Association of preS/S Mutations with Occult Hepatitis B Virus (HBV) Infection in South Korea: Transmission Potential of Distinct Occult HBV Variants
Abstract
:1. Introduction
2. Occult Hepatitis B Virus Infection
3. Occult HBV Infections in Korean Subjects
| No. of HBsAg (−) Subjects | No. of Subjects Positive for Nested PCR | Prevalence | ||
|---|---|---|---|---|
| 624 | 41 | 6.6% | ||
| No. of Serotype (41 subjects a) | ||||
| adr | adw | Untypeable | ||
| 39 (95.2%) | 1 (2.4%) | 1 (2.4%) | ||
| No. of HBeAg (+) Subjects (21 subjects b) | ||||
| 15 (71.4%) | ||||
4. Mutations of the Hepatitis B Virus preS Region Related to Occult Infection in South Korea
| Regions | Types | Polymerase | Occult (n = 41) | Carriers (n = 40) | p-Value | Clinical Relevance |
|---|---|---|---|---|---|---|
| preS1 | Hepatocyte receptor binding region | - | 29 (70.7%) | 7 (17.5%) | <0.001 | [58] |
| Start codon deletion | spacer6-12 Deletion | 11 (26.8%) | 3 (7.5%) | <0.05 | [14,46] | |
| W4P/R | spacerL9D | 7 (17.1%) | 2 (5.0%) | 0.087 | [21,23] | |
| K/Q10R | - | 6 (14.6%) | 0 (0%) | <0.05 | - | |
| S17A | spacerF22C | 5 (12.2%) | 0 (0%) | <0.05 | - | |
| P32L | - | 5 (12.2%) | 0 (0%) | <0.05 | - | |
| W43L/R | spacerL48S | 6 (14.6%) | 0 (0%) | <0.05 | - | |
| H51P | - | 5 (12.2%) | 1 (2.5%) | 0.096 | - | |
| I84T | - | 7 (17.1%) | 0 (0%) | <0.01 | - | |
| preS2 | Deletion | spacer132-147 Deletion | 7 (17.1%) | 1 (2.5%) | <0.05 | [17,44,47] |
| M1L/T/V | - | 4 (9.8%) | 1 (2.5%) | 0.175 | - | |
| W3R/* | - | 6 (14.6%) | 0 (0%) | <0.05 | - | |
| S5A | - | 5 (12.2%) | 0 (0%) | <0.05 | - | |
| S | “a” determinant | - | 31 (75.6%) | 5 (12.5%) | <0.001 | - |
| I/T126N/S | - | 11 (26.8%) | 3 (7.5%) | <0.05 | - | |
| W182L/* | - | 15 | 0 (0%) | <0.001 | [19] |
5. Mutations in the Hepatitis B Virus S Region Related to Occult Infection in South Korea
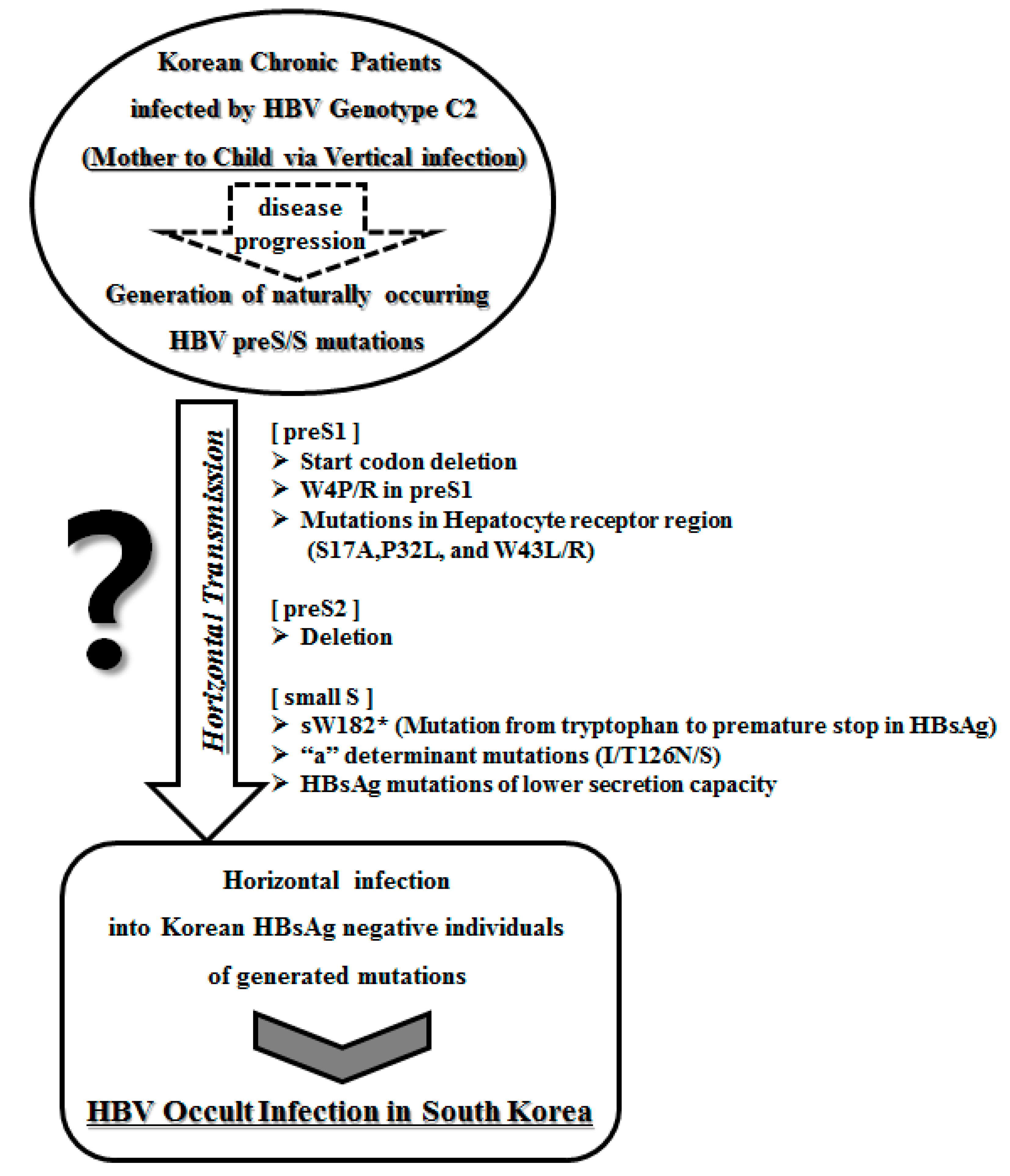
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Orito, E.; Mizokami, M.; Sakugawa, H.; Michitaka, K.; Ishikawa, K.; Ichida, T.; Okanoue, T.; Yotsuyanagi, H.; Iino, S. A case-control study for clinical and molecular biological differences between hepatitis B viruses of genotypes B and C. Hepatology 2001, 33, 218–223. [Google Scholar] [PubMed]
- Kao, J.H.; Chen, D.S. Global control of hepatitis B virus infection. Lancet Infect. Dis. 2002, 2, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [CrossRef]
- Ganem, D.; Prince, A.M. Hepatitis B virus infection—Natural history and clinical consequences. N. Engl. J. Med. 2004, 350, 1118–1129. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.C.; Ip, H.M.; Reesink, H.W.; Lelie, P.N.; Reerink-Brongers, E.E.; Yeung, C.Y.; Ma, H.K. Prevention of the HBsAg carrier state in newborn infants of mothers who are chronic carriers of HBsAg and HBeAg by administration of hepatitis-B vaccine and hepatitis-B immunoglobulin. Double-blind randomised placebo-controlled study. Lancet 1984, 1, 921–926. [Google Scholar] [CrossRef]
- De la Hoz, F.; Perez, L.; de Neira, M.; Hall, A.J. Eight years of hepatitis B vaccination in Colombia with a recombinant vaccine: Factors influencing hepatitis B virus infection and effectiveness. Int. J. Infect. Dis. 2008, 12, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Prevention, K.C. Korea national health and nutrition examination survey. Int. J. Epidemiol. 2011, 43, 69–77. [Google Scholar]
- Chun, B.Y.; Lee, M.K.; Rho, Y.K. The prevalence of hepatitis B surface antigen among Korean by literature review. Korean J. Epidemiol. 1992, 14, 54–62. [Google Scholar]
- Kim, H.; Jee, Y.M.; Song, B.C.; Shin, J.W.; Yang, S.H.; Mun, H.S.; Kim, H.J.; Oh, E.J.; Yoon, J.H.; Kim, Y.J.; et al. Molecular epidemiology of hepatitis B virus (HBV) genotypes and serotypes in patients with chronic HBV infection in Korea. Intervirology 2007, 50, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Song, B.C.; Kim, H.; Kim, S.H.; Cha, C.Y.; Kook, Y.H.; Kim, B.J. Comparison of full length sequences of hepatitis B virus isolates in hepatocellular carcinoma patients and asymptomatic carriers of Korea. J. Med. Virol. 2005, 75, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Song, B.C.; Kim, S.H.; Kim, H.; Ying, Y.H.; Kim, H.J.; Kim, Y.J.; Yoon, J.H.; Lee, H.S.; Cha, C.Y.; Kook, Y.H.; et al. Prevalence of naturally occurring surface antigen variants of hepatitis B virus in Korean patients infected chronically. J. Med. Virol. 2005, 76, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Jee, Y.; Mun, H.S.; Park, J.H.; Yoon, J.H.; Kim, Y.J.; Lee, H.S.; Hyun, J.W.; Hwang, E.S.; Cha, C.Y.; et al. Characterization of two hepatitis B virus populations in a single Korean hepatocellular carcinoma patient with an HBeAg-negative serostatus: A novel X-Gene-deleted strain with inverted duplication sequences of upstream enhancer site II. Intervirology 2007, 50, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Jee, Y.; Mun, H.S.; Song, B.C.; Park, J.H.; Hyun, J.W.; Hwang, E.S.; Cha, C.Y.; Kook, Y.H.; Kim, B.J. Comparison of full genome sequences between two hepatitis B virus strains with or without preC mutation (A1896) from a single Korean hepatocellular carcinoma patient. J. Microb. Biotechnol. 2007, 17, 701–704. [Google Scholar]
- Kim, H.J.; Park, J.H.; Jee, Y.; Lee, S.A.; Kim, H.; Song, B.C.; Yang, S.; Lee, M.; Yoon, J.H.; Kim, Y.J.; et al. Hepatitis B virus X mutations occurring naturally associated with clinical severity of liver disease among Korean patients with chronic genotype C infection. J. Med. Virol. 2008, 80, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Mun, H.S.; Lee, S.A.; Jee, Y.; Kim, H.; Park, J.H.; Song, B.C.; Yoon, J.H.; Kim, Y.J.; Lee, H.S.; Hyun, J.W.; et al. The prevalence of hepatitis B virus preS deletions occurring naturally in Korean patients infected chronically with genotype C. J. Med. Virol. 2008, 80, 1189–1194. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.A.; Cho, Y.K.; Lee, K.H.; Hwang, E.S.; Kook, Y.H.; Kim, B.J. Gender disparity in distribution of the major hydrophilic region variants of hepatitis B virus genotype C according to hepatitis B e antigen serostatus. J. Med. Virol. 2011, 83, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.A.; Mun, H.S.; Kim, H.; Lee, H.K.; Kim, B.J.; Hwang, E.S.; Kook, Y.H.; Kim, B.J. Naturally occurring hepatitis B virus X deletions and insertions among Korean chronic patients. J. Med. Virol. 2011, 83, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Mun, H.S.; Lee, S.A.; Kim, H.; Hwang, E.S.; Kook, Y.H.; Kim, B.J. Novel F141L pre-S2 mutation in hepatitis B virus increases the risk of hepatocellular carcinoma in patients with chronic genotype C infections. J. Virol. 2011, 85, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Lee, S.A.; Hwang, E.S.; Kook, Y.H.; Kim, B.J. Naturally occurring precore/core region mutations of hepatitis B virus genotype C related to hepatocellular carcinoma. PLoS ONE 2012, 7, e47372. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.A.; Kim, K.; Kim, H.; Kim, B.J. Nucleotide change of codon 182 in the surface gene of hepatitis B virus genotype C leading to truncated surface protein is associated with progression of liver diseases. J. Hepatol. 2012, 56, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, S.A.; Kim, D.W.; Lee, S.H.; Kim, B.J. Naturally occurring mutations in large surface genes related to occult infection of hepatitis B virus genotype C. PLoS ONE 2013, 8, e54486. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.A.; Kim, K.J.; Kim, D.W.; Kim, B.J. Male-specific W4P/R mutation in the pre-S1 region of hepatitis B virus, increasing the risk of progression of liver diseases in chronic patients. J. Clin. Microbiol. 2013, 51, 3928–3936. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J. Hepatitis B virus mutations related to liver disease progression of Korean patients. World J. Gastroenterol. 2014, 20, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.A.; Kim, H.; Won, Y.S.; Seok, S.H.; Na, Y.; Shin, H.B.; Inn, K.S.; Kim, B.J. Male-specific hepatitis B virus large surface protein variant W4P potentiates tumorigenicity and induces gender disparity. Mol. Cancer 2015, 14. [Google Scholar] [CrossRef] [PubMed]
- Raimondo, G.; Balsano, C.; Craxi, A.; Farinati, F.; Levrero, M.; Mondelli, M.; Pollicino, T.; Squadrito, G.; Tiribelli, C. Occult hepatitis B virus infection. Dig. Liver Dis. 2000, 32, 822–826. [Google Scholar] [CrossRef]
- Torbenson, M.; Thomas, D.L. Occult hepatitis B. Lancet Infect. Dis. 2002, 2, 479–486. [Google Scholar] [CrossRef]
- Conjeevaram, H.S.; Lok, A.S. Occult hepatitis B virus infection: A hidden menace? Hepatology 2001, 34, 204–206. [Google Scholar] [CrossRef] [PubMed]
- Raimondo, G.; Allain, J.P.; Brunetto, M.R.; Buendia, M.A.; Chen, D.S.; Colombo, M.; Craxi, A.; Donato, F.; Ferrari, C.; Gaeta, G.B.; et al. Statements from the Taormina expert meeting on occult hepatitis B virus infection. J. Hepatol. 2008, 49, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Hansson, B.G.; Kuo, L.S.; Widell, A.; Nordenfelt, E. Hepatitis B virus DNA in serum and liver is commonly found in Chinese patients with chronic liver disease despite the presence of antibodies to HBsAg. Hepatology 1993, 17, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.J.; Baruch, Y.; Ben-Porath, E.; Enat, R.; Bassan, L.; Brown, N.V.; Rimon, N.; Blum, H.E.; Wands, J.R. Hepatitis B virus infection in patients with idiopathic liver disease. Hepatology 1991, 13, 1044–1051. [Google Scholar] [PubMed]
- Koike, K.; Kobayashi, M.; Gondo, M.; Hayashi, I.; Osuga, T.; Takada, S. Hepatitis B virus DNA is frequently found in liver biopsy samples from hepatitis C virus-infected chronic hepatitis patients. J. Med. Virol. 1998, 54, 249–255. [Google Scholar] [CrossRef]
- Bréchot, C.; Hadchouel, M.; Scotto, J.; Fonck, M.; Potet, F.; Vyas, G.N.; Tiollais, P. State of hepatitis B virus DNA in hepatocytes of patients with hepatitis B surface antigen-positive and -negative liver diseases. Proc. Natl. Acad. Sci. USA 1981, 78, 3906–3910. [Google Scholar] [CrossRef] [PubMed]
- Samal, J.; Kandpal, M.; Vivekanandan, P. Molecular mechanisms underlying occult hepatitis B virus infection. Clin. Microbiol. Rev. 2012, 25, 142–163. [Google Scholar] [CrossRef] [PubMed]
- Shafritz, D.A.; Shouval, D.; Sherman, H.I.; Hadziyannis, S.J.; Kew, M.C. Integration of hepatitis B virus DNA into the genome of liver cells in chronic liver disease and hepatocellular carcinoma. Studies in percutaneous liver biopsies and post-mortem tissue specimens. N. Engl. J. Med. 1981, 305, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- De Maria, N.; Colantoni, A.; Friedlander, L.; Leandro, G.; Idilman, R.; Harig, J.; van Thiel, D.H. The impact of previous HBV infection on the course of chronic hepatitis C. Am. J. Gastroenterol. 2000, 95, 3529–3536. [Google Scholar] [CrossRef]
- Sagnelli, E.; Coppola, N.; Scolastico, C.; Mogavero, A.R.; Filippini, P.; Piccinino, F. HCV genotype and “silent” HBV coinfection: Two main risk factors for a more severe liver disease. J. Med. Virol. 2001, 64, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Chemin, I.; Zoulim, F.; Merle, P.; Arkhis, A.; Chevallier, M.; Kay, A.; Cova, L.; Chevallier, P.; Mandrand, B.; Trépo, C. High incidence of hepatitis B infections among chronic hepatitis cases of unknown aetiology. J. Hepatol. 2001, 34, 447–454. [Google Scholar] [CrossRef]
- Fang, Z.L.; Zhuang, H.; Wang, X.Y.; Ge, X.M.; Harrison, T.J. Hepatitis B virus genotypes, phylogeny and occult infection in a region with a high incidence of hepatocellular carcinoma in China. World J. Gastroenterol. 2004, 10, 3264–3268. [Google Scholar] [PubMed]
- Luo, K.X.; Zhou, R.; He, C.; Liang, Z.S.; Jiang, S.B. Hepatitis B virus DNA in sera of virus carriers positive exclusively for antibodies to the hepatitis B core antigen. J. Med. Virol. 1991, 35, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Shih, L.N.; Sheu, J.C.; Wang, J.T.; Huang, G.T.; Yang, P.M.; Lee, H.S.; Sung, J.L.; Wang, T.H.; Chen, D.S. Serum hepatitis B virus DNA in healthy HBsAg-negative Chinese adults evaluated by polymerase chain reaction. J. Med. Virol. 1990, 32, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.T.; Wang, T.H.; Sheu, J.C.; Shih, L.N.; Lin, J.T.; Chen, D.S. Detection of hepatitis B virus DNA by polymerase chain reaction in plasma of volunteer blood donors negative for hepatitis B surface antigen. J. Infect. Dis. 1991, 163, 397–399. [Google Scholar] [CrossRef] [PubMed]
- Mu, S.C.; Lin, Y.M.; Jow, G.M.; Chen, B.F. Occult hepatitis B virus infection in hepatitis B vaccinated children in Taiwan. J. Hepatol. 2009, 50, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Ye, X.; Zhang, L.; Wang, W.; Shuai, L.; Wang, A.; Zeng, J.; Candotti, D.; Allain, J.P.; Li, C. Characterization of occult hepatitis B virus infection from blood donors in China. J. Clin. Microbiol. 2011, 49, 1730–1737. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Ou, S.H.; Chen, C.R.; Ge, S.X.; Pei, B.; Chen, Q.R.; Yan, Q.; Lin, Y.C.; Ni, H.Y.; Huang, C.H.; et al. Molecular characteristics of occult hepatitis B virus from blood donors in southeast China. J. Clin. Microbiol. 2010, 48, 357–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuen, M.F.; Wong, D.K.; Lee, C.K.; Tanaka, Y.; Allain, J.P.; Fung, J.; Leung, J.; Lin, C.K.; Sugiyama, M.; Sugauchi, F.; et al. Transmissibility of hepatitis B virus (HBV) infection through blood transfusion from blood donors with occult HBV infection. Clin. Infect. Dis. 2011, 52, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.H.; Li, L.; Hung, Y.S.; Hung, C.S.; Allain, J.P.; Lin, K.S.; Tsai, S.J. The efficacy of individual-donation and minipool testing to detect low-level hepatitis B virus DNA in Taiwan. Transfusion 2010, 50, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, P.J.; Chen, M.H.; Chak, K.F.; Lin, K.S.; Tsai, S.J. A pilot study for screening blood donors in Taiwan by nucleic acid amplification technology: Detecting occult hepatitis B virus infections and closing the serologic window period for hepatitis C virus. Transfusion 2008, 48, 1198–1206. [Google Scholar] [CrossRef] [PubMed]
- Phikulsod, S.; Oota, S.; Tirawatnapong, T.; Sakuldamrongpanich, T.; Chalermchan, W.; Louisirirotchanakul, S.; Tanprasert, S.; Chongkolwatana, V.; Kitpoka, P.; Phanuphak, P.; et al. One-year experience of nucleic acid technology testing for human immunodeficiency virus Type 1, hepatitis C virus, and hepatitis B virus in Thai blood donations. Transfusion 2009, 49, 1126–1135. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Lee, K.S.; Park, C.J.; Lee, J.Y.; Kim, K.H.; Park, J.Y.; Lee, J.H.; Kim, H.Y.; Yoo, J.Y.; Jang, M.K. Prevalence of occult HBV infection among subjects with normal serum ALT levels in Korea. J. Infect. 2007, 54, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Song, E.Y.; Yun, Y.M.; Park, M.H.; Seo, D.H. Prevalence of occult hepatitis B virus infection in a general adult population in Korea. Intervirology 2009, 52, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.Y.; Kim, M.H.; Lee, W.I. The prevalence of “anti-HBc alone” and HBV DNA detection among anti-HBc alone in Korea. J. Med. Virol. 2010, 82, 1508–1514. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, H.; Lee, S.A.; Won, Y.S.; Kim, H.I.; Inn, K.S.; Kim, B.J. Upregulation of endoplasmic reticulum stress and reactive oxygen species by naturally occurring mutations in HBcAg of the hepatitis B virus. J. Gen. Virol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, S.A.; Won, Y.S.; Lee, H.; Kim, B.J. Occult infection related hepatitis B surface antigen variants showing lowered secretion capacity. World J. Gastroenterol. 2015, 21, 1794–1803. [Google Scholar] [CrossRef] [PubMed]
- Klingmüller, U.; Schaller, H. Hepadnavirus infection requires interaction between the viral pre-S domain and a specific hepatocellular receptor. J. Virol. 1993, 67, 7414–7422. [Google Scholar] [PubMed]
- Dyson, M.R.; Murray, K. Selection of peptide inhibitors of interactions involved in complex protein assemblies: Association of the core and surface antigens of hepatitis B virus. Proc. Natl. Acad. Sci. USA 1995, 92, 2194–2198. [Google Scholar] [CrossRef] [PubMed]
- Trautwein, C.; Schrem, H.; Tillmann, H.L.; Kubicka, S.; Walker, D.; Böker, K.H.; Maschek, H.J.; Pichlmayr, R.; Manns, M.P. Hepatitis B virus mutations in the pre-S genome before and after liver transplantatio. Hepatology 1996, 24, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Bock, C.T.; Tillmann, H.L.; Maschek, H.J.; Manns, M.P.; Trautwein, C. A preS mutation isolated from a patient with chronic hepatitis B infection leads to virus retention and misassembly. Gastroenterology 1997, 113, 1976–1982. [Google Scholar] [CrossRef]
- Hsieh, Y.-H.; Su, I.-J.; Wang, H.-C.; Chang, W.-W.; Lei, H.-Y.; Lai, M.-D.; Chang, W.-T.; Huang, W. Pre-S mutant surface antigens in chronic hepatitis B virus infection induce oxidative stress and DNA damage. Carcinogenesis 2004, 25, 2023–2032. [Google Scholar] [CrossRef] [PubMed]
- Hung, J.H.; Su, I.J.; Lei, H.Y.; Wang, H.C.; Lin, W.C.; Chang, W.T.; Huang, W.; Chang, W.C.; Chang, Y.S.; Chen, C.C.; et al. Endoplasmic reticulum stress stimulates the expression of cyclooxygenase-2 through activation of NF-κB and pp38 mitogen-activated protein kinase. J. Biol. Chem. 2004, 279, 46384–46392. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.C.; Wu, H.C.; Chen, C.F.; Fausto, N.; Lei, H.Y.; Su, I.J. Different types of ground glass hepatocytes in chronic hepatitis B virus infection contain specific pre-S mutants that may induce endoplasmic reticulum stress. Am. J. Pathol. 2003, 163, 2441–2449. [Google Scholar] [CrossRef]
- Wang, H.-C.; Huang, W.; Lai, M.-D.; Su, I.-J. Hepatitis B virus pre-S mutants, endoplasmic reticulum stress and hepatocarcinogenesis. Cancer Sci. 2006, 97, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Caselmann, W.H.; Meyer, M.; Kekule, A.S.; Lauer, U.; Hofschneider, P.H.; Koshy, R. A trans-activator function is generated by integration of hepatitis B virus preS/S sequences in human hepatocellular carcinoma DNA. Proc. Natl. Acad. Sci. USA 1990, 87, 2970–2974. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.A.; Ki, K.J.; Kim, H.; Choi, W.H.; Won, Y.S.; Kim, B.J. Hepatitis B virus preS1 deletion is related to viral replication increase and disease progression. World J. Gastroenterol. 2015, in press. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Teng, X.; Xu, W.Z.; Li, D.; Zhao, H.W.; Fu, L.J.; Zhang, F.M.; Gu, H.X. Molecular characterization and functional analysis of occult hepatitis B virus infection in Chinese patients infected with genotype C. J. Med. Virol. 2009, 81, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Peng, B.; He, W.; Zhong, G.; Qi, Y.; Ren, B.; Gao, Z.; Jing, Z.; Song, M.; Xu, G.; et al. Molecular determinants of hepatitis B and D virus entry restriction in mouse sodium taurocholate cotransporting polypeptide. J. Virol. 2013, 87, 7977–7991. [Google Scholar] [CrossRef] [PubMed]
- Lada, O.; Benhamou, Y.; Poynard, T.; Thibault, V. Coexistence of hepatitis B surface antigen (HBsAg) and anti-HBs antibodies in chronic hepatitis B virus carriers: Influence of “a” determinant variants. J. Virol. 2006, 80, 2968–2975. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Chandra, P.K.; Datta, S.; Biswas, A.; Bhattacharya, P.; Chakraborty, S.; Chakrabarti, S.; Bhattacharya, S.K.; Chakravarty, R. Frequency and significance of hepatitis B virus surface gene variant circulating among “antiHBc only” individuals in Eastern India. J. Clin. Virol. 2007, 40, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Amini-Bavil-Olyaee, S.; Vucur, M.; Luedde, T.; Trautwein, C.; Tacke, F. Differential impact of immune escape mutations G145R and P120T on the replication of lamivudine-resistant hepatitis B virus e antigen-positive and -negative strains. J. Virol. 2010, 84, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Zuckerman, A.J. Effect of hepatitis B virus mutants on efficacy of vaccination. Lancet 2000, 355, 1382–1384. [Google Scholar] [CrossRef]
- Cooreman, M.P.; van Roosmalen, M.H.; te Morsche, R.; Sunnen, C.M.; de Ven, E.M.; Jansen, J.B.; Tytgat, G.N.; de Wit, P.L.; Paulij, W.P. Characterization of the reactivity pattern of murine monoclonal antibodies against wild-type hepatitis B surface antigen to G145R and other naturally occurring “a” loop escape mutations. Hepatology 1999, 30, 1287–1292. [Google Scholar] [CrossRef] [PubMed]
- Oon, C.J.; Chen, W.N.; Goo, K.S.; Goh, K.T. Intra-familial evidence of horizontal transmission of hepatitis B virus surface antigen mutant G145R. J. Infect. 2000, 41, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Tsubota, A.; Hirokawa, T.; Kumada, H.; Yang, Z.; Tanaka, H. A unique amino acid substitution, T126I, in human genotype C of hepatitis B virus S gene and its possible influence on antigenic structural change. Gene 2006, 383, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Chua, P.K.; Wang, R.Y.; Lin, M.H.; Masuda, T.; Suk, F.M.; Shih, C. Reduced secretion of virions and hepatitis B virus (HBV) surface antigen of a naturally occurring HBV variant correlates with the accumulation of the small S envelope protein in the endoplasmic reticulum and Golgi apparatus. J. Virol. 2005, 79, 13483–13496. [Google Scholar] [CrossRef] [PubMed]
- Jeantet, D.; Chemin, I.; Mandrand, B.; Tran, A.; Zoulim, F.; Merle, P.; Trepo, C.; Kay, A. Cloning and expression of surface antigens from occult chronic hepatitis B virus infections and their recognition by commercial detection assays. J. Med. Virol. 2004, 73, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, V.; Tayal, R.; Nayak, B.; Acharya, S.K.; Panda, S.K. Occult hepatitis B virus infection in chronic liver disease: Full-length genome and analysis of mutant surface promoter. Gastroenterology 2004, 127, 1356–1371. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Rehman, S.; Durgapal, H.; Acharya, S.K.; Panda, S.K. Role of surface promoter mutations in hepatitis B surface antigen production and secretion in occult hepatitis B virus infection. J. Med. Virol. 2007, 79, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.K.; Kim, H.; Won, Y.S.; Kim, B.J. Induction of ER-derived oxidative stress by an occult infection related S surface antigen variant. World J. Gastroenterol. 2015, in press. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Kim, B.-J. Association of preS/S Mutations with Occult Hepatitis B Virus (HBV) Infection in South Korea: Transmission Potential of Distinct Occult HBV Variants. Int. J. Mol. Sci. 2015, 16, 13595-13609. https://doi.org/10.3390/ijms160613595
Kim H, Kim B-J. Association of preS/S Mutations with Occult Hepatitis B Virus (HBV) Infection in South Korea: Transmission Potential of Distinct Occult HBV Variants. International Journal of Molecular Sciences. 2015; 16(6):13595-13609. https://doi.org/10.3390/ijms160613595
Chicago/Turabian StyleKim, Hong, and Bum-Joon Kim. 2015. "Association of preS/S Mutations with Occult Hepatitis B Virus (HBV) Infection in South Korea: Transmission Potential of Distinct Occult HBV Variants" International Journal of Molecular Sciences 16, no. 6: 13595-13609. https://doi.org/10.3390/ijms160613595






