The Effect of Diagnostic Absorbed Doses from 131I on Human Thyrocytes in Vitro
Abstract
:1. Introduction
2. Results
2.1. Apoptosis
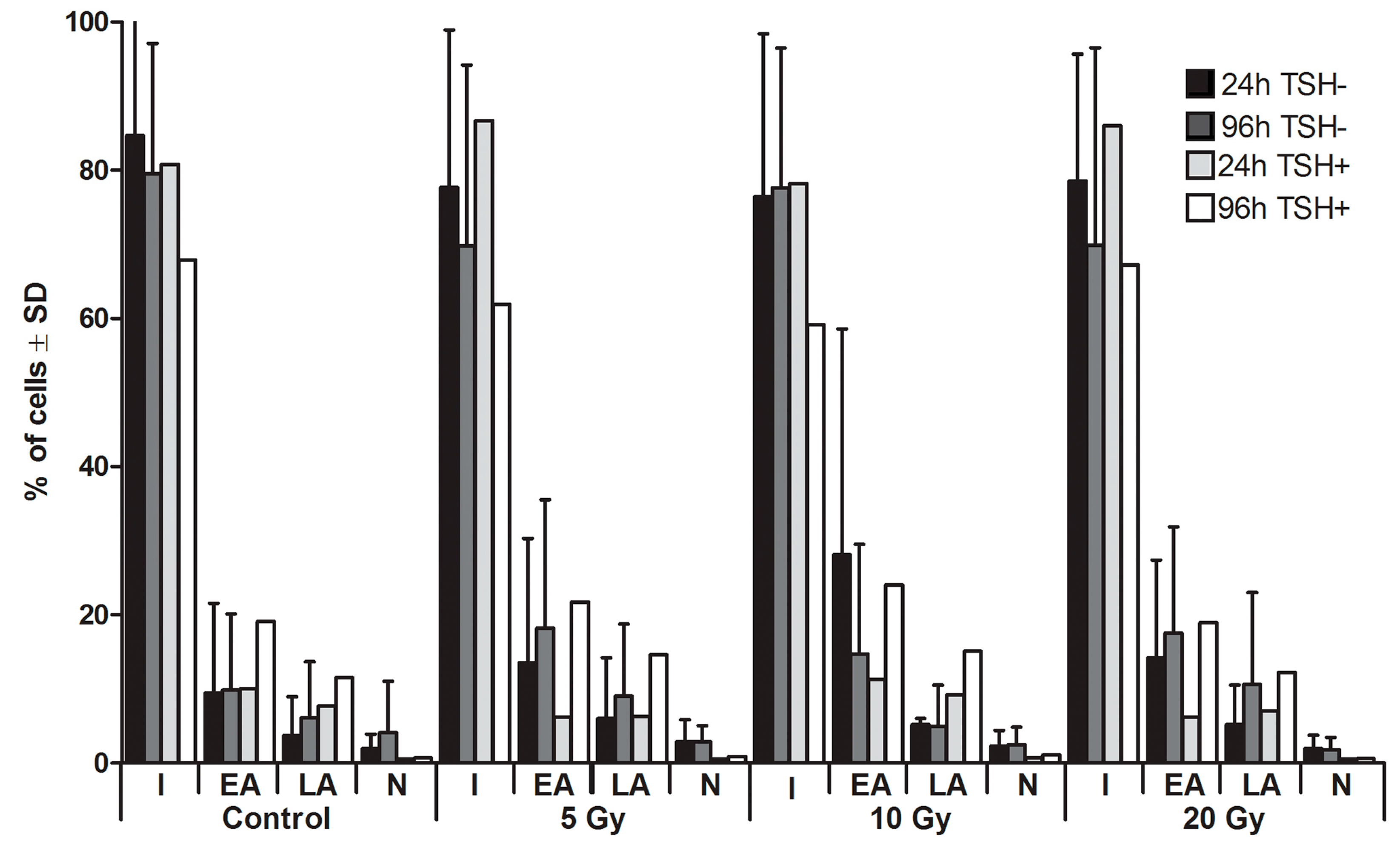
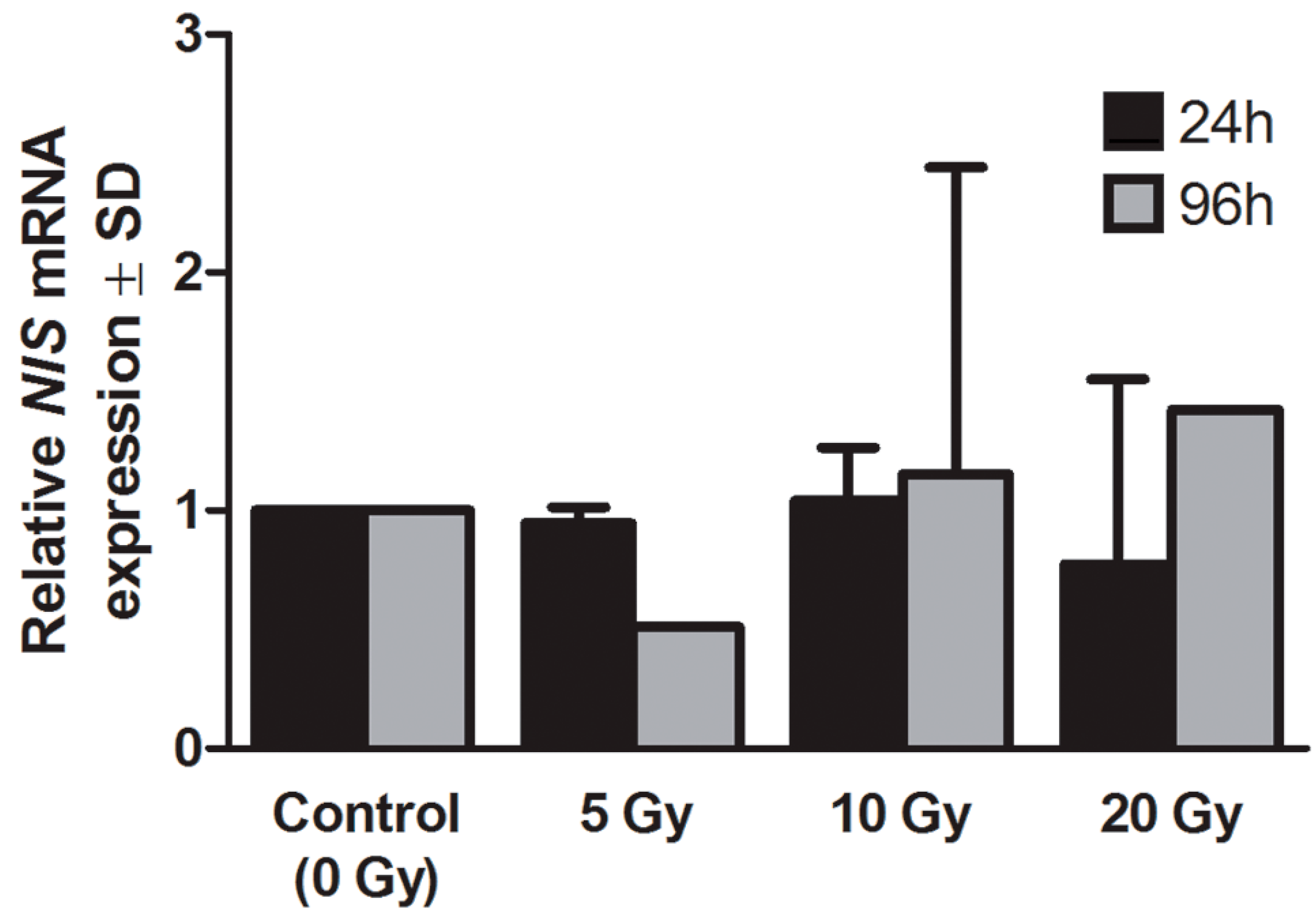
2.2. Expression of Sodium Iodide Symporter (NIS) Gene
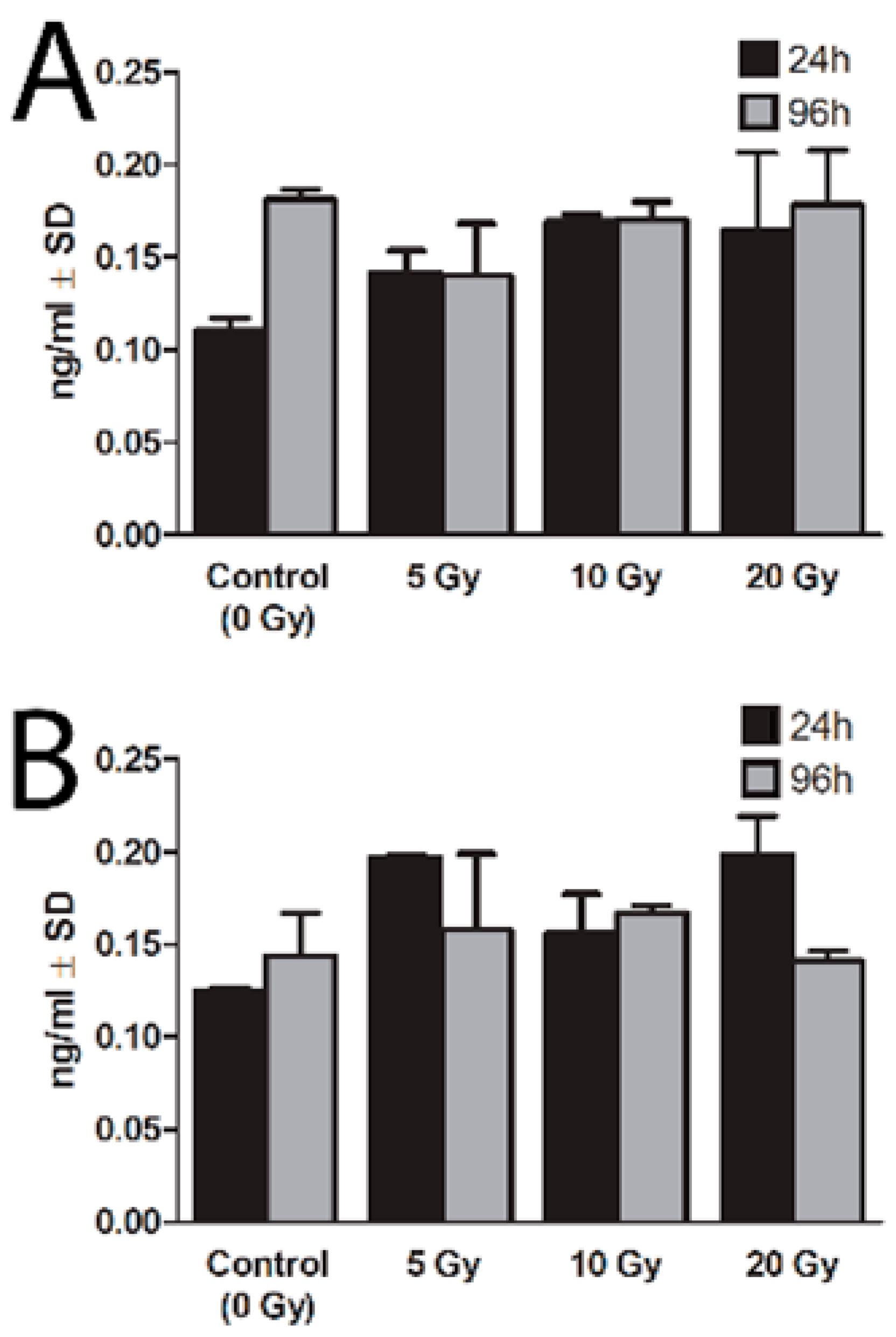
2.3. Expression of NIS Protein
2.4. DNA Damage
2.4.1. Comet Assay
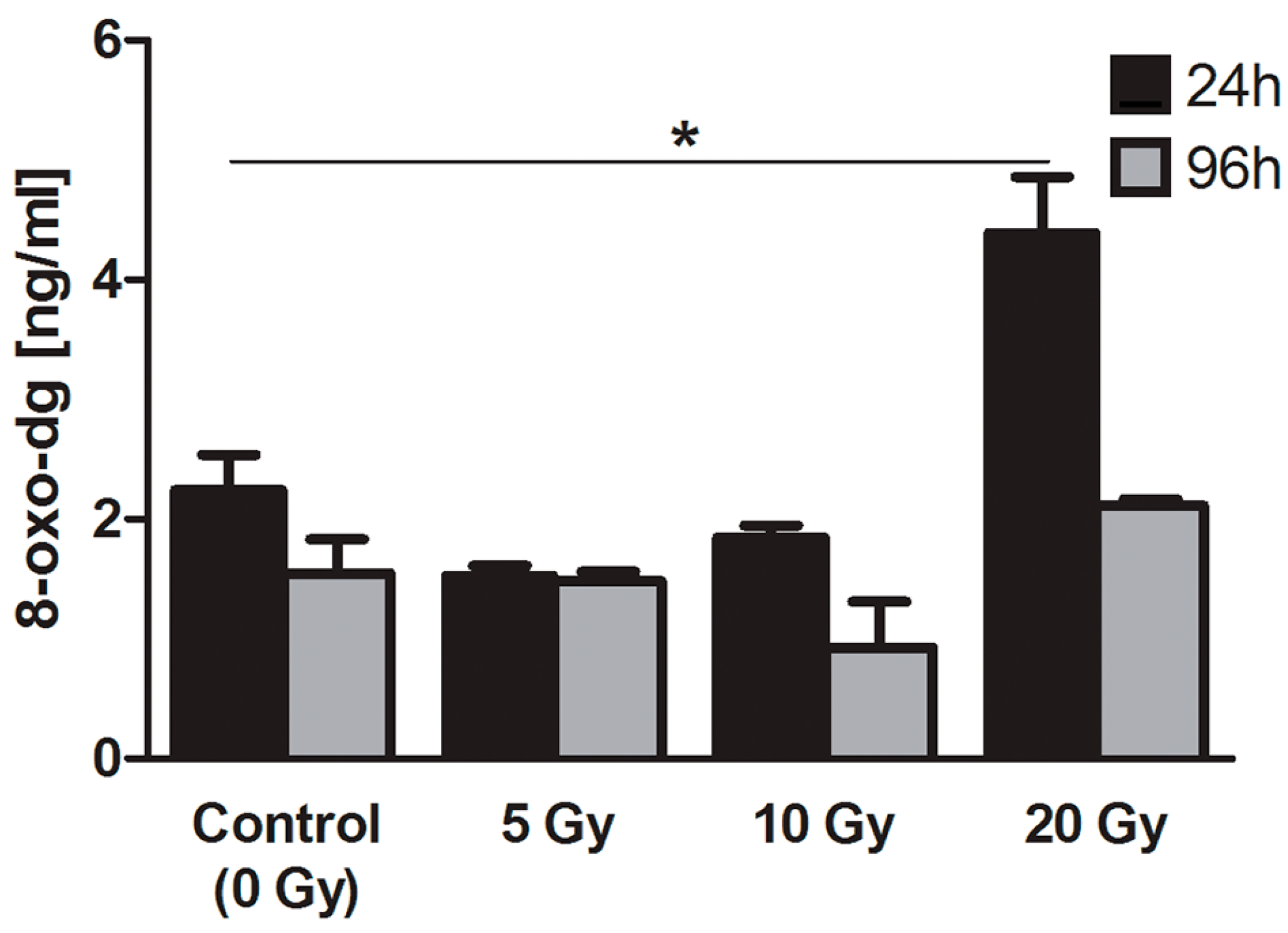
2.4.2. 8-Oxo-7,8-dihydro-2′deoxyguanosine (8-oxo-dG)
2.4.3. Apurinic/Apirymidinic Sites (AP-Site)

3. Discussion
4. Experimental Section
4.1. Ethics Statement
4.2. Thyrocyte Isolation and Culture
4.3. Fluorescence-Activated Cell Sorting (FACS) Analysis
4.4. RT-qPCR
4.5. Comet Assay
4.6. 8-Oxo-7,8-dihydro-2'deoxyguanosine (8-oxo-dG) Measurement
4.7. Apurinic/Apirymidinic Sites (AP-Site) Determination
4.8. Enzyme-Linked Immunoabsorbent Assay (ELISA)
4.9. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pellegriti, G.; Frasca, F.; Regalbuto, C.; Squatrito, S.; Vigneri, R. Worldwide increasing incidence of thyroid cancer: Update on epidemiology and risk factors. J. Cancer Epidemiol. 2013, 2013, 965212. [Google Scholar] [CrossRef] [PubMed]
- Durante, C.; Costante, G.; Filetti, S. Differentiated thyroid carcinoma: Defining new paradigms for postoperative management. Endocr. Relat. Cancer 2013, 20, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Pacini, F.; Schlumberger, M.; Dralle, H.; Elisei, R.; Smit, J.W.; Wiersinga, W.; the European Thyroid Cancer Taskforce. European consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. Eur. J. Endocrinol. 2006, 154, 787–803. [Google Scholar] [CrossRef] [PubMed]
- Rawson, R.W.; Rall, J.E.; Peacock, W. Limitations and indications in the treatment of cancer of the thyroid with radioactive iodine. J. Clin. Endocrinol. Metab. 1951, 11, 1128–1131. [Google Scholar] [CrossRef] [PubMed]
- McDougall, I.R. 74 MBq radioiodine 131I does not prevent uptake of therapeutic doses of 131I (i.e., it does not cause stunning) in differentiated thyroid cancer. Nucl. Med. Commun. 1997, 18, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Morris, L.F.; Waxman, A.D.; Braunstein, G.D. The nonimpact of thyroid stunning: Remnant ablation rates in 131I-scanned and nonscanned individuals. J. Clin. Endocrinol. Metab. 2001, 86, 3507–3511. [Google Scholar] [CrossRef] [PubMed]
- Silberstein, E.B. Comparison of outcomes after 123I versus 131I preablation imaging before radioiodine ablation in differentiated thyroid carcinoma. J. Nucl. Med. 2007, 48, 1043–1046. [Google Scholar] [CrossRef] [PubMed]
- Lassmann, M.; Luster, M.; Hanscheid, H.; Reiners, C. Impact of 131I diagnostic activities on the biokinetics of thyroid remnants. J. Nucl. Med. 2004, 45, 619–625. [Google Scholar] [PubMed]
- Jeevanram, R.K.; Shah, D.H.; Sharma, S.M.; Ganatra, R.D. Influence of initial large dose on subsequent uptake of therapeutic radioiodine in thyroid cancer patients. Int. J. Radiat. Appl. Instrum. B 1986, 13, 277–279. [Google Scholar] [CrossRef]
- Muratet, J.P.; Daver, A.; Minier, J.F.; Larra, F. Influence of scanning doses of iodine-131 on subsequent first ablative treatment outcome in patients operated on for differentiated thyroid carcinoma. J. Nucl. Med. 1998, 39, 1546–1550. [Google Scholar] [PubMed]
- Lees, W.; Mansberg, R.; Roberts, J.; Towson, J.; Chua, E.; Turtle, J. The clinical effects of thyroid stunning after diagnostic whole-body scanning with 185 MBq 131I. Eur. J. Nucl. Med. Mol. Imaging 2002, 29, 1421–1427. [Google Scholar] [CrossRef] [PubMed]
- Park, H.M.; Perkins, O.W.; Edmondson, J.W.; Schnute, R.B.; Manatunga, A. Influence of diagnostic radioiodines on the uptake of ablative dose of iodine-131. Thyroid 1994, 4, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Medvedec, M. Thyroid stunning in vivo and in vitro. Nucl. Med. Commun. 2005, 26, 731–735. [Google Scholar] [CrossRef] [PubMed]
- Lundh, C.; Norden, M.M.; Nilsson, M.; Forssell-Aronsson, E. Reduced iodide transport (stunning) and DNA synthesis in thyrocytes exposed to low absorbed doses from 131I in vitro. J. Nucl. Med. 2007, 48, 481–486. [Google Scholar] [PubMed]
- Park, H.M. Stunned thyroid after high-dose I-131 imaging. Clin. Nucl. Med. 1992, 17, 501–502. [Google Scholar] [CrossRef] [PubMed]
- Park, H.M.; Park, Y.H.; Zhou, X.H. Detection of thyroid remnant/metastasis without stunning: An ongoing dilemma. Thyroidology 1997, 7, 277–280. [Google Scholar] [CrossRef]
- Huic, D.; Medvedec, M.; Dodig, D.; Popović, S.; Ivancević, D.; Pavlinovic, Z.; Zuvic, M. Radioiodine uptake in thyroid cancer patients after diagnostic application of low-dose 131I. Nucl. Med. Commun. 1996, 17, 839–842. [Google Scholar] [CrossRef] [PubMed]
- Yeung, H.W.; Humm, J.L.; Larson, S.M. Radioiodine uptake in thyroid remnants during therapy after tracer dosimetry. J. Nucl. Med. 2000, 41, 1082–1085. [Google Scholar] [PubMed]
- Leger, F.A.; Izembart, M.; Dagousset, F.; Barritault, L.; Baillet, G.; Chevalier, A.; Clerc, J. Decreased uptake of therapeutic doses of iodine-131 after 185-MBq iodine-131 diagnostic imaging for thyroid remnants in differentiated thyroid carcinoma. Eur. J. Nucl. Med. 1998, 25, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Verburg, F.A.; Verkooijen, R.B.; Stokkel, M.P.; van Isselt, J.W. The success of 131I ablation in thyroid cancer patients is significantly reduced after a diagnostic activity of 40 MBq 131I. Nuklearmedizin 2009, 48, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.S.; Hseu, H.H.; Lin, W.Y.; Wang, S.J.; Liu, Y.C. Decreased uptake after fractionated ablative doses of iodine-131. Eur. J. Nucl. Med. Mol. Imaging 2005, 32, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Arad, E.; Flannery, K.; Wilson, G.A.; O’Mara, R.E. Fractionated doses of radioiodine for ablation of postsurgical thyroid tissue remnants. Clin. Nucl. Med. 1990, 15, 676–677. [Google Scholar] [CrossRef] [PubMed]
- Leger, A.F.; Pellan, M.; Dagousset, F.; Chevalier, A.; Keller, I.; Clerc, J. A case of stunning of lung and bone metastases of papillary thyroid cancer after a therapeutic dose (3.7 GBq) of 131I and review of the literature: Implications for sequential treatments. Br. J. Radiol. 2005, 78, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Dohán, O.; de la Vieja, A.; Paroder, V.; Riedel, C.; Artani, M.; Reed, M.; Ginter, C.S.; Carrasco, N. The sodium/iodide symporter (NIS): Characterization, regulation, and medical significance. Endocr. Rev. 2003, 24, 48–77. [Google Scholar] [CrossRef] [PubMed]
- Kogai, T.; Brent, G.A. The sodium iodide symporter (NIS): Regulation and approaches to targeting for cancer therapeutics. Pharmacol. Ther. 2012, 135, 355–370. [Google Scholar] [CrossRef] [PubMed]
- Nordén, M.M.; Larsson, F.; Tedelind, S.; Carlsson, T.; Lundh, C.; Forssell-Aronsson, E.; Nilsson, M. Down-regulation of the sodium/iodide symporter explains 131I-induced thyroid stunning. Cancer Res. 2007, 67, 7512–7517. [Google Scholar] [CrossRef] [PubMed]
- Postgard, P.; Himmelman, J.; Lindencrona, U.; Bhogal, N.; Wiberg, D.; Berg, G.; Jansson, S.; Nyström, E.; Forssell-Aronsson, E.; Nilsson, M. Stunning of iodide transport by 131I irradiation in cultured thyroid epithelial cells. J. Nucl. Med. 2002, 43, 828–834. [Google Scholar] [PubMed]
- Lundh, C.; Lindencrona, U.; Postgård, P.; Carlsson, T.; Nilsson, M.; Forssell-Aronsson, E. Radiation-induced thyroid stunning: differential effects of 123I, 131I, 99mTc, and 211At on iodide transport and NIS mRNA expression in cultured thyroid cells. J. Nucl. Med. 2009, 50, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef]
- Cadet, J.; Loft, S.; Olinski, R.; Evans, M.D.; Bialkowski, K.; Richard Wagner, J.; Dedon, P.C.; Møller, P.; Greenberg, M.M.; Cooke, M.S. Biologically relevant oxidants and terminology, classification and nomenclature of oxidatively generated damage to nucleobases and 2-deoxyribose in nucleic acids. Free Radic. Res. 2012, 46, 367–381. [Google Scholar] [CrossRef] [PubMed]
- Cholewinski, S.P.; Yoo, K.S.; Klieger, P.S.; O’Mara, R.E. Absence of thyroid stunning after diagnostic whole-body scanning with 185 MBq 131I. J. Nucl. Med. 2000, 41, 1198–1202. [Google Scholar] [PubMed]
- Bajén, M.T.; Mañé, S.; Muñoz, A.; García, J.R. Effect of a diagnostic dose of 185 MBq 131I on postsurgical thyroid remnants. J. Nucl. Med. 2000, 41, 2038–2042. [Google Scholar] [PubMed]
- Rosário, P.W.; Barroso, A.L.; Rezende, L.L.; Padrão, E.L.; Maia, F.F.; Fagundes, T.A.; Purisch, S. 5 mCi pretreatment scanning does not cause stunning when the ablative dose is administered within 72 h. Arq. Bras. Endocrinol. Metab. 2005, 49, 420–424. [Google Scholar] [CrossRef]
- Karam, M.; Gianoukakis, A.; Feustel, P.J.; Cheema, A.; Postal, E.S.; Cooper, J.A. Influence of diagnostic and therapeutic doses on thyroid remnant ablation rates. Nucl. Med. Commun. 2003, 24, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Adamczewski, Z.; Makarewicz, J.; Lewinski, A. Effects of absorbed dose of 131I isotope on the effectiveness of ablation of thyroid remnant tissue. Arch. Med. Sci. 2007, 2, 136–141. [Google Scholar]
- McCready, V.R. A different approach to the use of unsealed radionuclides for cancer therapy. Nucl. Med. 1995, 22, 1–3. [Google Scholar] [CrossRef]
- Riccabona, G. 131I Therapy of Thyroid Disease: Why? When? How? EANM-Continuing Education: Düsseldorf, Germany, 1994. [Google Scholar]
- Dahlmann, H.A.; Vaidyanathan, V.G.; Sturla, S.J. Investigating the biochemical impact of DNA damage with structure-based probes: Abasic sites, photodimers, alkylation adducts, and oxidative lesions. Biochemistry 2009, 48, 9347–9359. [Google Scholar] [CrossRef] [PubMed]
- Slupphaug, G.; Kavli, B.; Krokan, H.E. The interacting pathways for prevention and repair of oxidative DNA damage. Mutat. Res. 2003, 531, 231–251. [Google Scholar] [CrossRef] [PubMed]
- Maynard, S.; Schurman, S.H.; Harboe, C.; de Souza-Pinto, N.C.; Bohr, V.A. Base excision repair of oxidative DNA damage and association with cancer and aging. Carcinogenesis 2009, 30, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Dikilitas, M.; Kocyigit, A. Mononuclear leukocyte DNA damage on higher cells caused by eco-friendly pesticides and their analysis using CAPS® programme. J. Agric. Fac. 2010, 14, 47–56. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamczewski, Z.; Stasiołek, M.; Karwowski, B.; Dedecjus, M.; Orszulak-Michalak, D.; Merecz, A.; Śliwka, P.W.; Puła, B.; Lewiński, A. The Effect of Diagnostic Absorbed Doses from 131I on Human Thyrocytes in Vitro. Int. J. Mol. Sci. 2015, 16, 14608-14622. https://doi.org/10.3390/ijms160714608
Adamczewski Z, Stasiołek M, Karwowski B, Dedecjus M, Orszulak-Michalak D, Merecz A, Śliwka PW, Puła B, Lewiński A. The Effect of Diagnostic Absorbed Doses from 131I on Human Thyrocytes in Vitro. International Journal of Molecular Sciences. 2015; 16(7):14608-14622. https://doi.org/10.3390/ijms160714608
Chicago/Turabian StyleAdamczewski, Zbigniew, Mariusz Stasiołek, Bolesław Karwowski, Marek Dedecjus, Daria Orszulak-Michalak, Anna Merecz, Przemysław W. Śliwka, Bartosz Puła, and Andrzej Lewiński. 2015. "The Effect of Diagnostic Absorbed Doses from 131I on Human Thyrocytes in Vitro" International Journal of Molecular Sciences 16, no. 7: 14608-14622. https://doi.org/10.3390/ijms160714608





