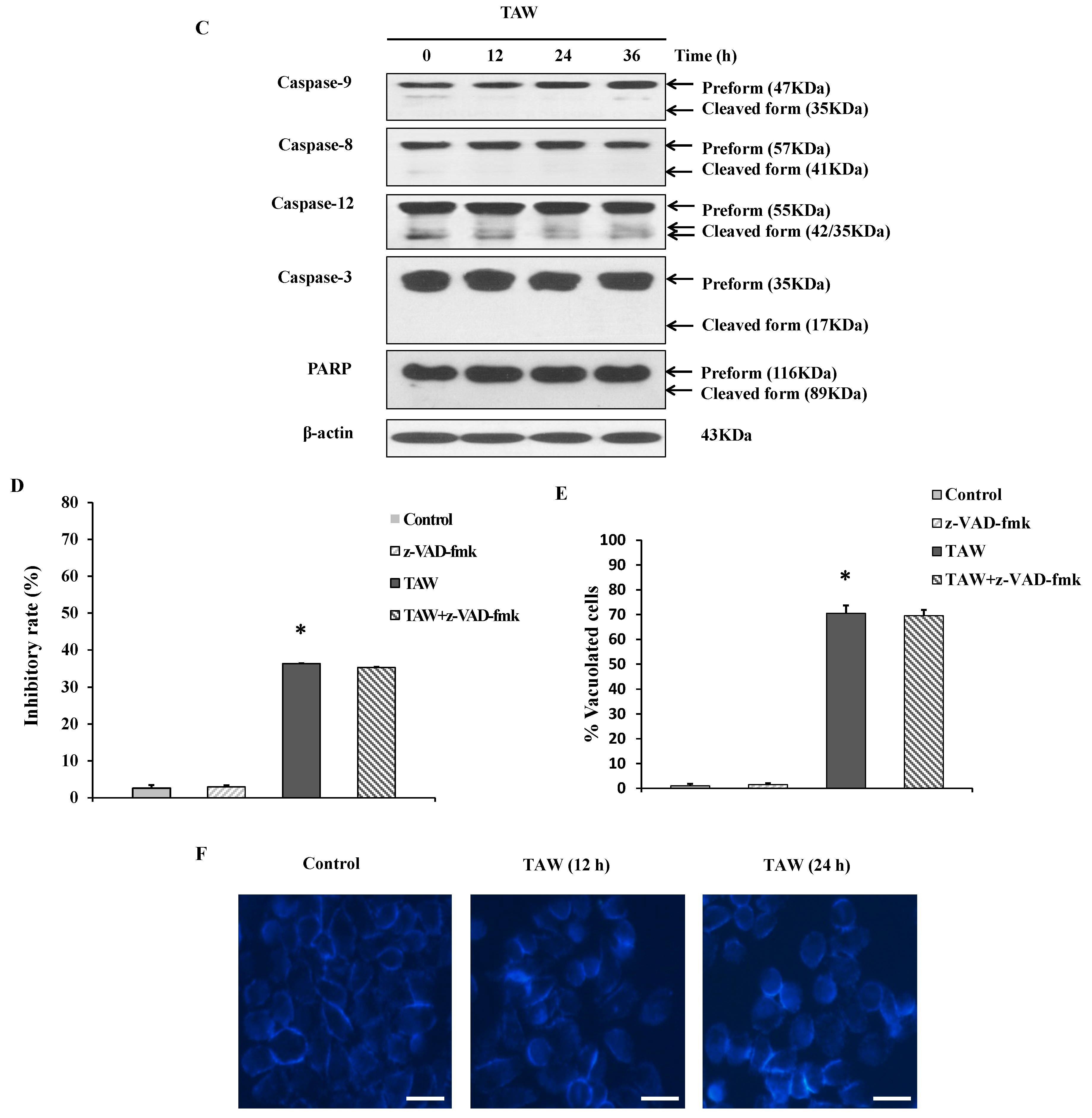
2.4. 8-p-Hdroxybenzoyl Tovarol (TAW) Induced Vacuolation Is Reversed by Treatment with Cycloheximide in HeLa Cells

2.5. 8-p-Hdroxybenzoyl Tovarol (TAW)-Mediated ER-Stress and Unfolded Protein Response (UPR) Lead to Paraptosis Like Cell Death
2.6. 8-p-Hdroxybenzoyl Tovarol (TAW) Induces Autophagy in HeLa Cells
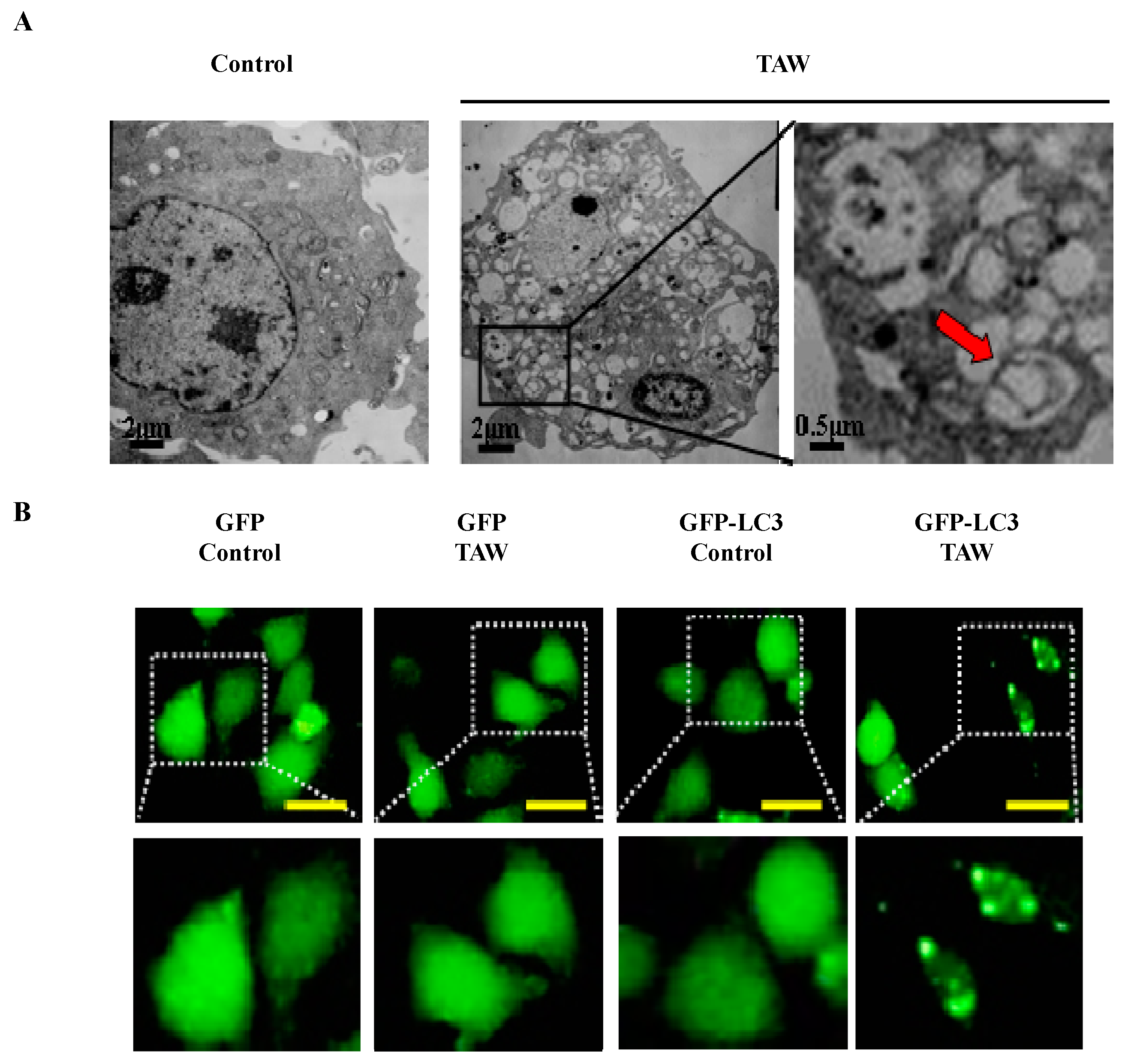

2.7. Autophagy Antagonizes Paraptosis in 8-p-Hdroxybenzoyl Tovarol (TAW) Treated HeLa Cells

3. Discussion
4. Experimental Section
4.1. Plant Materials and Reagents
4.2. Extraction and Isolation of TAW
4.3. Cell Culture
4.4. Cell Viability Assay
4.5. Endoplasmic Reticulum (ER)-Tracker and Mito-Tracker
4.6. Hoechst 33258, MDC and Rhodamine 123 Staining
4.7. Transmission Electron Microscopy
4.8. Vacuolated Cell Counting
4.9. Western Blot Analysis
4.10. Transfection of siRNA
4.11. Transfection of EGFP-LC3 Plasmid
4.12. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Valle, G.M.; Appendino, G.; Nano, G.M.; Picci, V. Prenylated coumarins and sesquiterpenoids from Ferula communis. Phytochemistry 1987, 26, 253–258. [Google Scholar] [CrossRef]
- Ahmed, A.A. Sesquiterpene coumarins and sesquiterpenes from Ferula sinaica. Phytochemistry 1999, 50, 109–112. [Google Scholar] [CrossRef]
- Ahmed, A.A.; AbdEl-Razek, M.H.; Nassar, M.I.; Izuma, S.; Ohta, S.; Hirata, T. Sesquiterpene coumarins from the roots of Ferula assa-foetida. Phytochemistry 2001, 58, 1289–1295. [Google Scholar]
- Iranshahi, M.; Arfa, P.; Ramezani, M.; Jaafari, M.R.; Sadeghian, H.; Bassarello, C.; Piacente, S.; Pizza, C. Sesquiterpene coumarins from Ferula szowitsiana and in vitro antileishmanial activity of 7-prenyloxycoumarins against promas-tigotes. Phytochemistry 2007, 68, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Okasaka, M.; Kashiwada, Y.; Takaishi, Y.; Honda, G.; Ito, M.; Takeda, Y.; Kodzhimatov, O.K.; Ashurmetov, O.; Sekiya, M.; et al. Sesquiterpene lactones from the roots of Ferula varia and their cytotoxic activity. J. Nat. Prod. 2007, 70, 1915–1918. [Google Scholar] [CrossRef] [PubMed]
- Alkhatib, R.; Hennebelle, T.; Joha, S.; Idziorek, T.; Preudhomme, C.; Quesnel, B.; Sahpaz, S.; Bailleul, F. Activity of elaeochytrin A from Ferula elaeochytris on leukemia cell lines. Phytochemistry 2008, 69, 2979–2983. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Liu, J.H.; Zhang, J.; Zhang, N.; Wang, Z.H. Blockade of autophagy aggravates endoplasmic reticulum stress and improves Paclitaxel cytotoxicity in humancervical cancer cells. Cancer Res. Treat. 2015, 47, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Suh, D.H.; Kim, J.W.; Kim, K.; Kim, H.J.; Lee, K.H. Major clinical research advances in gynecologic cancer in 2012. J. Gynecol. Oncol. 2013, 24, 66–82. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Galluzzi, L.; Vandenabeele, P.; Abrams, J.; Alnemri, E.S.; Baehrecke, E.H.; Blagosklonny, M.V.; El-Deiry, W.S.; Golstein, P.; Green, D.R. Classification of cell death: Recommendations of the Nomenclature Committee on Cell Death 2009. Cell. Death Differ. 2009, 16, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Sperandio, S.; Poksay, K.S.; Schilling, B.; Crippen, D.; Gibson, B.W.; Bredesen, D.E. Identification of new modulators and protein alterations in non-apoptotic programmed cell death. J. Cell. Biochem. 2010, 111, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Bröker, L.E.; Kruyt, F.A.; Giaccone, G. Cell death independent of caspases: A review. Clin. Cancer Res. 2005, 11, 3155–3162. [Google Scholar] [CrossRef] [PubMed]
- Sperandio, S.; de Belle, I.; Bredesen, D.E. An alternative, nonapoptotic form of programmed cell death. Proc. Natl. Acad. Sci. USA 2000, 97, 14376–14381. [Google Scholar] [CrossRef] [PubMed]
- Gerl, R.; Vaux, D.L. Apoptosis in the development and treatment of cancer. Carcinogenesis 2005, 26, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T.; Serrano, M.; Blasco, M.A. The common biology of cancer and ageing. Nature 2007, 448, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H. Discovery and development of natural product-derived chemotherapeutic agents based on a medicinal chemistry approach. J. Nat. Prod. 2010, 73, 500–516. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.K.; Basu, S.; Sarkar, N.; Ghosh, A.C. Advances in cancer therapy with plant based natural products. Curr. Med. Chem. 2001, 8, 1467–1486. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Han, H.Y.; Li, G.Y.; Wang, H.Y.; Zhang, C.; Zhang, K.; Tan, Y.; Li, P.Y.; Wang, J.H. Two new terpenoid benzoates with antitumor activity from the roots of Ferula dissecta. J. Asian Nat. Prod. Res. 2013, 15, 1100–1106. [Google Scholar] [CrossRef] [PubMed]
- Constantinou, C.; Papas, K.A.; Constantinou, A.I. Caspase-independent pathways of programmed cell death: The unraveling of new targets of cancer therapy? Curr. Cancer Drug Targets 2009, 9, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Mathiasen, I.S.; Jäättelä, M. Triggering caspase-independent cell death to combat cancer. Trends Mol. Med. 2002, 8, 212–220. [Google Scholar] [CrossRef]
- Bury, M.; Girault, A.; Mégalizzi, V.; Spiegl-Kreinecker, S.; Mathieu, V.; Berger, W.; Evidente, A.; Kornienko, A.; Gailly, P.; Vandier, C. Ophiobolin A induces paraptosis-like cell death in human glioblastoma cells by decreasing BKCa channel activity. Cell Death Dis. 2013, 4, e561. [Google Scholar] [CrossRef] [PubMed]
- Tardito, S.; Isella, C.; Medico, E.; Marchiò, L.; Bevilacqua, E.; Hatzoglou, M.; Bussolati, O.; Franchi-Gazzola, R. The thioxotriazole copper(II) complex A0 induces endoplasmic reticulum stress and paraptotic death in human cancer cells. J. Biol. Chem. 2009, 284, 24306–24319. [Google Scholar] [CrossRef] [PubMed]
- Yumnam, S.; Park, H.S.; Kim, M.K.; Nagappan, A.; Hong, G.E.; Lee, H.J.; Lee, W.S.; Kim, E.H.; Cho, J.H.; Shin, S.C. Hesperidin induces paraptosis like cell death in hepatoblatoma, HepG2 cells: Involvement of ERK1/2 MAPK. PLoS ONE 2014, 9, e101321. [Google Scholar] [CrossRef] [PubMed]
- Jambrina, E.; Alonso, R.; Alcalde, M.; del Carmen Rodríguez, M.; Serrano, A.; Martínez-A, C.; García-Sancho, J.; Izquierdo, M. Calcium influx through receptor-operated channel induces mitochondria-triggered paraptotic cell death. J. Biol. Chem. 2003, 278, 14134–14145. [Google Scholar] [CrossRef] [PubMed]
- Ram, B.M.; Ramakrishna, G. Endoplasmic reticulum vacuolation and unfolded protein response leading to paraptosis like cell death in cyclosporine A treated cancer cervix cells is mediated by cyclophilin B inhibition. Biochim. Biophys. Acta 2014, 1843, 2497–2512. [Google Scholar] [CrossRef] [PubMed]
- Calfon, M.; Zeng, H.; Urano, F.; Till, J.H.; Hubbard, S.R.; Harding, H.P.; Clark, S.G.; Ron, D. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 2002, 415, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Finnerty, C.C.; Herndon, D.N.; Boehning, D.; Jeschke, M.G. Severe burninduced endoplasmic reticulum stress and hepatic damage in mice. Mol. Med. 2009, 15, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.V.; Ellerby, H.M.; Bredesen, D.E. Coupling endoplasmic reticulum stress to the cell death program. Cell Death Differ. 2004, 11, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Bernales, S.; Papa, F.R.; Walter, P. Intracellular signaling by the unfolded protein response. Annu. Rev. Cell Dev. Biol. 2006, 22, 487–508. [Google Scholar] [CrossRef] [PubMed]
- Bertolotti, A.; Zhang, Y.; Hendershot, L.M.; Harding, H.P.; Ron, D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat. Cell. Biol. 2000, 2, 326–332. [Google Scholar] [PubMed]
- Sato, Y.; Nadanaka, S.; Okada, T.; Okawa, K.; Mori, K. Luminal domain of ATF6 alone is sufficient for sensing endoplasmic reticulum stress and subsequent transport to the Golgi apparatus. Cell Struct. Funct. 2011, 36, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.A.; Groenendyk, J.; Michalak, M. Endoplasmic reticulum stress associated responses in cancer. Biochim. Biophys. Acta 2014, 1843, 2143–2149. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.X.; Ni, H.M.; Yin, X.M. Absence of Bax switched MG132-induced apoptosis to non-apoptotic cell death that could be suppressed by transcriptional or translational inhibition. Apoptosis 2007, 12, 2233–2244. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N. Autophagy: Process and function. Genes Dev. 2007, 21, 2861–2873. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.; Kroemer, G. Autophagy in the pathogenesis of disease. Cell 2008, 132, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Mariño, G.; Levine, B. Autophagy and the integrated stress response. Mol. Cell 2010, 40, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Carew, J.S.; Nawrocki, S.T.; Cleveland, J.L. Modulating autophagy for therapeutic benefit. Autophagy 2007, 3, 464–467. [Google Scholar] [CrossRef] [PubMed]
- Miski, M.; Mabry, T.J.; Saya, O. New daucane and germacrane esters from Ferula orientalis var. orientalis. J. Nat. Prod. 1987, 50, 829–834. [Google Scholar] [CrossRef]
- Wang, W.B.; Feng, L.X.; Yue, Q.X.; Wu, W.Y.; Guan, S.H.; Jiang, B.H.; Yang, M.; Liu, X.; Guo, D.A. Paraptosis accompanied by autophagy and apoptosis was induced by celastrol, a natural compound with influence on proteasome, ER stress and Hsp90. J. Cell. Physiol. 2012, 227, 2196–2206. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kang, J.G.; Kim, C.S.; Ihm, S.H.; Choi, M.G.; Yoo, H.J.; Lee, S.J. The hsp70 inhibitor VER155008 induces paraptosis requiring de novo protein synthesis in anaplastic thyroid carcinoma cells. Biochem. Biophys. Res. Commun. 2014, 454, 36–41. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Jiang, Y.; Zhang, J.; Huang, J.; Wang, J. 8-p-Hdroxybenzoyl Tovarol Induces Paraptosis Like Cell Death and Protective Autophagy in Human Cervical Cancer HeLa Cells. Int. J. Mol. Sci. 2015, 16, 14979-14996. https://doi.org/10.3390/ijms160714979
Zhang C, Jiang Y, Zhang J, Huang J, Wang J. 8-p-Hdroxybenzoyl Tovarol Induces Paraptosis Like Cell Death and Protective Autophagy in Human Cervical Cancer HeLa Cells. International Journal of Molecular Sciences. 2015; 16(7):14979-14996. https://doi.org/10.3390/ijms160714979
Chicago/Turabian StyleZhang, Cui, Yingnan Jiang, Jin Zhang, Jian Huang, and Jinhui Wang. 2015. "8-p-Hdroxybenzoyl Tovarol Induces Paraptosis Like Cell Death and Protective Autophagy in Human Cervical Cancer HeLa Cells" International Journal of Molecular Sciences 16, no. 7: 14979-14996. https://doi.org/10.3390/ijms160714979