Supramolecular Approaches to Nanoscale Morphological Control in Organic Solar Cells
Abstract
:1. Introduction
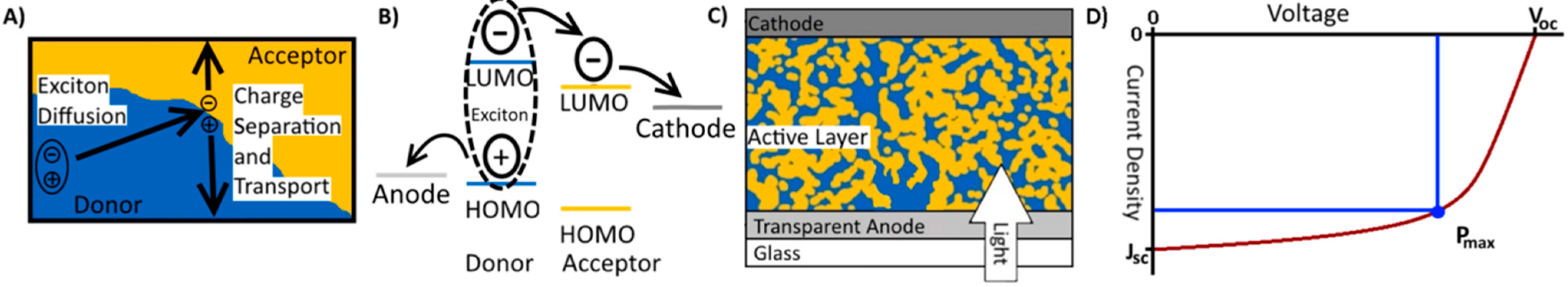
2. Working Principles of Organic Solar Cells
3. Aromatic Stacking
3.1. Self-Assembled Nanowires



3.2. Tuning Bulk Heterojunction Domain Size
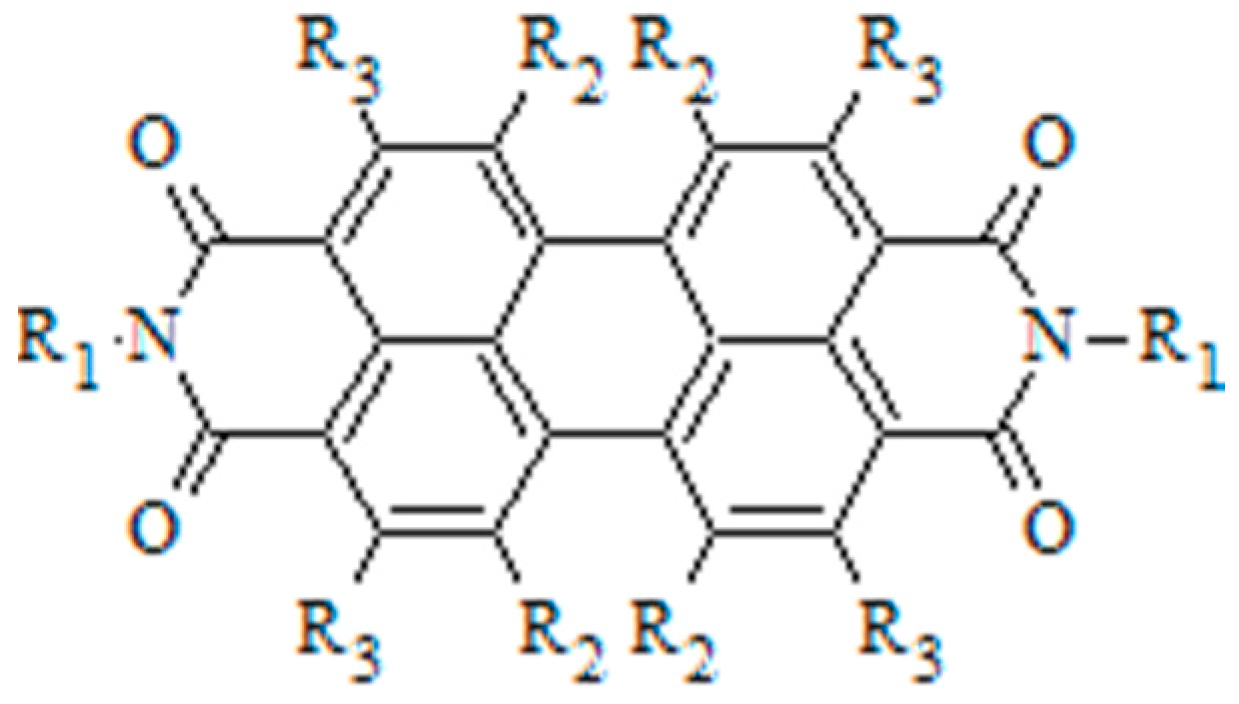
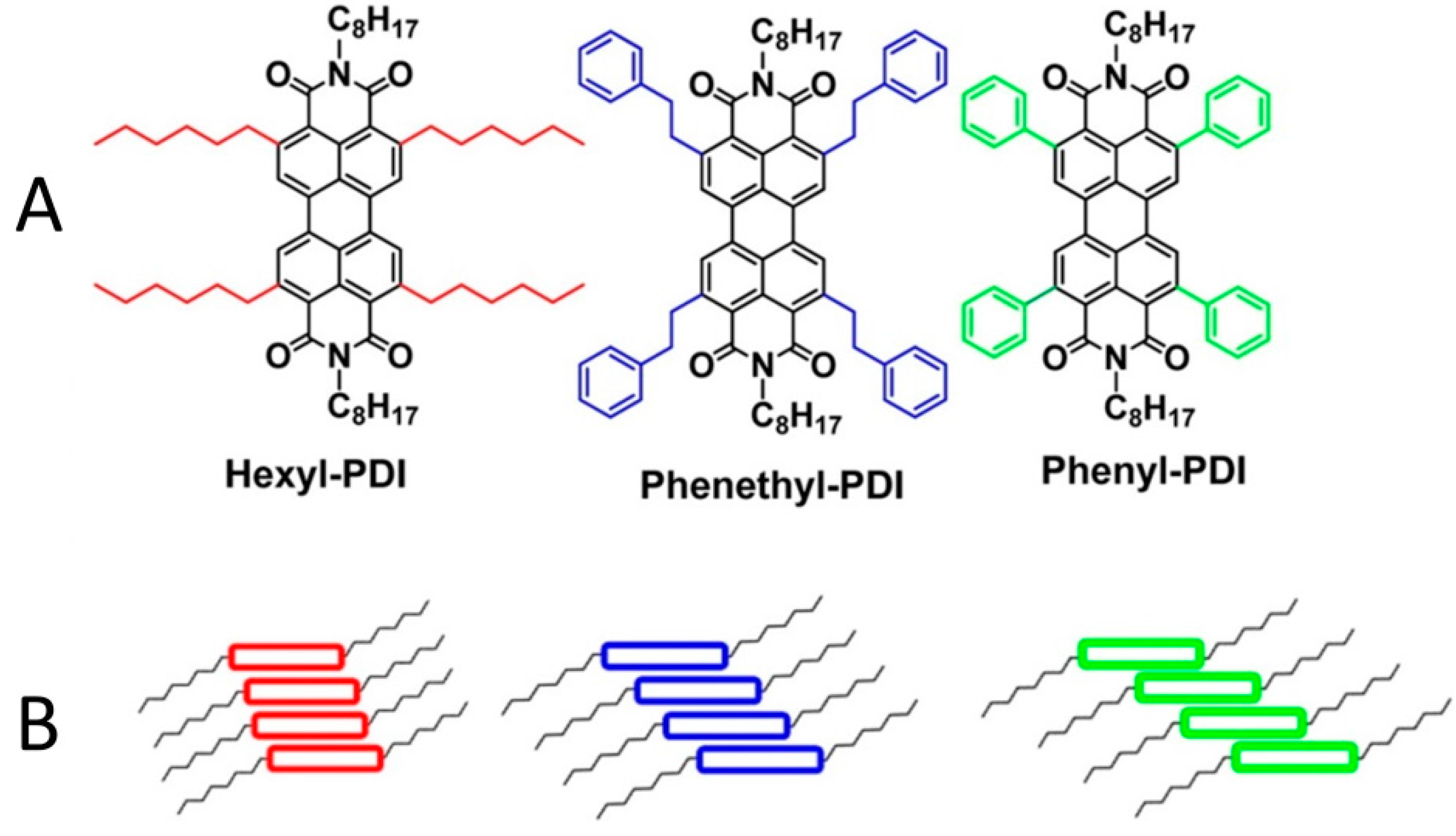
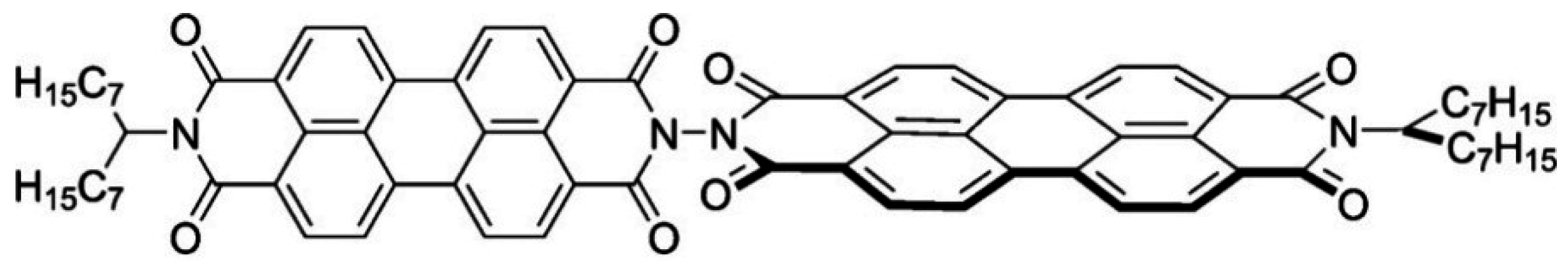
3.3. Selective Donor–Acceptor Interactions

3.4. Covalently Attached Donors and Acceptors
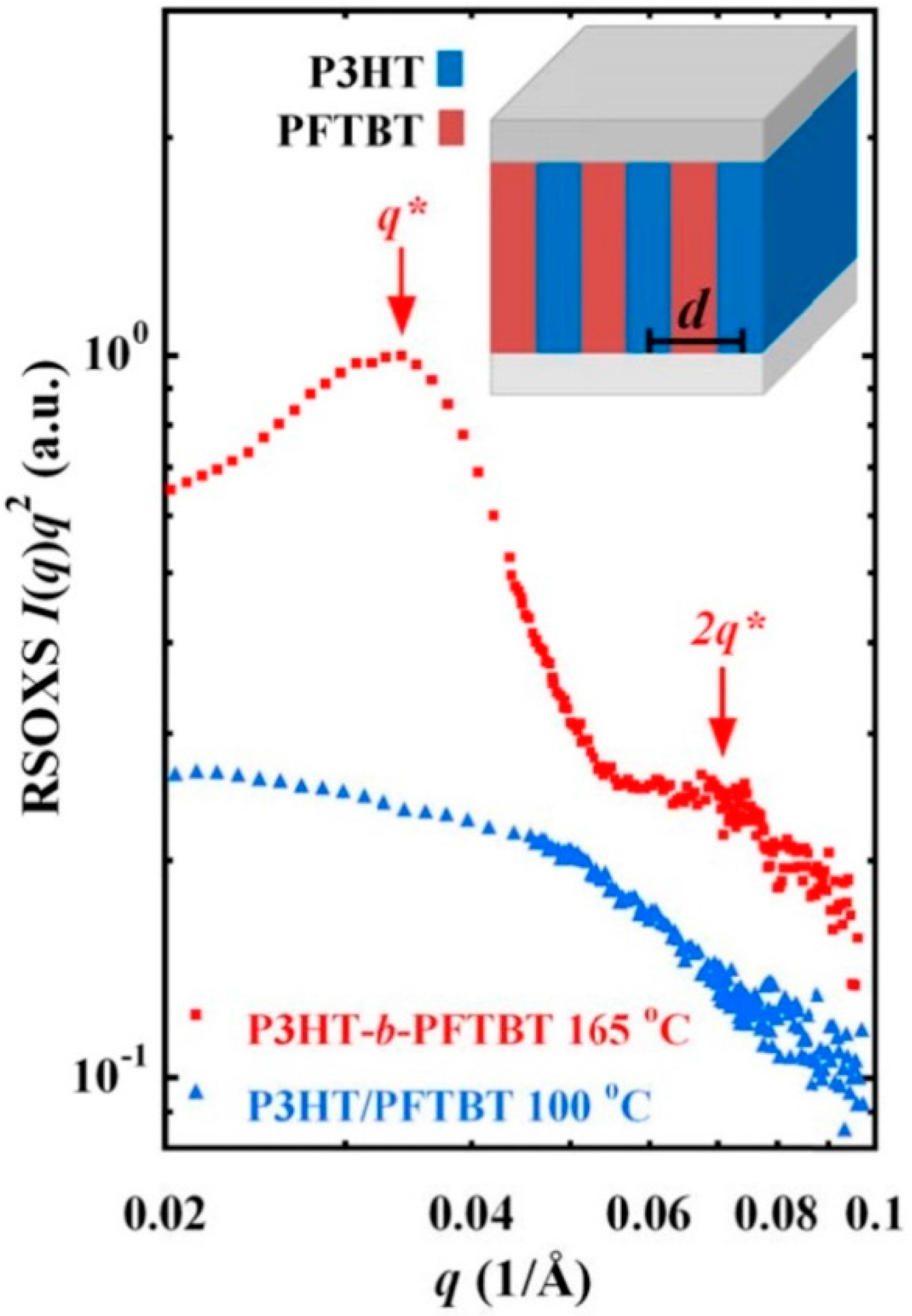
4. Hydrogen Bonding
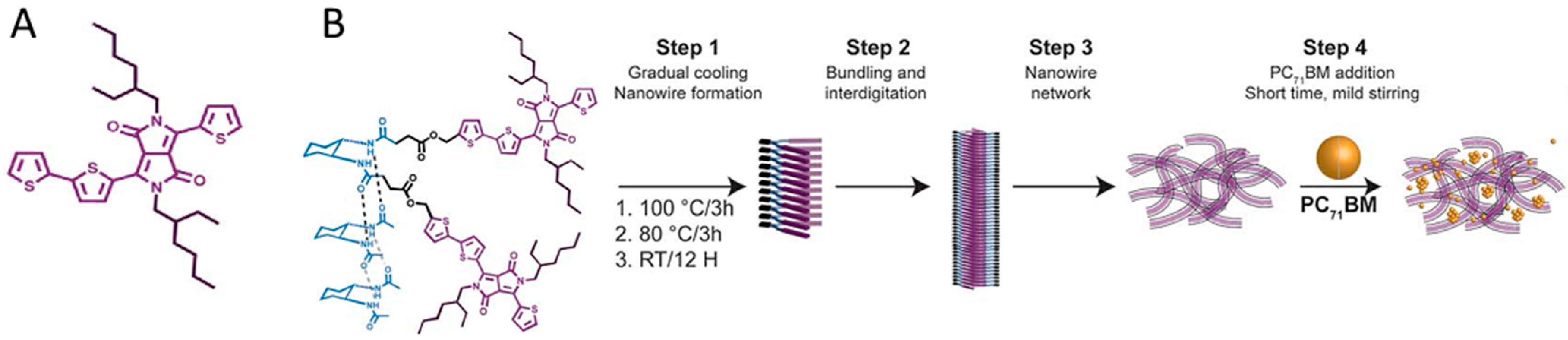
4.1. Tuning Self-Assembly with Hydrogen Bonding

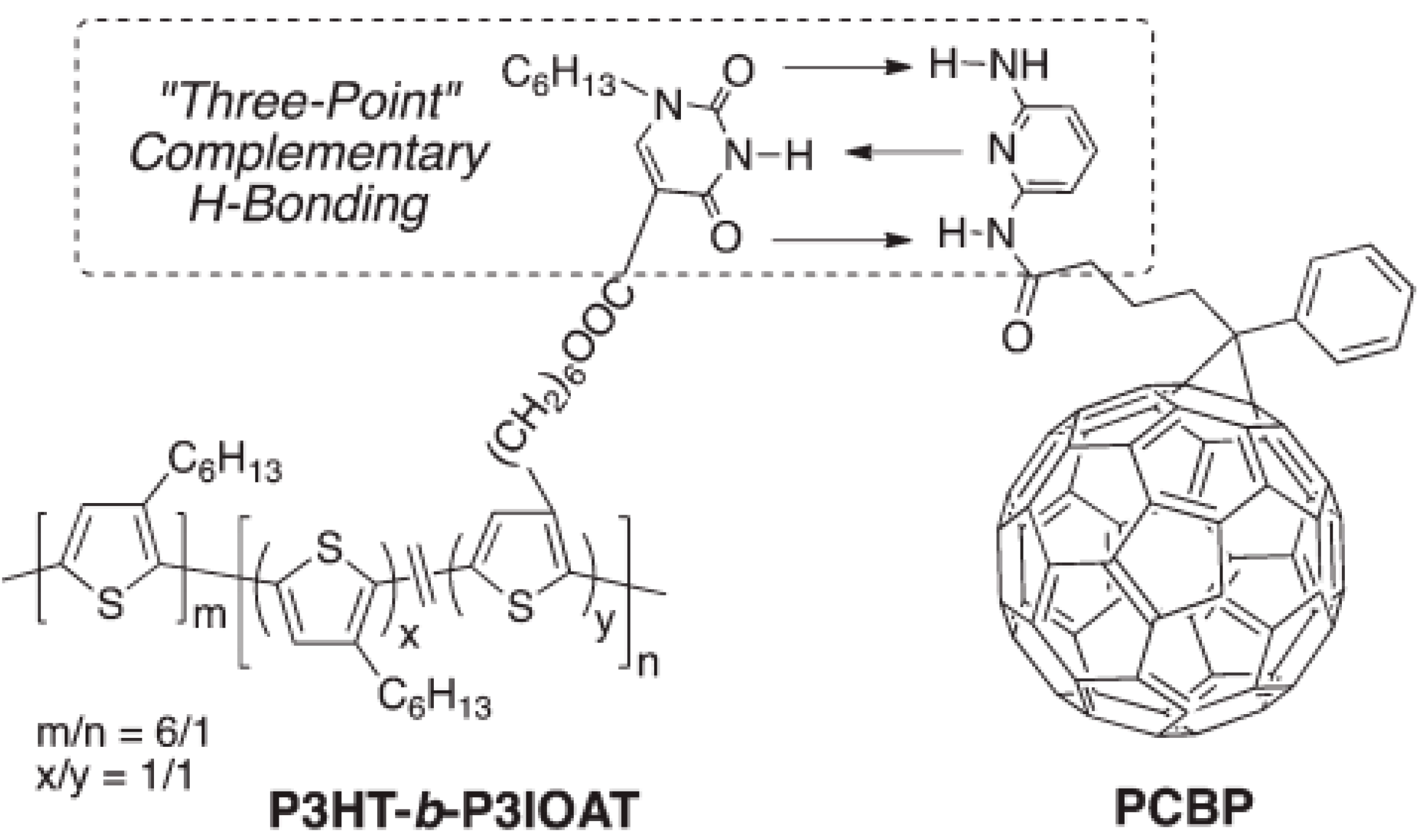
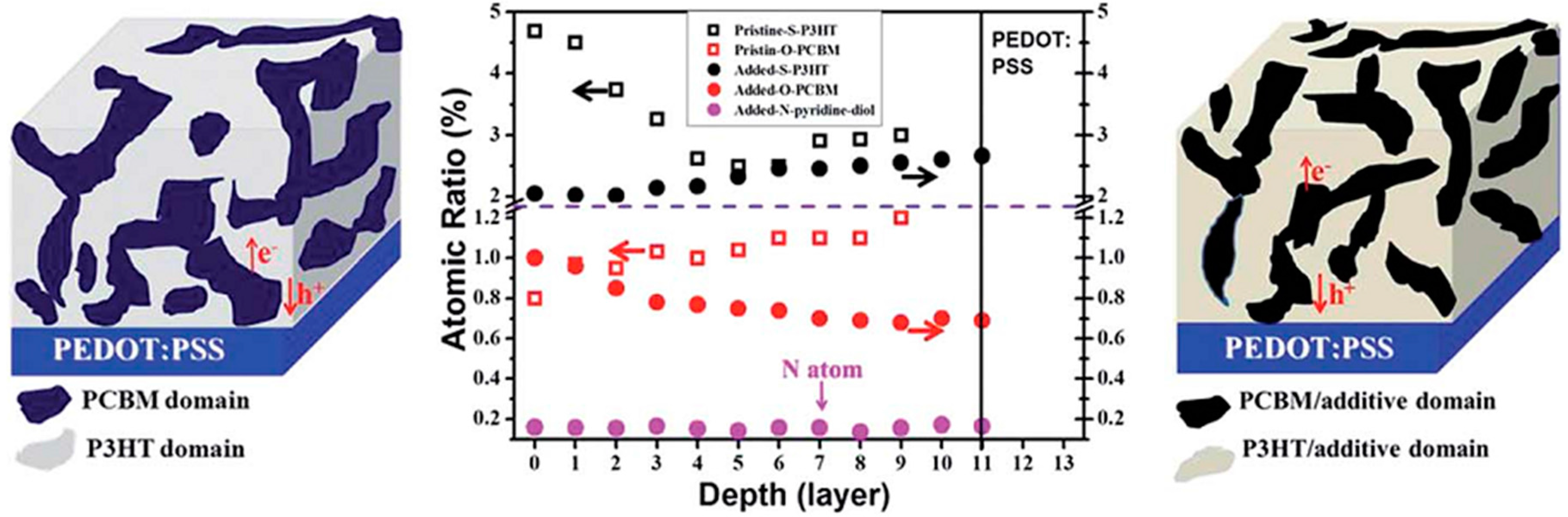
4.2. Active Layer Stabilization
4.3. Molecular Additives
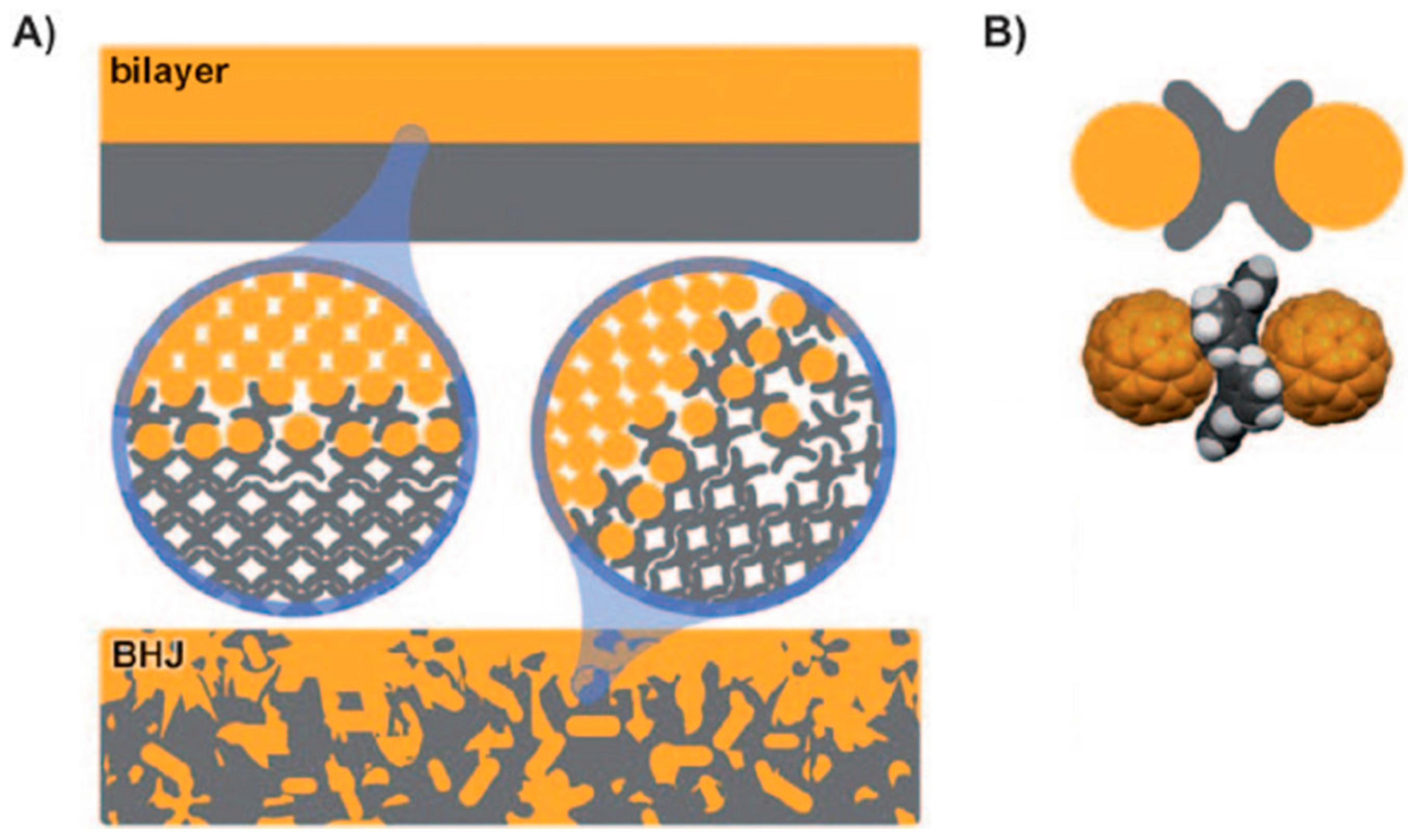
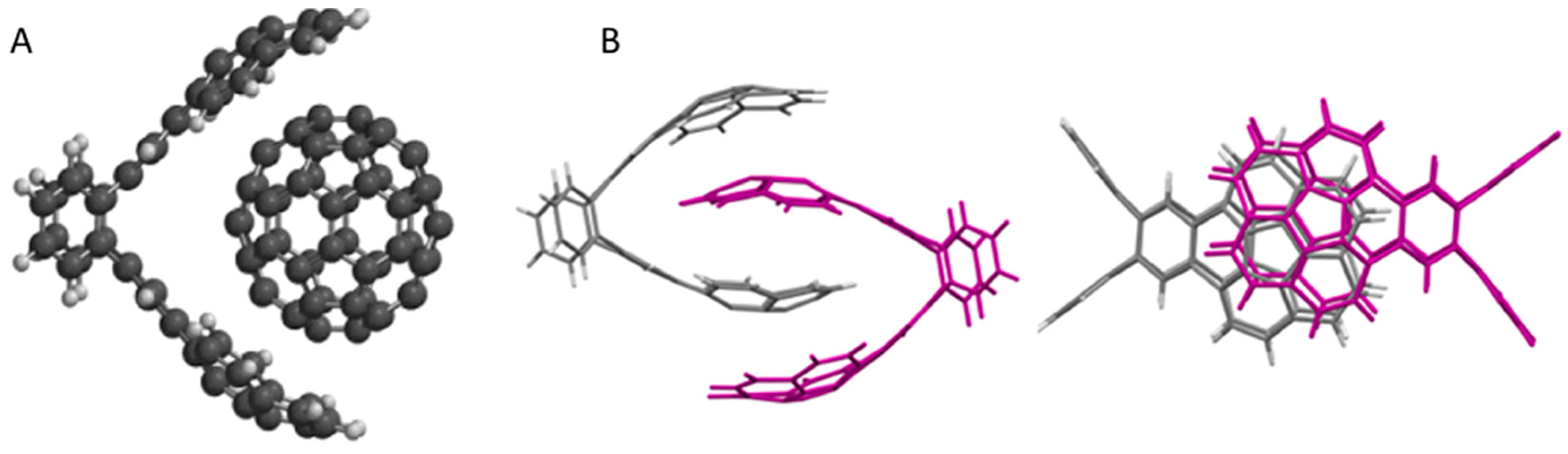
5. Shape Complementarity
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dennler, G.; Scharber, M.C.; Brabec, C.J. Polymer-fullerene bulk-heterojunction solar cells. Adv. Mater. 2009, 21, 1323–1338. [Google Scholar] [CrossRef]
- Scharber, M.C.; Sariciftci, N.S. Efficiency of bulk-heterojunction organic solar cells. Prog. Polym. Sci. 2013, 38, 1929–1940. [Google Scholar] [CrossRef] [PubMed]
- Mazzio, K.A.; Luscombe, C.K. The future of organic photovoltaics. Chem. Soc. Rev. 2014, 44, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Lyons, B.P.; Clarke, N.; Groves, C. The relative importance of domain size, domain purity and domain interfaces to the performance of bulk-heterojunction organic photovoltaics. Energy Environ. Sci. 2012, 5, 7657–7663. [Google Scholar] [CrossRef] [Green Version]
- Darling, S.B.; You, F. The case for organic photovoltaics. RSC Adv. 2013, 3, 17633–17648. [Google Scholar] [CrossRef]
- Hoppe, H.; Sariciftci, N.S. Organic solar cells: An overview. J. Mater. Res. 2004, 19, 1924–1945. [Google Scholar] [CrossRef]
- Servaites, J.D.; Ratner, M.A.; Marks, T.J. Organic solar cells: A new look at traditional models. Energy Environ. Sci. 2011, 4, 4410–4422. [Google Scholar] [CrossRef]
- Walker, B.; Kim, C.; Nguyen, T.-Q. Small molecule solution—Processed bulk heterojunction solar cells. Chem. Mater. 2011, 23, 470–482. [Google Scholar] [CrossRef]
- Krebs, F.C. Fabrication and processing of polymer solar cells: A review of printing and coating techniques. Sol. Energy Mater. Sol. Cells 2009, 93, 394–412. [Google Scholar] [CrossRef]
- Krebs, F.C.; Tromholt, T.; Jørgensen, M. Upscaling of polymer solar cell fabrication using full roll-to-roll processing. Nanoscale 2010, 2, 873–886. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Xiao, B.; Liu, F.; Wu, H.; Yang, Y.; Xiao, S.; Wang, C.; Russell, T.P.; Cao, Y. Single-junction polymer solar cells with high efficiency and photovoltage. Nat. Photonics 2015, 9, 174–179. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, J.; Li, Z.; Mu, C.; Ma, W.; Hu, H.; Jiang, K.; Lin, H.; Ade, H.; Yan, H. Aggregation and morphology control enables multiple cases of high-efficiency polymer solar cells. Nat. Commun. 2014, 5, 5293. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Chang, W.H.; Yoshimura, K.; Ohya, K.; You, J.; Gao, J.; Hong, Z.; Yang, Y. An efficient triple-junction polymer solar cell having a power conversion efficiency exceeding 11%. Adv. Mater. 2014, 26, 5670–5677. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Saunders, B.R. Third-generation solar cells: A review and comparison of polymer: Fullerene, hybrid polymer and perovskite solar cells. RSC Adv. 2014, 4, 43286–43314. [Google Scholar] [CrossRef]
- Nagarjuna, G.; Venkataraman, D. Strategies for controlling the active layer morphologies in OPVs. J. Polym. Sci. B 2012, 50, 1045–1056. [Google Scholar] [CrossRef]
- Alam, M.A.; Ray, B.; Khan, M.R.; Dongaonkar, S. The essence and efficiency limits of bulk-heterostructure organic solar cells: A polymer-to-panel perspective. J. Mater. Res. 2013, 28, 541–557. [Google Scholar] [CrossRef]
- Müller-Buschbaum, P. The active layer morphology of organic solar cells probed with grazing incidence scattering techniques. Adv. Mater. 2014, 26, 7692–7709. [Google Scholar] [CrossRef] [PubMed]
- Tsoi, W.C.; Nicholson, P.G.; Kim, J.S.; Roy, D.; Burnett, T.L.; Murphy, C.E.; Nelson, J.; Bradley, D.D.C.; Kim, J.-S.; Castro, F.A. Surface and subsurface morphology of operating nanowire: Fullerene solar cells revealed by photoconductive-AFM. Energy Environ. Sci. 2011, 4, 3646–3651. [Google Scholar] [CrossRef]
- Vakhshouri, K.; Kesava, S.V.; Kozub, D.R.; Gomez, E.D. Characterization of the mesoscopic structure in the photoactive layer of organic solar cells: A focused review. Mater. Lett. 2013, 90, 97–102. [Google Scholar] [CrossRef]
- Giridharagopal, R.; Ginger, D.S. Characterizing morphology in bulk heterojunction organic photovoltaic systems. J. Phys. Chem. Lett. 2010, 1, 1160–1169. [Google Scholar] [CrossRef]
- DeLongchamp, D.M.; Kline, R.J.; Herzing, A. Nanoscale structure measurements for polymer-fullerene photovoltaics. Energy Environ. Sci. 2012, 5, 5980–5993. [Google Scholar] [CrossRef]
- Brady, M.A.; Su, G.M.; Chabinyc, M.L. Recent progress in the morphology of bulk heterojunction photovoltaics. Soft Matter 2011, 7, 11065–11077. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, H.; Yuan, S.; Lee, Y.; Gan, L.; Yu, L. Conjugated block copolymers and co-oligomers: From supramolecular assembly to molecular electronics. J. Mater. Chem. 2007, 17, 2183–2194. [Google Scholar] [CrossRef]
- Babu, S.S.; Möhwald, H.; Nakanishi, T. Recent progress in morphology control of supramolecular fullerene assemblies and its applications. Chem. Soc. Rev. 2010, 39, 4021–4035. [Google Scholar] [CrossRef] [PubMed]
- Hoeben, F.J.M.; Jonkheijm, P.; Meijer, E.W.; Schenning, A.P.H.J. About supramolecular assemblies of π-conjugated systems. Chem. Rev. 2005, 105, 1491–1546. [Google Scholar] [CrossRef] [PubMed]
- Ciesielski, A.; Palma, C.A.; Bonini, M.; Samori, P. Towards supramolecular engineering of functional nanomaterials: Pre-programming multi-component 2D self-assembly at solid-liquid interfaces. Adv. Mater. 2010, 22, 3506–3520. [Google Scholar] [CrossRef] [PubMed]
- Elemans, J.A.A.W.; Lei, S.; de Feyter, S. Molecular and supramolecular networks on surfaces: From two-dimensional crystal engineering to reactivity. Angew. Chem. Int. Ed. 2009, 48, 7298–7333. [Google Scholar] [CrossRef] [PubMed]
- Kondratuk, D.V.; Perdigão, L.M.A.; Esmail, A.M.S.; O’Shea, J.N.; Beton, P.H.; Anderson, H.L. Supramolecular nesting of cyclic polymers. Nat. Chem. 2015, 7, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Bruni, F.; Sassi, M.; Campione, M.; Giovanella, U.; Ruffo, R.; Luzzati, S.; Meinardi, F.; Beverina, L.; Brovelli, S. Post-deposition activation of latent hydrogen-bonding: A new paradigm for enhancing the performances of bulk heterojunction solar cells. Adv. Funct. Mater. 2014, 24, 7410–7419. [Google Scholar] [CrossRef]
- Kim, F.S.; Ren, G.; Jenekhe, S.A. One-dimensional nanostructures of π-conjugated molecular systems: Assembly, properties, and applications from photovoltaics, sensors, and nanophotonics to nanoelectronics. Chem. Mater. 2011, 23, 682–732. [Google Scholar] [CrossRef]
- Yu, G.; Gao, J.; Hummelen, J.C.; Wudl, F.; Heeger, A.J. Polymer photovoltaic cells: Enhanced efficiencies via a network of internal donor–acceptor heterojunctions. Science 1995, 270, 1789–1791. [Google Scholar] [CrossRef]
- Collins, B.A.; Gann, E.; Guignard, L.; He, X.; McNeill, C.R.; Ade, H. Molecular miscibility of polymer-fullerene blends. J. Phys. Chem. Lett. 2010, 1, 3160–3166. [Google Scholar] [CrossRef]
- Treat, N.D.; Varotto, A.; Takacs, C.J.; Batara, N.; Al-Hashimi, M.; Heeney, M.J.; Heeger, A.J.; Wudl, F.; Hawker, C.J.; Chabinyc, M.L. Polymer-fullerene miscibility: A metric for screening new materials for high-performance organic solar cells. J. Am. Chem. Soc. 2012, 134, 15869–15879. [Google Scholar] [CrossRef] [PubMed]
- Miller, N.C.; Cho, E.; Gysel, R.; Risko, C.; Coropceanu, V.; Miller, C.E.; Sweetnam, S.; Sellinger, A.; Heeney, M.; McCulloch, I.; et al. Factors governing intercalation of fullerenes and other small molecules between the side chains of semiconducting polymers used in solar cells. Adv. Energy Mater. 2012, 2, 1208–1217. [Google Scholar] [CrossRef]
- Bartelt, J.A.; Beiley, Z.M.; Hoke, E.T.; Mateker, W.R.; Douglas, J.D.; Collins, B.A.; Tumbleston, J.R.; Graham, K.R.; Amassian, A.; Ade, H.; et al. The importance of fullerene percolation in the mixed regions of polymer–fullerene bulk heterojunction solar cells. Adv. Energy Mater. 2013, 3, 364–374. [Google Scholar] [CrossRef]
- Coropceanu, V.; Cornil, J.; da Silva Filho, D.A.; Olivier, Y.; Silbey, R.; Bredas, J.-L. Charge transport in organic semiconductors. Chem. Rev. 2007, 107, 926–952. [Google Scholar] [CrossRef] [PubMed]
- Mas-Torrent, M.; Rovira, C. Role of molecular order and solid-state structure in organic field-effect transistors. Chem. Rev. 2011, 111, 4833–4856. [Google Scholar] [CrossRef] [PubMed]
- Mativetsky, J.M.; Wang, H.; Lee, S.S.; Whittaker-Brooks, L.; Loo, Y.-L. Face-on stacking and enhanced out-of-plane hole mobility in graphene-templated copper phthalocyanine. Chem. Commun. 2014, 50, 5319–5321. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Wu, S.-X.; Li, H.-B.; Tang, X.-D.; Wu, Y.; Su, Z.-M.; Liao, Y. A theoretical discussion on the relationships among molecular packings, intermolecular interactions, and electron transport properties for naphthalene tetracarboxylic diimide derivatives. J. Mater. Chem. 2011, 21, 15558–15566. [Google Scholar] [CrossRef]
- Olivier, Y.; Lemaur, V.; Brédas, J.L.; Cornil, J. Charge hopping in organic semiconductors: Influence of molecular parameters on macroscopic mobilities in model one-dimensional stacks. J. Phys. Chem. A 2006, 110, 6356–6364. [Google Scholar] [CrossRef] [PubMed]
- Facchetti, A. π-Conjugated polymers for organic electronics and photovoltaic cell applications. Chem. Mater. 2011, 23, 733–758. [Google Scholar] [CrossRef]
- Gershenson, M.E.; Podzorov, V.; Morpurgo, A.F. Colloquium: Electronic transport in single-crystal organic transistors. Rev. Mod. Phys. 2006, 78, 973–989. [Google Scholar] [CrossRef]
- Pfattner, R.; Mas-Torrent, M.; Bilotti, I.; Brillante, A.; Milita, S.; Liscio, F.; Biscarini, F.; Marszalek, T.; Ulanski, J.; Nosal, A.; et al. High-performance single crystal organic field-effect transistors based on two dithiophene-tetrathiafulvalene (DT-TTF) polymorphs. Adv. Mater. 2010, 22, 4198–4203. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Mativetsky, J.M.; Loth, M.A.; Anthony, J.E.; Loo, Y.L. Quantifying resistances across nanoscale low- and high-angle interspherulite boundaries in solution-processed organic semiconductor thin films. ACS Nano 2012, 6, 9879–9886. [Google Scholar] [CrossRef] [PubMed]
- Hiszpanski, A.M.; Baur, R.M.; Kim, B.; Tremblay, N.J.; Nuckolls, C.; Woll, A.R.; Loo, Y. Tuning polymorphism and orientation in organic semiconductor thin films via post-deposition processing. J. Am. Chem. Soc. 2014, 136, 15749–15756. [Google Scholar] [CrossRef] [PubMed]
- Etxebarria, I.; Ajuria, J.; Pacios, R. Polymer: Fullerene solar cells: Materials, processing issues, and cell layouts to reach power conversion efficiency over 10%, a review. J. Photonics Energy 2015, 5, 057214. [Google Scholar] [CrossRef]
- Marsh, R.A.; Groves, C.; Greenham, N.C. A microscopic model for the behavior of nanostructured organic photovoltaic devices. J. Appl. Phys. 2007, 101, 083509. [Google Scholar] [CrossRef] [Green Version]
- Gadisa, A.; Svensson, M.; Andersson, M.R.; Inganas, O. Correlation between oxidation potential and open-circuit voltage of composite solar cells based on blends of polythiophenes/fullerene derivative. Appl. Phys. Lett. 2004, 84, 1609–1611. [Google Scholar] [CrossRef]
- Brabec, C.J.; Cravino, A.; Meissner, D.; Serdar Sariciftci, N.; Fromherz, T.; Rispens, M.T.; Sanchez, L.; Hummelen, J.C. Origin of the open circuit voltage of plastic solar cells. Adv. Funct. Mater. 2001, 11, 374–380. [Google Scholar] [CrossRef]
- Brabec, C.J.; Gowrisanker, S.; Halls, J.J.M.; Laird, D.; Jia, S.; Williams, S.P. Polymer-fullerene bulk-heterojunction solar cells. Adv. Mater. 2010, 22, 3839–3856. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.C.; Ho, C.C.; Chang, C.Y.; Jao, M.H.; Darling, S.B.; Su, W.F. Additives for morphology control in high-efficiency organic solar cells. Mater. Today 2013, 16, 326–336. [Google Scholar] [CrossRef]
- Chu, C.-W.; Yang, H.; Hou, W.-J.; Huang, J.; Li, G.; Yang, Y. Control of the nanoscale crystallinity and phase separation in polymer solar cells. Appl. Phys. Lett. 2008, 92, 103306. [Google Scholar] [CrossRef]
- Kim, D.H.; Lee, D.Y.; Lee, H.S.; Lee, W.H.; Kim, Y.H.; Han, J.I.; Cho, K. High-mobility organic transistors based on single-crystalline microribbons of triisopropylsilylethynyl pentacene via solution-phase self-assembly. Adv. Mater. 2007, 19, 678–682. [Google Scholar] [CrossRef]
- Reese, C.; Bao, Z. High-resolution measurement of the anisotropy of charge transport in single crystals. Adv. Mater. 2007, 19, 4535–4538. [Google Scholar] [CrossRef]
- Anthony, J.E.; Brooks, J.S.; Eaton, D.L.; Parkin, S.R. Functionalized pentacene: Improved electronic properties from control of solid-state order. J. Am. Chem. Soc. 2001, 123, 9482–9483. [Google Scholar] [CrossRef] [PubMed]
- Sundar, V.C.; Zaumseil, J.; Podzorov, V.; Menard, E.; Willett, R.L.; Someya, T.; Gershenson, M.E.; Rogers, J.A. Elastomeric transistor stamps : Transport in organic crystals. Science 2004, 303, 1644–1646. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Lee, J.H.; Park, J.H.; Shim, C.; Sim, M.; Cho, K. High-efficiency organic solar cells based on preformed poly(3-hexylthiophene) nanowires. Adv. Funct. Mater. 2011, 21, 480–486. [Google Scholar] [CrossRef]
- Jo, S.B.; Lee, W.H.; Qiu, L.; Cho, K. Polymer blends with semiconducting nanowires for organic electronics. J. Mater. Chem. 2012, 22, 4244–4260. [Google Scholar] [CrossRef]
- Ren, G.; Wu, P.-T.; Jenekhe, S.A. Solar cells based on block copolymer semiconductor nanowires: Effects of nanowire aspect ratio. ACS Nano 2011, 5, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Kim, M.; Jinnai, H.; Shin, T.J.; Kim, H.; Park, J.H.; Jo, S.B.; Cho, K. Organic solar cells based on three-dimensionally percolated polythiophene nanowires with enhanced charge transport. ACS Appl. Mater. Interfaces 2014, 6, 5640–5650. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Park, Y.; Lee, D.Y.; Lee, J.H.; Park, J.H.; Kim, J.K.; Cho, K. Poly(3-hexylthiophene) nanorods with aligned chain orientation for organic photovoltaics. Adv. Funct. Mater. 2010, 20, 540–545. [Google Scholar] [CrossRef]
- Ren, G.; Ahmed, E.; Jenekhe, S.A. Nanowires of oligothiophene-functionalized naphthalene diimides: Self assembly, morphology, and all-nanowire bulk heterojunction solar cells. J. Mater. Chem. 2012, 22, 24373–24379. [Google Scholar] [CrossRef]
- Briseno, A.L.; Mannsfeld, S.C.B.; Reese, C.; Hancock, J.M.; Xiong, Y.; Jenekhe, S.A.; Bao, Z.; Xia, Y. Perylenediimide nanowires and their use in fabricating field-effect transistors and complementary inverters. Nano Lett. 2007, 7, 2847–2853. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, J.-H.; Choi, H.H.; Park, J.H.; Jo, S.B.; Sim, M.; Kim, J.S.; Jinnai, H.; Park, Y.D.; Cho, K. Electrical performance of organic solar cells with additive-assisted vertical phase separation in the photoactive layer. Adv. Energy Mater. 2014, 4, 1300612. [Google Scholar] [CrossRef]
- Mativetsky, J.M.; Orgiu, E.; Lieberwirth, I.; Pisula, W.; Samorì, P. Charge transport over multiple length scales in supramolecular fiber transistors: Single fiber versus ensemble performance. Adv. Mater. 2014, 26, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Koster, L.J.A.; Mihailetchi, V.D.; Xie, H.; Blom, P.W.M. Origin of the light intensity dependence of the short-circuit current of polymer/fullerene solar cells. Appl. Phys. Lett. 2005, 87, 203502. [Google Scholar] [CrossRef]
- Mihailetchi, V.D.; Wildeman, J.; Blom, P.W.M. Space-charge limited photocurrent. Phys. Rev. Lett. 2005, 94, 126602. [Google Scholar] [CrossRef] [PubMed]
- Ooi, Z.E.; Chan, K.L.; Lombardo, C.J.; Dodabalapur, A. Analysis of photocurrents in lateral-geometry organic bulk heterojunction devices. Appl. Phys. Lett. 2012, 101, 053301. [Google Scholar] [CrossRef]
- Briseno, A.L.; Holcombe, T.W.; Boukai, A.I.; Garnett, E.C.; Shelton, S.W.; Fréchet, J.J.M.; Yang, P. Oligo- and polythiophene/ZnO hybrid nanowire solar cells. Nano Lett. 2010, 10, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Shin, J.W.; Lee, Y.B.; Cho, M.Y.; Lee, S.H.; Park, D.H.; Jang, D.K.; Lee, C.J.; Joo, J. Poly(3-hexylthiophene)/multiwalled carbon hybrid coaxial nanotubes: Nanoscale rectification and photovoltaic characteristics. ACS Nano 2010, 4, 4197–4205. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Park, J.H.; Kim, J.H.; Sung, J.H.; Jo, S.B.; Jo, M.-H.; Cho, K. Lateral organic solar cells with self-assembled semiconductor nanowires. Adv. Energy Mater. 2014, 5, 1401317. [Google Scholar] [CrossRef]
- Ren, G.; Ahmed, E.; Jenekhe, S.A. Non-fullerene acceptor-based bulk heterojunction polymer solar cells: Engineering the nanomorphology via processing additives. Adv. Energy Mater. 2011, 1, 946–953. [Google Scholar] [CrossRef]
- Anthony, J.E. Small-molecule, nonfullerene acceptors for polymer bulk heterojunction organic photovoltaics. Chem. Mater. 2011, 23, 583–590. [Google Scholar] [CrossRef]
- Huang, C.; Barlow, S.; Marder, S.R. Perylene-3,4,9,10-tetracarboxylic acid diimides: Synthesis, physical properties, and use in organic electronics. J. Org. Chem. 2011, 76, 2386–2407. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Brandt, R.G.; Gu, Z.; Wu, S.; Andersen, T.R.; Shi, M.; Yu, D.; Chen, H. The effect of molecular geometry on the photovoltaic property of diketopyrrolopyrrole based non-fullerene acceptors. Synth. Met. 2015, 203, 249–254. [Google Scholar] [CrossRef]
- Wu, J.; Ma, Y.; Wu, N.; Lin, Y.; Lin, J.; Wang, L.; Ma, C.-Q. 2,2-Dicyanovinyl-end-capped oligothiophenes as electron acceptor in solution processed bulk-heterojunction organic solar cells. Org. Electron. 2015, 23, 28–38. [Google Scholar] [CrossRef]
- Liu, W.; Shi, H.; Andersen, T.R.; Zawacka, N.K.; Cheng, P.; Bundgaard, E.; Shi, M.; Zhan, X.; Krebs, F.C.; Chen, H. Roll-coating fabrication of ITO-free flexible solar cells based on a non-fullerene small molecule acceptor. RSC Adv. 2015, 5, 36001–36006. [Google Scholar] [CrossRef]
- Sherman, J.B.; Purushothaman, B.; Parkin, S.R.; Kim, C.; Collins, S.; Anthony, J.; Nguyen, T.-Q.; Chabinyc, M.L. Role of crystallinity of non-fullerene acceptors in bulk heterojunctions. J. Mater. Chem. A 2015, 3, 9989–9998. [Google Scholar] [CrossRef]
- Abhijith, T.; Ameen, M.Y.; Reddy, V.S. Synthesis of PTCDI-C 8 one dimensional nanostructures for photovoltaic applications. IOP Conf. Ser. Mater. Sci. Eng. 2015, 73, 012052. [Google Scholar] [CrossRef]
- Savage, R.C.; Orgiu, E.; Mativetsky, J.M.; Pisula, W.; Schnitzler, T.; Eversloh, C.L.; Li, C.; Müllen, K.; Samorì, P. Charge transport in fibre-based perylene-diimide transistors: Effect of the alkyl substitution and processing technique. Nanoscale 2012, 4, 2387–2393. [Google Scholar] [CrossRef] [PubMed]
- Ostrick, J.R.; Dodabalapur, A.; Torsi, L.; Lovinger, A.J.; Kwock, E.W.; Miller, T.M.; Galvin, M.; Berggren, M.; Katz, H.E. Conductivity-type anisotropy in molecular solids. J. Appl. Phys. 1997, 81, 6804–6808. [Google Scholar] [CrossRef]
- Malenfant, P.R.L.; Dimitrakopoulos, C.D.; Gelorme, J.D.; Kosbar, L.L.; Graham, T.O.; Curioni, A.; Andreoni, W. N-type organic thin-film transistor with high field-effect mobility based on a N,N′-dialkyl-3,4,9,10-perylene tetracarboxylic diimide derivative. Appl. Phys. Lett. 2002, 80, 2517–2519. [Google Scholar] [CrossRef]
- Liscio, A.; de Luca, G.; Nolde, F.; Palermo, V.; Müllen, K.; Samorì, P. Photovoltaic charge generation visualized at the nanoscale: A proof of principle. J. Am. Chem. Soc. 2008, 130, 780–781. [Google Scholar] [CrossRef] [PubMed]
- Dittmer, J.J.; Lazzaroni, R.; Leclere, P.; Moretti, P.; Granstrom, M.; Petritsch, K.; Marseglia, E.A.; Friend, R.H.; Bredas, J.L.; Rost, H.; et al. Crystal network formation in organic solar cells. Sol. Energy Mater. Sol. Cells 2000, 61, 53–61. [Google Scholar] [CrossRef]
- Oh, J.H.; Liu, S.; Bao, Z.; Schmidt, R.; Würthner, F. Air-stable N-channel organic thin-film transistors with high field-effect mobility based on N,N′-bis(heptafluorobutyl)-3,4:9,10-perylene diimide. Appl. Phys. Lett. 2007, 91, 123–126. [Google Scholar] [CrossRef]
- Chen, H.Z.; Ling, M.M.; Mo, X.; Shi, M.M.; Wang, M.; Bao, Z. Air stable N-channel organic semiconductors for thin film transistors based on fluorinated derivatives of perylene diimides. Chem. Mater. 2007, 19, 816–824. [Google Scholar] [CrossRef]
- De Luca, G.; Treossi, E.; Liscio, A.; Mativetsky, J.M.; Scolaro, L.M.; Palermo, V.; Samorì, P. Solvent vapour annealing of organic thin films: Controlling the self-assembly of functional systems across multiple length scales. J. Mater. Chem. 2010, 20, 2493–2498. [Google Scholar] [CrossRef]
- Datar, A.; Oitker, R.; Zang, L. Surface-assisted one-dimensional self-assembly of a perylene based semiconductor molecule. Chem. Commun. 2006, 1649–1651. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, K.; Datar, A.; Naddo, T.; Huang, J.; Oitker, R.; Yen, M.; Zhao, J.; Zang, L. Effect of side-chain substituents on self-assembly of perylene diimide molecules: Morphology control. J. Am. Chem. Soc. 2006, 128, 7390–7398. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, K.; Datar, A.; Oitker, R.; Chen, H.; Zuo, J.; Zang, L. Nanobelt self-assembly from an organic N-type semiconductor: Propoxyethyl-PTCDI. J. Am. Chem. Soc. 2005, 127, 10496–10497. [Google Scholar] [CrossRef] [PubMed]
- Zang, L.; Che, Y.; Moore, J.S. One-dimensional self-assembly of planar π-conjugated molecules: Adaptable building blocks for organic nanodevices. Acc. Chem. Res. 2008, 41, 1596–1608. [Google Scholar] [CrossRef] [PubMed]
- Palermo, V.; Liscio, A.; Gentilini, D.; Nolde, F.; Müllen, K.; Samori, P. Scanning probe microscopy investigation of self-organized perylenetetracarboxdiimide nanostructures at surfaces: Structural and electronic properties. Small 2007, 3, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Engel, E.; Leo, K.; Hoffmann, M. Ultrafast relaxation and exciton-exciton annihilation in PTCDA thin films at high excitation densities. Chem. Phys. 2006, 325, 170–177. [Google Scholar] [CrossRef]
- Margulies, E.A.; Shoer, L.E.; Eaton, S.W.; Wasielewski, M.R. Excimer formation in cofacial and slip-stacked perylene-3,4:9,10-bis(dicarboximide) dimers on a redox-inactive triptycene scaffold. Phys. Chem. Chem. Phys. 2014, 16, 23735–23742. [Google Scholar] [CrossRef] [PubMed]
- Schubert, A.; Settels, V.; Liu, W.; Würthner, F.; Meier, C.; Fink, R.F.; Schindlbeck, S.; Lochbrunner, S.; Engels, B.; Engel, V. Ultrafast exciton self-trapping upon geometry deformation in perylene-based molecular aggregates. J. Phys. Chem. Lett. 2013, 4, 792–796. [Google Scholar] [CrossRef]
- Marciniak, H.; Li, X.Q.; Würthner, F.; Lochbrunner, S. One-dimensional exciton diffusion in perylene bisimide aggregates. J. Phys. Chem. A 2011, 115, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Bu, L.; Zhao, Y.; Xie, Z.; Geng, Y.; Wang, L. Controlled phase separation for efficient energy conversion in dye/polymer blend bulk heterojunction photovoltaic cells. Thin Solid Films 2009, 517, 4654–4657. [Google Scholar] [CrossRef]
- Li, J.; Dierschke, F.; Wu, J.; Grimsdale, A.C.; Müllen, K. Poly(2,7-carbazole) and perylene tetracarboxydiimide: A promising donor/acceptor pair for polymer solar cells. J. Mater. Chem. 2006, 16, 96–100. [Google Scholar] [CrossRef]
- Hartnett, P.E.; Timalsina, A.; Matte, H.S.S.R.; Zhou, N.; Guo, X.; Marks, T.J. Slip-stacked perylenediimides as an alternative strategy for high efficiency nonfullerene acceptors in organic photovoltaics. J. Am. Chem. Soc. 2014, 136, 16345–16356. [Google Scholar] [CrossRef] [PubMed]
- Rajaram, S.; Shivanna, R.; Kandappa, S.K.; Narayan, K.S. Nonplanar perylene diimides as potential alternatives to fullerenes in organic solar cells. J. Phys. Chem. Lett. 2012, 3, 2405–2408. [Google Scholar] [CrossRef]
- Shivanna, R.; Shoaee, S.; Dimitrov, S.; Kandappa, S.K.; Rajaram, S.; Durrant, J.R.; Narayan, K.S. Charge generation and transport in efficient organic bulk heterojunction solar cells with a perylene acceptor. Energy Environ. Sci. 2014, 7, 435–441. [Google Scholar] [CrossRef]
- Jiang, W.; Ye, L.; Li, X.; Xiao, C.; Tan, F.; Zhao, W.; Hou, J.; Wang, Z. Bay-linked perylene bisimides as promising non-fullerene acceptors for organic solar cells. Chem. Commun. 2014, 50, 1024–1026. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Li, C.-Z.; Chueh, C.-C.; Williams, S.T.; Jiang, W.; Wang, Z.H.; Yu, J.S.; Jen, A.K.-Y. Integrated molecular, interfacial, and device engineering towards high-performance non-fullerene based organic solar cells. Adv. Mater. 2014, 26, 5708–5714. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, Y.; Lin, H.; Liu, Y.; Jiang, K.; Mu, C.; Ma, T.; Lin Lai, J.Y.; Hu, H.; Yu, D.; et al. High-efficiency non-fullerene organic solar cells enabled by a difluorobenzothiadiazole-based donor polymer combined with a properly matched small molecule acceptor. Energy Environ. Sci. 2015, 8, 520–525. [Google Scholar] [CrossRef]
- Zhong, Y.; Trinh, M.T.; Chen, R.; Wang, W.; Khlyabich, P.P.; Kumar, B.; Xu, Q.; Nam, C.; Sfeir, M.Y.; Black, C.; et al. Efficient organic solar cells with helical perylene diimide electron acceptors. J. Am. Chem. Soc. 2014, 136, 15215–15221. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Peng, S.; Chen, Y. Cooperative assembly of pyrene-functionalized donor/acceptor blend for ordered nanomorphology by intermolecular noncovalent π–π interactions. ACS Appl. Mater. Interfaces 2014, 6, 8115–8123. [Google Scholar] [CrossRef] [PubMed]
- Beltrán, E.; Garzoni, M.; Feringán, B.; Vancheri, A.; Barberá, J.; Serrano, J.L.; Pavan, G.M.; Giménez, R.; Sierra, T. Self-organization of star-shaped columnar liquid crystals with a coaxial nanophase segregation revealed by a combined experimental and simulation approach. Chem. Commun. 2015, 51, 1811–1814. [Google Scholar] [CrossRef] [PubMed]
- Mativetsky, J.M.; Kastler, M.; Savage, R.C.; Gentilini, D.; Palma, M.; Pisula, W.; Müllen, K.; Samorì, P. Self-assembly of a donor–acceptor dyad across multiple length scales: Functional architectures for organic electronics. Adv. Funct. Mater. 2009, 19, 2486–2494. [Google Scholar] [CrossRef]
- Possamai, G.; Marcuz, S.; Mageini, M.; Menna, E.; Franco, L.; Ruzzi, M.; Ceola, S.; Corvaja, C.; Ridolfi, G.; Geri, A.; et al. Synthesis, photophysics, and photoresponse of fullerene-based azoaromatic dyads. Chem. A Eur. J. 2005, 11, 5765–5776. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, P.O.; Biniek, L.; Zaborova, E.; Heinrich, B.; Brinkmann, M.; Leclerc, N.; Méry, S. Perylenediimide-based donor–acceptor dyads and triads: Impact of molecular architecture on self-assembling properties. J. Am. Chem. Soc. 2014, 136, 5981–5992. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.B.; Allen, K.; Oh, S.J.; Lee, S.; Toney, M.F.; Kim, Y.S.; Kagan, C.R.; Nuckolls, C.; Loo, Y.L. Small-molecule thiophene-C60 dyads as compatibilizers in inverted polymer solar cells. Chem. Mater. 2010, 22, 5762–5773. [Google Scholar] [CrossRef]
- Nierengarten, J.F. Fullerene-(π-conjugated oligomer) dyads as active photovoltaic materials. Sol. Energy Mater. Sol. Cells 2004, 83, 187–199. [Google Scholar] [CrossRef]
- Rajaram, S.; Armstrong, P.B.; Bumjoon, J.K.; Fréchet, J.M.J. Effect of addition of a diblock copolymer on blend morphology and performance of poly(3-hexylthiophene):perylene diimide solar cells. Chem. Mater. 2009, 21, 1775–1777. [Google Scholar] [CrossRef]
- Sivula, K.; Ball, Z.T.; Watanabe, N.; Fréchet, J.M.J. Amphiphilic diblock copolymer compatibilizers and their effect on the morphology and performance of polythiophene: Fullerene solar cells. Adv. Mater. 2006, 18, 206–210. [Google Scholar] [CrossRef]
- Kamkar, D.A.; Wang, M.; Wudl, F.; Nguyen, T.-Q. Single nanowire OPV properties of a photoconductive AFM. ACS Nano 2012, 6, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.U.; Jung, J.W.; Emrick, T.; Russell, T.P.; Jo, W.H. Synthesis of C60-end capped P3HT and its application for high performance of P3HT/PCBM bulk heterojunction solar cells. J. Mater. Chem. 2010, 20, 3287–3294. [Google Scholar] [CrossRef]
- Zhang, Q.; Cirpan, A.; Russell, T.P.; Emrick, T. Donor–acceptor poly(thiophene-block-perylene diimide) copolymers: Synthesis and solar cell fabrication. Macromolecules 2009, 42, 1079–1082. [Google Scholar] [CrossRef]
- Miyanishi, S.; Zhang, Y.; Tajima, K.; Hashimoto, K. Fullerene attached all-semiconducting diblock copolymers for stable single-component polymer solar cells. Chem. Commun. 2010, 46, 6723–6725. [Google Scholar] [CrossRef] [PubMed]
- Leibler, L. Theory of microphase separation in block copolymers. Macromolecules 1980, 13, 1602–1617. [Google Scholar] [CrossRef]
- Topham, P.D.; Parnell, A.J.; Hiorns, R.C. Block copolymer strategies for solar cell technology. J. Polym. Sci. B 2011, 49, 1131–1156. [Google Scholar] [CrossRef]
- Matsen, M.W.; Bates, F.S. Unifying weak- and strong-segregation block copolymer theories. Macromolecules 1996, 29, 1091–1098. [Google Scholar] [CrossRef]
- Verduzco, R.; Botiz, I.; Pickel, D.L.; Kilbey, S.M.; Hong, K.; Dimasi, E.; Darling, S.B. Polythiophene-block-polyfluorene and polythiophene-blockpoly(fluorene-co-benzothiadiazole): Insights into the self-assembly of all-conjugated block copolymers. Macromolecules 2011, 44, 530–539. [Google Scholar] [CrossRef]
- Sommer, M.; Komber, H.; Huettner, S.; Mulherin, R.; Kohn, P.; Greenham, N.C.; Huck, W.T.S. Synthesis, purification, and characterization of well-defined all-conjugated diblock copolymers PF8TBT-b-P3HT. Macromolecules 2012, 45, 4142–4151. [Google Scholar] [CrossRef]
- Sommer, M.; Huettner, S.; Thelakkat, M. Donor–acceptor block copolymers for photovoltaic applications. J. Mater. Chem. 2010, 20, 10788–10797. [Google Scholar] [CrossRef]
- Guo, C.; Lin, Y.H.; Witman, M.D.; Smith, K.A.; Wang, C.; Hexemer, A.; Strzalka, J.; Gomez, E.D.; Verduzco, R. Conjugated block copolymer photovoltaics with near 3% efficiency through microphase separation. Nano Lett. 2013, 13, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.; Dodabalapur, A.; Lovinger, A.J. Soluble and processable regioregular poly(3-hexylthiophene) for thin film field-effect transistor applications with high mobility. Appl. Phys. Lett. 1996, 69, 4108–4110. [Google Scholar] [CrossRef]
- Kim, D.H.; Park, Y.D.; Jang, Y.; Yang, H.; Kim, Y.H.; Han, J.I.; Moon, D.G.; Park, S.; Chang, T.; Chang, C.; et al. Enhancement of field-effect mobility due to surface-mediated molecular ordering in regioregular polythiophene thin film transistors. Adv. Funct. Mater. 2005, 15, 77–82. [Google Scholar] [CrossRef]
- Yang, H.; Shin, T.J.; Yang, L.; Cho, K.; Ryu, C.Y.; Bao, Z. Effect of mesoscale crystalline structure on the field-effect mobility of regioregular poly(3-hexyl thiophene) in thin-film transistors. Adv. Funct. Mater. 2005, 15, 671–676. [Google Scholar] [CrossRef]
- Woo, C.H.; Thompson, B.C.; Kim, B.J.; Toney, M.F.; Fréchet, J.M.J. The influence of poly(3-hexylthiophene) regioregularity on fullerene-composite solar cell performance. J. Am. Chem. Soc. 2008, 130, 16324–16329. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.; Shin, M.; Park, S.; Lee, S.; Kim, H.; Kim, Y. All-polymer solar cells with bulk heterojunction nanolayers of chemically doped electron-donating and electron-accepting polymers. Phys. Chem. Chem. Phys. 2012, 14, 15046–15053. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.; Huang, Y.-S.; Huettner, S.; Sommer, M.; Brinkmann, M.; Mulherin, R.; Niedzialek, D.; Beljonne, D.; Clark, J.; Huck, W.T.S.; et al. Control of intrachain charge transfer in model systems for block copolymer photovoltaic materials. J. Am. Chem. Soc. 2013, 135, 5074–5083. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Carretero, A.; Aytun, T.; Bruns, C.J.; Newcomb, C.J.; Tsai, W.-W.; Stupp, S.I. Stepwise self-assembly to improve solar cell morphology. J. Mater. Chem. A 2013, 1, 11674–11681. [Google Scholar] [CrossRef]
- Aytun, T.; Barreda, L.; Ruiz-Carretero, A.; Lehrman, J.A.; Stupp, S.I. Improving solar cell efficiency through hydrogen bonding: A method for tuning active layer morphology. Chem. Mater. 2015, 27, 1201–1209. [Google Scholar] [CrossRef]
- Noriega, R.; Rivnay, J.; Vandewal, K.; Koch, F.P.V.; Stingelin, N.; Smith, P.; Toney, M.F.; Salleo, A. A general relationship between disorder, aggregation and charge transport in conjugated polymers. Nat. Mater. 2013, 12, 1038–1044. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, M.; Norrman, K.; Krebs, F.C. Stability/degradation of polymer solar cells. Sol. Energy Mater. Sol. Cells 2008, 92, 686–714. [Google Scholar] [CrossRef]
- Petersen, M.H.; Hagemann, O.; Nielsen, K.T.; Jørgensen, M.; Krebs, F.C. Low band gap poly-thienopyrazines for solar cells-Introducing the 11-thia-9,13-diaza-cyclopenta[b]triphenylenes. Sol. Energy Mater. Sol. Cells 2007, 91, 996–1009. [Google Scholar] [CrossRef]
- Krebs, F.C.; Spanggard, H.; Kjær, T.; Biancardo, M.; Alstrup, J. Large area plastic solar cell modules. Mater. Sci. Eng. B 2007, 138, 106–111. [Google Scholar] [CrossRef]
- Lin, Y.; Lim, J.A.; Wei, Q.; Stefan, C.B.; Briseno, A.L.; Watkins, J.J.; Mannsfeld, S.C.B.; Briseno, A.L.; Watkins, J.J. Cooperative assembly of hydrogen-bonded diblock copolythiophene/fullerene blends for photovoltaic devices with well-defined morphologies and enhanced stability. Chem. Mater. 2012, 24, 622–632. [Google Scholar] [CrossRef]
- Li, F.; Yager, K.G.; Dawson, N.M.; Yang, J.; Malloy, K.J.; Qin, Y. Complementary hydrogen bonding and block copolymer self-assembly in cooperation toward stable solar cells with tunable morphologies. Macromolecules 2013, 46, 9021–9031. [Google Scholar] [CrossRef]
- Li, F.; Yager, K.G.; Dawson, N.M.; Jiang, Y.B.; Malloy, K.J.; Qin, Y. Stable and controllable polymer/fullerene composite nanofibers through cooperative noncovalent interactions for organic photovoltaics. Chem. Mater. 2014, 26, 3747–3756. [Google Scholar] [CrossRef]
- Li, F.; Yager, K.G.; Dawson, N.M.; Jiang, Y.B.; Malloy, K.J.; Qin, Y. Nano-structuring polymer/fullerene composites through the interplay of conjugated polymer crystallization, block copolymer self-assembly and complementary hydrogen bonding interactions. Polym. Chem. 2015, 6, 721–731. [Google Scholar] [CrossRef]
- Hiszpanski, A.M.; Lee, S.S.; Wang, H.; Woll, A.R.; Nuckolls, C.; Loo, Y.L. Post-deposition processing methods to induce preferential orientation in contorted hexabenzocoronene thin films. ACS Nano 2013, 7, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.H.; Wang, J.; Du, G.T.; Shi, S.H.; Du, Z.J.; Fan, Z.Q.; Bian, J.M.; Wang, M.S. Organic solar cells with remarkable enhanced efficiency by using a CuI buffer to control the molecular orientation and modify the anode. Appl. Phys. Lett. 2010, 97, 083305. [Google Scholar] [CrossRef]
- Diao, Y.; Tee, B.C.-K.; Giri, G.; Xu, J.; Kim, D.H.; Becerril, H.A.; Stoltenberg, R.M.; Lee, T.H.; Xue, G.; Mannsfeld, S.C.B.; et al. Solution coating of large-area organic semiconductor thin films with aligned single-crystalline domains. Nat. Mater. 2013, 12, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Witte, G.; Wöll, C. Growth of aromatic molecules on solid substrates for applications in organic electronics. J. Mater. Res. 2004, 19, 1889–1916. [Google Scholar] [CrossRef]
- Piris, J.; Debije, M.G.; Stutzmann, N.; Laursen, B.W.; Pisula, W.; Watson, M.D.; Bjørnholm, T.; Müllen, K.; Warman, J.M. Aligned thin films of discotic hexabenzocoronenes: Anisotropy in the optical and charge transport properties. Adv. Funct. Mater. 2004, 14, 1053–1061. [Google Scholar] [CrossRef]
- Lee, J.U.; Jung, J.W.; Emrick, T.; Russell, T.P.; Jo, W.H. Morphology control of a polythiophene-fullerene bulk heterojunction for enhancement of the high-temperature stability of solar cell performance by a new donor–acceptor diblock copolymer. Nanotechnology 2010, 21, 105201. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Lee, J.K.; Heeger, A.J.; Wudl, F. Well-defined donor–acceptor rod–coil diblock copolymers based on P3HT containing C60: The morphology and role as a surfactant in bulk-heterojunction solar cells. J. Mater. Chem. 2009, 19, 5416–5423. [Google Scholar] [CrossRef] [Green Version]
- Jeong, S.; Kwon, Y.; Choi, B.D.; Ade, H.; Han, Y.S. Improved efficiency of bulk heterojunction poly(3-hexylthiophene):[6,6]-phenyl-C61-butyric acid methyl ester photovoltaic devices using discotic liquid crystal additives. Appl. Phys. Lett. 2010, 96, 2008–2011. [Google Scholar] [CrossRef]
- Paci, B.; Generosi, A.; Albertini, V.R.; Spyropoulos, G.D.; Stratakis, E.; Kymakis, E. Enhancement of photo/thermal stability of organic bulk heterojunction photovoltaic devices via gold nanoparticles doping of the active layer. Nanoscale 2012, 4, 7452–7459. [Google Scholar] [CrossRef] [PubMed]
- Nalwa, K.S.; Carr, J.A.; Mahadevapuram, R.C.; Kodali, H.K.; Bose, S.; Chen, Y.; Petrich, J.W.; Ganapathysubramanian, B.; Chaudhary, S. Enhanced charge separation in organic photovoltaic films doped with ferroelectric dipoles. Energy Environ. Sci. 2012, 5, 7042–7049. [Google Scholar] [CrossRef]
- Wu, C.-G.; Chiang, C.-H.; Han, H.-C. Manipulating the horizontal morphology and vertical distribution of the active layer in BHJ-PSC with a multi-functional solid organic additive. J. Mater. Chem. A 2014, 2, 5295–5303. [Google Scholar] [CrossRef]
- Campoy-Quiles, M.; Ferenczi, T.; Agostinelli, T.; Etchegoin, P.G.; Kim, Y.; Anthopoulos, T.D.; Stavrinou, P.N.; Bradley, D.D.C.; Nelson, J. Morphology evolution via self-organization and lateral and vertical diffusion in polymer: Fullerene solar cell blends. Nat. Mater. 2008, 7, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Germack, D.S.; Chan, C.K.; Hamadani, B.H.; Richter, L.J.; Fischer, D.A.; Gundlach, D.J.; Delongchamp, D.M. Substrate-dependent interface composition and charge transport in films for organic photovoltaics. Appl. Phys. Lett. 2009, 94, 233303. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, L.M.; Yang, G.; Huang, C.H.; Hou, J.; Wu, Y.; Li, G.; Hsu, C.S.; Yang, Y. Vertical phase separation in poly(3-hexylthiophene): Fullerene derivative blends and its advantage for inverted structure solar cells. Adv. Funct. Mater. 2009, 19, 1227–1234. [Google Scholar] [CrossRef]
- Wang, H.; Gomez, E.D.; Kim, J.; Guan, Z.; Jaye, C.; Fischer, D.A.; Kahn, A.; Loo, Y. Device characteristics of bulk-heterojunction polymer solar cells are independent of interfacial segregation of active layers. Chem. Mater. 2011, 23, 2020–2023. [Google Scholar] [CrossRef]
- Tremblay, N.J.; Gorodetsky, A.A.; Cox, M.P.; Schiros, T.; Kim, B.; Steiner, R.; Bullard, Z.; Sattler, A.; So, W.Y.; Itoh, Y.; et al. Photovoltaic universal joints: Ball-and-socket interfaces in molecular photovoltaic cells. Chem. Phys. Chem. 2010, 11, 799–803. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.J.; Kim, J.B.; Chiu, C.Y.; Ahn, S.; Schiros, T.; Lee, S.S.; Yager, K.G.; Toney, M.F.; Loo, Y.L.; Nuckolls, C. A supramolecular complex in small-molecule solar cells based on contorted aromatic molecules. Angew. Chem. Int. Ed. 2012, 51, 8594–8597. [Google Scholar] [CrossRef] [PubMed]
- Zabula, A.V.; Sevryugina, Y.V.; Spisak, S.N.; Kobryn, L.; Sygula, R.; Sygula, A.; Petrukhina, M.A. An unsolvated buckycatcher and its first dianion. Chem. Commun. 2014, 50, 2657–2659. [Google Scholar] [CrossRef] [PubMed]
- Filatov, A.S.; Ferguson, M.V.; Spisak, S.N.; Li, B.; Campana, C.F.; Petrukhina, M.A. Bowl-shaped polyarenes as concave–convex shape complementary hosts for C60- and C70-fullerenes. Cryst. Growth Des. 2014, 14, 756–762. [Google Scholar] [CrossRef]
- Sygula, A.; Yanney, M.; Henry, W.P.; Fronczek, F.R.; Zabula, A.V.; Petrukhina, M.A. Inclusion complexes and solvates of buckycatcher, a versatile molecular host with two corannulene pincers. Cryst. Growth Des. 2014, 14, 2633–2639. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haruk, A.M.; Mativetsky, J.M. Supramolecular Approaches to Nanoscale Morphological Control in Organic Solar Cells. Int. J. Mol. Sci. 2015, 16, 13381-13406. https://doi.org/10.3390/ijms160613381
Haruk AM, Mativetsky JM. Supramolecular Approaches to Nanoscale Morphological Control in Organic Solar Cells. International Journal of Molecular Sciences. 2015; 16(6):13381-13406. https://doi.org/10.3390/ijms160613381
Chicago/Turabian StyleHaruk, Alexander M., and Jeffrey M. Mativetsky. 2015. "Supramolecular Approaches to Nanoscale Morphological Control in Organic Solar Cells" International Journal of Molecular Sciences 16, no. 6: 13381-13406. https://doi.org/10.3390/ijms160613381