Phylogeography of Thlaspi arvense (Brassicaceae) in China Inferred from Chloroplast and Nuclear DNA Sequences and Ecological Niche Modeling
Abstract
:1. Introduction
2. Results
2.1. Sequence Variation of T. arvense cpDNA and ZIP
2.2. Population Demography and Phylogeographic Structure
| Code | Locality (All in China) | Long. | Lat. | Alt. | cpDNA | nDNA | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| (E) | (N) | (m) | Chloroplast Haplotypes (No.) | π | Hd | Nuclear Alleles (No.) | π | Hd | ||
| LZ | Bujiu, Tibet | 94°24ʹ | 29°28ʹ | 2985 | C1(2), C2(3) | 0.00115 | 0.600 | N1(5) | 0.00000 | 0.000 |
| MRK | Maerkang, Sichuan | 102°42ʹ | 31°46ʹ | 3180 | C1(3), C2(1), C6(4), C9(1), C10(1) | 0.00106 | 0.800 | N1(2), N3(8) | 0.00145 | 0.356 |
| LH | Luhuo, Sichuan | 100°43ʹ | 31°36ʹ | 3447 | C1(2), C2(3), C4(1), C5(1), C9(3) | 0.00162 | 0.844 | N1(2), N3(9) | 0.00133 | 0.327 |
| KJ * | Kajun, Sichuan | 98°27ʹ | 29°43ʹ | 3806 | C1(4), C6(4), C8(1) | 0.00037 | 0.667 | N1(1), N3(4) | 0.00091 | 0.222 |
| TB | Tuoba, Sichuan | 97°29ʹ | 31°22ʹ | 3751 | C1(2), C2(4), C5(1), C9(2), C11(1) | 0.00133 | 0.822 | N1(3), N3(7), N4(1) | 0.00198 | 0.564 |
| XN | Xining,Qinghai | 101°45ʹ | 36°38ʹ | 2245 | C1(2), C2(18), C5(3) | 0.00084 | 0.379 | N1(8), N3(11), N5(1) | 0.00214 | 0.563 |
| LT | Litang, Sichuan | 100°19ʹ | 29°52ʹ | 4045 | C1(2), C2(3), C4(2), C6(1), C9(2) | 0.00176 | 0.867 | N1(3), N3(7) | 0.00190 | 0.467 |
| KD | Kangding, Sichuan | 101°57ʹ | 29°57ʹ | 3180 | C1(3), C4(1), C5(1), C6(4), C7(1) | 0.00133 | 0.800 | N1(2), N3(5), N4(2) | 0.00204 | 0.667 |
| LL * | Lulang, Tibet | 94°43ʹ | 29°43ʹ | 3436 | C1(2), C2(6), C5(3) | 0.00147 | 0.655 | N1(6), N3(4), N4(1) | 0.00226 | 0.618 |
| NLM | Nyalam, Tibet | 85°58ʹ | 28°9ʹ | 3763 | C1(12), C2(1) | 0.00030 | 0.154 | N1(8) | 0.00000 | 0.000 |
| MR * | MangRe, Tibet | 89°44ʹ | 29°42ʹ | 4406 | C1(10) | 0.00000 | 0.000 | N1(10) | 0.00000 | 0.000 |
| JD * | Jiangda, Tibet | 93°4ʹ | 29°59ʹ | 3570 | C1(3), C2(3) | 0.00115 | 0.600 | N3(6) | 0.00000 | 0.000 |
| RT * | Ritu, Tibet | 79°38ʹ | 33°25ʹ | 4292 | C1(10), C5(7) | 0.00123 | 0.515 | N4(13) | 0.00000 | 0.000 |
| HF | Hefei, Anhui | 117°11ʹ | 31°52ʹ | 31 | C1(8), C2(12) | 0.00097 | 0.505 | N1(13) | 0.00000 | 0.000 |
| ZH | Zhanhai, Hebei | 115°24ʹ | 41°14ʹ | 1771 | C2(4), C9(6) | 0.00026 | 0.533 | N3(10) | 0.00000 | 0.000 |
| XA * | Xiʹan, Shannxi | 109°7ʹ | 34°1ʹ | 766 | C1(5), C2(5) | 0.00107 | 0.556 | N1(7) | 0.00000 | 0.000 |
| YL * | Yilan, Harbin | 129°34ʹ | 46°19ʹ | 101 | C1(19), C2(1) | 0.00019 | 0.100 | N1(17), N2(2) | 0.00041 | 0.199 |
| BX | Benxi, Liaoning | 123°47ʹ | 41°15ʹ | 284 | C1(1), C2(6), C3(3) | 0.00125 | 0.600 | N1(7), N2(2) | 0.00079 | 0.389 |
| FS | Fansi, Shanxi | 113°29ʹ | 39°3ʹ | 2063 | C1(7), C2(3) | 0.00090 | 0.467 | N1(3), N3(7) | 0.00190 | 0.467 |
| Total | C1~C11(224) | 0.00132 | 0.696 | N1~N6(210) | 0.00211 | 0.604 | ||||
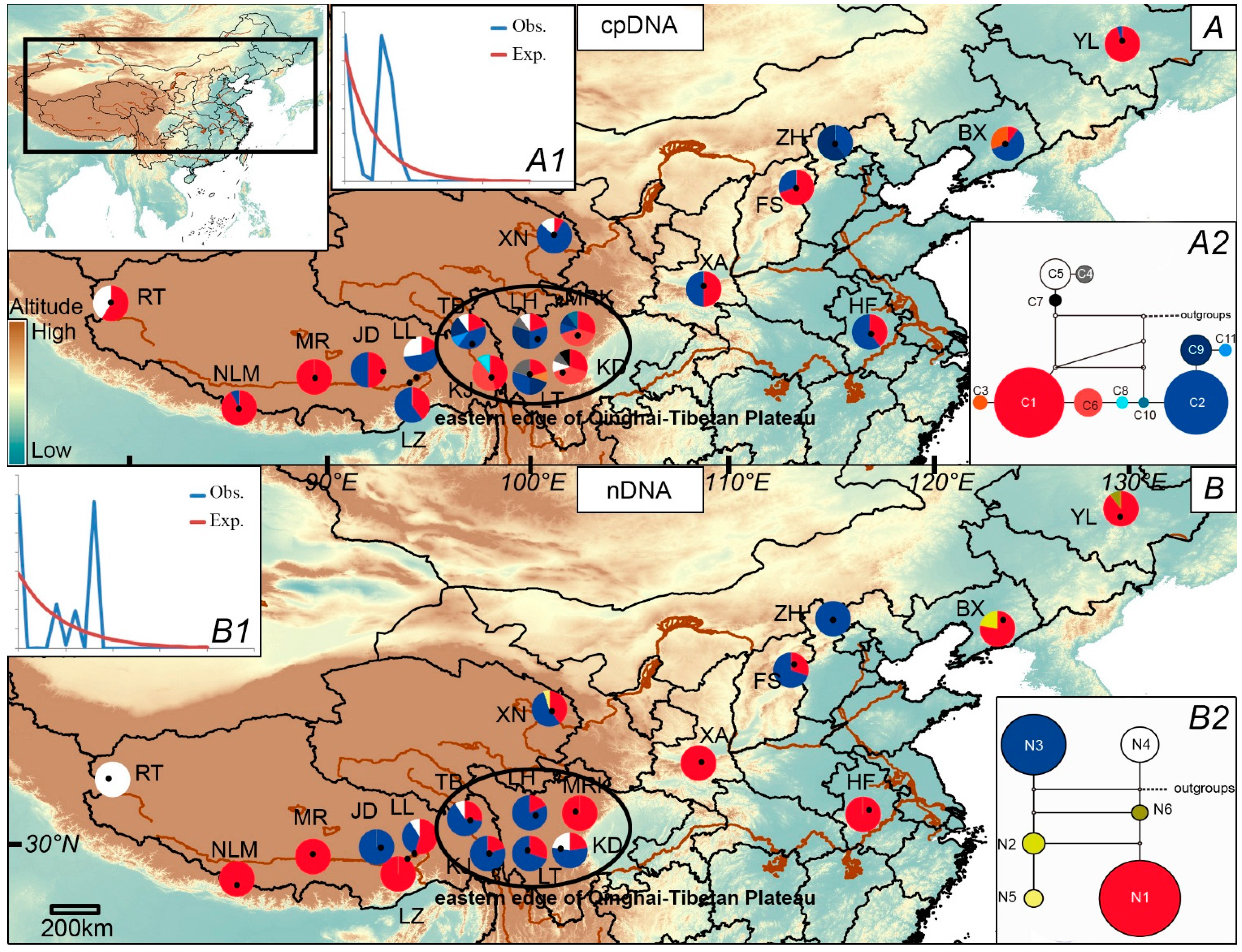
| NST | GST | hT | vT | π | Hd | |
|---|---|---|---|---|---|---|
| cpDNA | ||||||
| All populations | 0.285 | 0.235 | 0.719 | 0.721 | 0.00132 | 0.696 |
| Eastern edge of the QTP | 0.197 | 0.041 | 0.834 | 0.855 | 0.00151 | 0.831 |
| ZIP | ||||||
| All populations | 0.565 | 0.560 | 0.600 | 0.600 | 0.00211 | 0.604 |
| Eastern edge of the QTP | N/A | N/A | N/A | N/A | 0.00154 | 0.420 |
2.3. Phylogenetic Analyses and Divergence Time
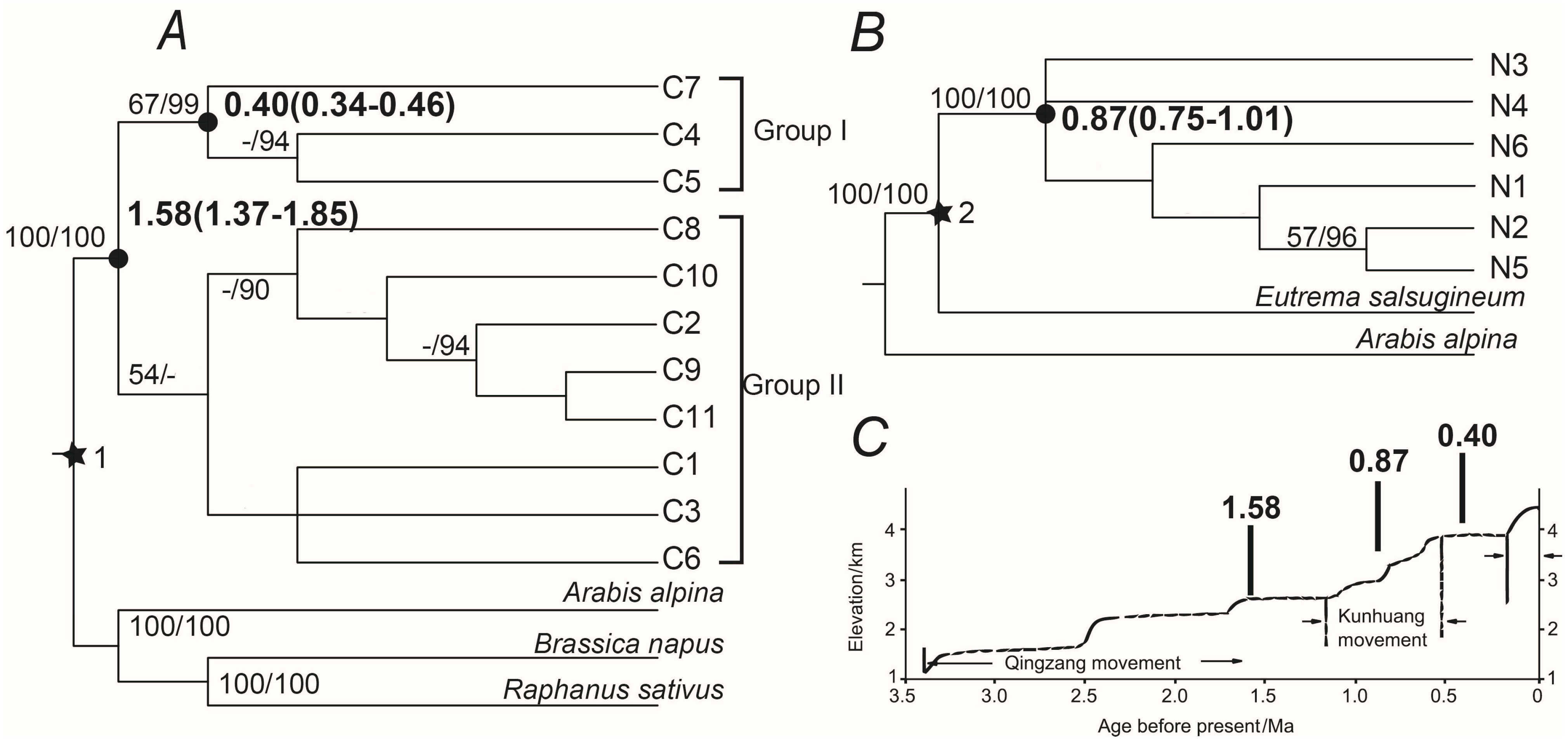
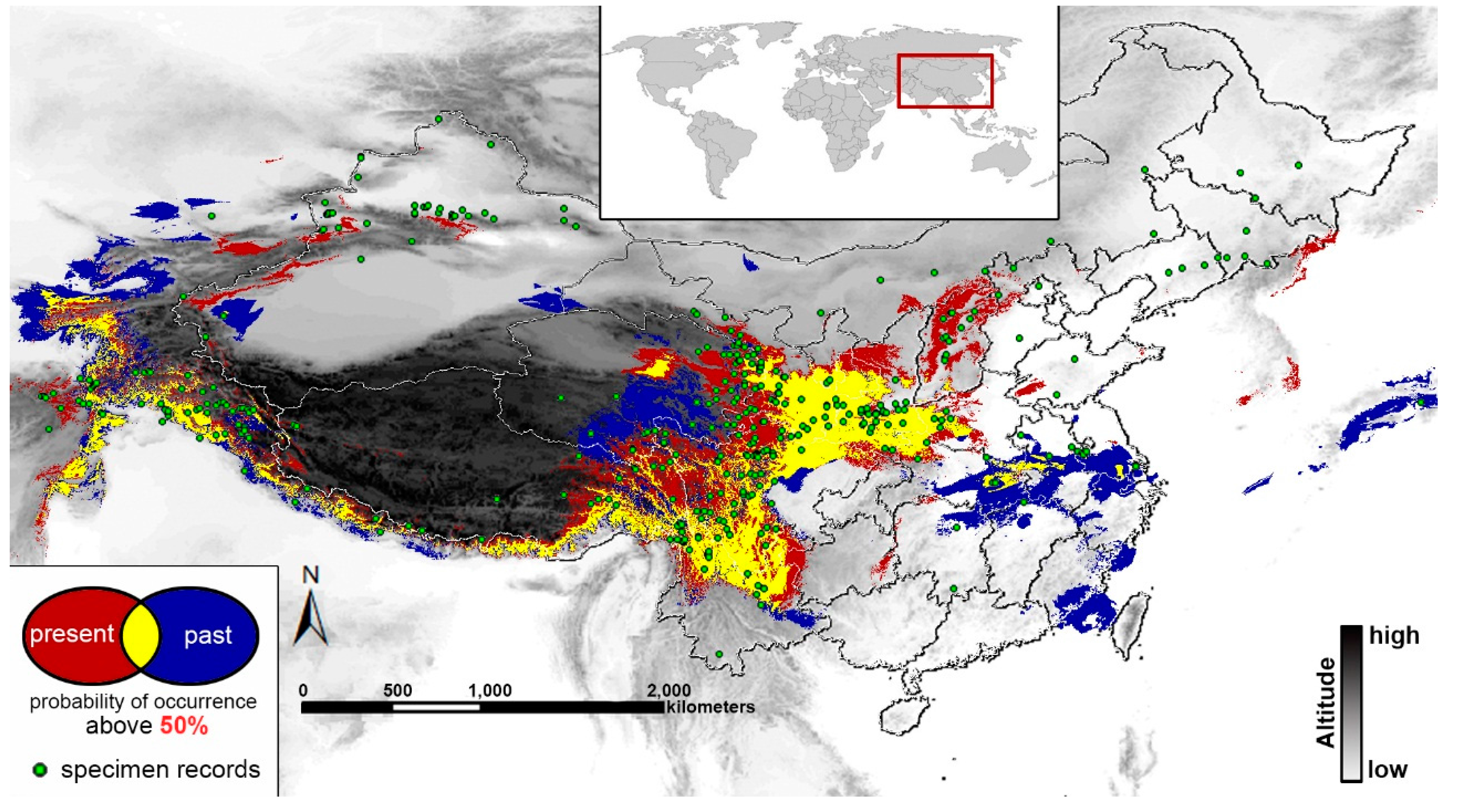
2.4. Past and Present Distributions
3. Discussion
3.1. Haplotype Divergence in T. arvense
3.2. Admixture Region of Diverged Haplotypes
3.3. Ecological Niche Modeling
4. Experimental Section
4.1. Population Sampling
4.2. Identification of Nuclear Marker
4.3. DNA Extraction, Amplification and Sequencing
4.4. Phylogenetic Analyses
4.5. Divergence Time Estimation
4.6. Population Genetic Diversity and Demography
4.7. Palaeo-Distribution Modeling
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Qiu, Y.; Fu, C.; Comes, H. Plant molecular phylogeography in China and adjacent regions: Tracing the genetic imprints of Quaternary climate and environmental change in the world’s most diverse temperate flora. Mol. Phylogenet. Evol. 2011, 59, 225–244. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; La, Q.; Sun, K.; Lu, F.; Wang, Y.; Song, Z.; Wu, Q.; Chen, J.; Zhang, W. Phylogeographic structure of Hippophae tibetana (Elaeagnaceae) highlights the highest microrefugia and the rapid uplift of the Qinghai-Tibetan Plateau. Mol. Ecol. 2010, 19, 2964–2979. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wen, S.; Zhang, Q.; Wang, F.; Zheng, B.; Li, B. A discussion on the period, amplitude and type of the uplift of the Qinghai-Xizang Plateau. Sci. Sin. 1979, 11, 1314–1328. [Google Scholar]
- Zhou, S.; Wang, X.; Wang, J.; Xu, L. A preliminary study on timing of the oldest Pleistocene glaciation in Qinghai-Tibetan Plateau. Quat. Int. 2006, 154, 44–51. [Google Scholar] [CrossRef]
- Shi, Y.; Li, J.; Li, B. Uplift of the Qinghai-Xizang (Tibetan) plateau and east Asia environmental change during late Cenozoic. Acta Geogr. Sin. Chin. Ed. 1999, 54, 20–28. [Google Scholar]
- Shi, Y. Uplift and Environmental Changes of Qinghai-Xizang (Tibetan) Plateau in the Late Cenozoic; Guangdong Science & Technology Press: Guangzhou, Guangdong, China, 1998. [Google Scholar]
- Liu, J.; Wang, Y.; Wang, A.; Hideaki, O.; Abbott, R. Radiation and diversification within the Ligularia-Cremanthodium-Parasenecio complex (Asteraceae) triggered by uplift of the Qinghai-Tibetan Plateau. Mol. Phylogenet. Evol. 2006, 38, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Wang, X.; Hong, D. Unexpected high divergence in nrDNA ITS and extensive parallelism in floral morphology of Pedicularis (Orobanchaceae). Plant Syst. Evol. 2003, 240, 91–105. [Google Scholar] [CrossRef]
- Xu, T.; Abbott, R.; Milne, R.; Mao, K.; Du, F.; Wu, G.; Ciren, Z.; Miehe, G.; Liu, J. Phylogeography and allopatric divergence of cypress species (Cupressus L.) in the Qinghai-Tibetan Plateau and adjacent regions. BMC Evol. Biol. 2010, 10, 194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Chiang, T.; George, M.; Liu, J.; Abbott, R. Phylogeography of the Qinghai-Tibetan Plateau endemic Juniperus przewalskii (Cupressaceae) inferred from chloroplast DNA sequence variation. Mol. Ecol. 2005, 14, 3513–3524. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Abbott, R.; Zheng, W.; Chen, P.; Wang, Y.; Liu, J. History and evolution of alpine plants endemic to the Qinghai-Tibetan Plateau: Aconitum gymnandrum (Ranunculaceae). Mol. Ecol. 2009, 18, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Best, K.; McIntyre, G. The biology of Canadian weeds: 9. Thlaspi arvense L. Can. J. Plant Sci. 1975, 55, 279–292. [Google Scholar] [CrossRef]
- Johnston, J.; Pepper, A.; Hall, A.; Chen, Z.; Hodnett, G.; Drabek, J.; Lopez, R.; Price, H. Evolution of genome size in Brassicaceae. Ann. Bot. Lond. 2005, 95, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Dorn, K.; Fankhauser, J.; Wyse, D.; Marks, M. De novo assembly of the pennycress (Thlaspi arvense) transcriptome provides tools for the development of a winter cover crop and biodiesel feedstock. Plant J. 2013, 75, 1028–1038. [Google Scholar] [CrossRef] [PubMed]
- Whiting, S.; Leake, J.; McGrath, S.; Baker, A. Hyperaccumulation of Zn by Thlaspi caerulescens can ameliorate Zn toxicity in the rhizosphere of cocropped Thlaspi arvense. Environ. Sci. Technol. 2001, 35, 3237–3241. [Google Scholar] [CrossRef] [PubMed]
- Tolrà, R.; Pongrac, P.; Poschenrieder, C.; Vogel-Mikuš, K.; Regvar, M.; Barceló, J. Distinctive effects of cadmium on glucosinolate profiles in Cd hyperaccumulator Thlaspi praecox and non-hyperaccumulator Thlaspi arvense. Plant Soil 2006, 288, 333–341. [Google Scholar] [CrossRef]
- Hazebroek, J.; Metzger, J.; Mansager, E. Thermoinductive regulation of gibberellin metabolism in Thlaspi arvense L. (II. Cold induction of enzymes in gibberellin biosynthesis). Plant Physiol. 1993, 102, 547–552. [Google Scholar] [PubMed]
- Metzger, J. Role of gibberellins in the environmental control of stem growth in Thlaspi arvense L. Plant Physiol. 1985, 78, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Moser, B.; Knothe, G.; Vaughn, S.; Isbell, T. Production and evaluation of biodiesel from field Pennycress (Thlaspi arvense L.) oil. Energy Fuel 2009, 23, 4149–4155. [Google Scholar] [CrossRef]
- Sharma, N.; Cram, D.; Huebert, T.; Zhou, N.; Parkin, I. Exploiting the wild crucifer Thlaspi arvense to identify conserved and novel genes expressed during a plant’s response to cold stress. Plant Mol. Biol. 2007, 63, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Robinson, S.; Huebert, T.; Bate, N.; Parkin, I. Comparative genome organization reveals a single copy of CBF in the freezing tolerant crucifer Thlaspi arvense. Plant Mol. Biol. 2007, 65, 693–705. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, G.; Best, K. Studies on the flowering of Thlaspi arvense L. IV. Genetic and ecological differences between early- and late-flowering strains. Bot. Gaz. 1978, 139, 190–195. [Google Scholar]
- Hume, L.; Devine, M.; Shirriff, S. The influence of temperature upon physiological processes in early-flowering and late-flowering strains of Thlaspi arvense L. Int. J. Plant Sci. 1995, 156, 445–449. [Google Scholar] [CrossRef]
- Huang, C.; Hung, K.; Wang, W.; Ho, C.; Huang, C.; Hsu, T.; Osada, N.; Hwang, C.; Chiang, T. Evolutionary rates of commonly used nuclear and organelle markers of Arabidopsis relatives (Brassicaceae). Gene 2012, 499, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, E.; Wen, J. Reprint of: Using nuclear gene data for plant phylogenetics: Progress and prospects. Mol. Phylogenet. Evol. 2013, 66, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Alvarado-Serrano, D.; Knowles, L. Ecological niche models in phylogeographic studies: Applications, advances and precautions. Mol. Ecol. Resour. 2014, 14, 233–248. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.; Anderson, R.; Schapire, R. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Warren, D.; Seifert, S. Ecological niche modeling in Maxent: The importance of model complexity and the performance of model selection criteria. Ecol. Appl. 2011, 21, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Pons, O.; Petit, R. Measuring and testing genetic differentiation with ordered vs. unordered alleles. Genetics 1996, 144, 1237–1245. [Google Scholar] [PubMed]
- Beilstein, M.; Nagalingum, N.; Clements, M.; Manchester, S.; Mathews, S. Dated molecular phylogenies indicate a Miocene origin for Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2010, 107, 18724–18728. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Fang, X. Uplift of the Tibetan Plateau and environmental changes. Chin. Sci. Bull. 1999, 44, 2117–2124. [Google Scholar] [CrossRef]
- Xue, B.; Wang, S.; Xia, W.; Wu, J.; Wang, Y.; Qian, J.; Hu, S.; Wu, Y.; Zhang, P. The uplifting and environmental change of Qinghai-Xizang (Tibetan) Plateau in the past 0.9 Ma inferred from core RM of Zoige Basin. Sci. China Ser. D: Earth Sci. 1998, 41, 165–170. [Google Scholar] [CrossRef]
- Bergsten, J. A review of long-branch attraction. Cladistics 2005, 21, 163–193. [Google Scholar] [CrossRef]
- Rota-Stabelli, O.; Telford, M.J. A multi criterion approach for the selection of optimal outgroups in phylogeny: Recovering some support for Mandibulata over Myriochelata using mitogenomics. Mol. Phylogenet. Evol. 2008, 48, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Petit, R.; Csaikl, U.; Bordács, S.; Burg, K.; Coart, E.; Cottrell, J.; van Dam, B.; Deans, J.; Dumolin-Lapègue, S.; Fineschi, S. Chloroplast DNA variation in European white oaks: Phylogeography and patterns of diversity based on data from over 2600 populations. For. Ecol. Manag. 2002, 156, 5–26. [Google Scholar] [CrossRef]
- Jadwiszczak, K.; Banaszek, A.; Jabłońska, E.; Sozinov, O. Chloroplast DNA variation of Betula humilis Schrk. in Poland and Belarus. Tree Genet. Genomes 2012, 8, 1017–1030. [Google Scholar] [CrossRef]
- Tajima, F. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 1989, 123, 585–595. [Google Scholar] [PubMed]
- Hedrick, P. Genetics of Populations, 3rd ed.; Jones and Bartlett Publishers: Sudbury, MA, USA, 2005. [Google Scholar]
- Nielsen, R. Molecular signatures of natural selection. Annu. Rev. Genet. 2005, 39, 197–218. [Google Scholar] [CrossRef] [PubMed]
- Koch, M.; Kiefer, C.; Ehrich, D.; Vogel, J.; Brochmann, C.; Mummenhoff, K. Three times out of Asia Minor: The phylogeography of Arabis alpina L. (Brassicaceae). Mol. Ecol. 2006, 15, 825–839. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Kang, D.; Ren, Y.; Qu, L.; Zhen, Y.; Gu, H. Genetic diversity of the natural populations of Arabidopsis thaliana in China. Heredity (Edinb) 2007, 99, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Yin, P.; Kang, J.; He, F.; Qu, L.; Gu, H. The origin of populations of Arabidopsis thaliana in China, based on the chloroplast DNA sequences. BMC Plant Biol. 2010, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Warwick, S.; Francis, A.; Susko, D. The biology of Canadian weeds. 9. Thlaspi arvense L. (updated). Can. J. Plant Sci. 2002, 82, 803–823. [Google Scholar] [CrossRef]
- Salisbury, S. Weeds and Aliens; Collins: London, UK, 1961. [Google Scholar]
- Petit, R.J.; Aguinagalde, I.; de Beaulieu, J.-L.; Bittkau, C.; Brewer, S.; Cheddadi, R.; Ennos, R.; Fineschi, S.; Grivet, D.; Lascoux, M. Glacial refugia: Hotspots but not melting pots of genetic diversity. Science 2003, 300, 1563–1565. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Li, Y.; Ding, X.; Wang, X. Extensive population expansion of Pedicularis longiflora (Orobanchaceae) on the Qinghai-Tibetan Plateau and its correlation with the Quaternary climate change. Mol. Ecol. 2008, 17, 5135–5145. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhai, S.; Qiu, Y.; Guo, Y.; Ge, X.; Comes, H. Glacial survival east and west of the “Mekong-Salween Divide” in the Himalaya-Hengduan Mountains region as revealed by AFLPs and cpDNA sequence variation in Sinopodophyllum hexandrum (Berberidaceae). Mol. Phylogenet. Evol. 2011, 59, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Cun, Y.; Wang, X. Plant recolonization in the Himalaya from the southeastern Qinghai-Tibetan Plateau: Geographical isolation contributed to high population differentiation. Mol. Phylogenet. Evol. 2010, 56, 972–982. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.; Yu, G.; Takahara, H.; Prentice, I. Palaeovegetation (Communications arising): Diversity of temperate plants in east Asia. Nature 2001, 413, 129–130. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.; Opgenoorth, L.; Holl, S.; Bastrop, R. Into the Himalayan exile: The phylogeography of the ground beetle Ethira clade supports the Tibetan origin of forest-dwelling Himalayan species groups. PLoS ONE 2012, 7, e45482. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, Z.; Bystriakova, N.; Ansell, S.; Xiang, Q.; Heinrichs, J.; Schneider, H.; Zhang, X. Phylogeography of the Sino-Himalayan fern Lepisorus clathratus on “the roof of the world”. PLoS ONE 2011, 6, e25896. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, G. The genetic legacy of the Quaternary ice ages. Nature 2000, 405, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Liu, K. Quaternary history of the temperate forests of China. Quat. Sci. Rev. 1988, 7, 1–20. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10. [Google Scholar] [CrossRef] [PubMed]
- Taberlet, P.; Gielly, L.; Pautou, G.; Bouvet, J. Universal primers for amplification of three non-coding regions of chloroplast DNA. Plant Mol. Biol. 1991, 17, 1105–1109. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.; Lickey, E.; Schilling, E.; Small, R. Comparison of whole chloroplast genome sequences to choose noncoding regions for phylogenetic studies in angiosperms: The tortoise and the hare III. Am. J. Bot. 2007, 94, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Small, R.; Ryburn, J.; Cronn, R.; Seelanan, T.; Wendel, J. The tortoise and the hare: Choosing between noncoding plastome and nuclear Adh sequences for phylogeny reconstruction in a recently diverged plant group. Am. J. Bot. 1998, 85, 1301–1315. [Google Scholar] [CrossRef] [PubMed]
- Larkin, M.; Blackshields, G.; Brown, N.; Chenna, R.; McGettigan, P.; McWilliam, H.; Valentin, F.; Wallace, I.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [PubMed]
- Librado, P.; Rozas, J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, A. RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 2006, 22, 2688–2690. [Google Scholar] [CrossRef] [PubMed]
- Huelsenbeck, J.; Ronquist, F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef] [PubMed]
- Swofford, D. PAUP 4.0: Phylogenetic Analysis Using Parsimony; Smithsonian Institution: Washington, DC, USA, 1998. [Google Scholar]
- Bandelt, H.; Forster, P.; Rohl, A. Median-joining networks for inferring intraspecific phylogenies. Mol. Biol. Evol. 1999, 16, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z. PAML: A program package for phylogenetic analysis by maximum likelihood. CABIOS 1997, 13, 555–556. [Google Scholar] [CrossRef] [PubMed]
- Excoffier, L.; Laval, G.; Schneider, S. Arlequin (version 3.0): An integrated software package for population genetics data analysis. Evol. Bioinform. Online 2005, 1, 47–50. [Google Scholar]
- Rogers, A.; Harpending, H. Population growth makes waves in the distribution of pairwise genetic differences. Mol. Biol. Evol. 1992, 9, 552–569. [Google Scholar] [PubMed]
- Hijmans, R.; Cameron, S.; Parra, J.; Jones, P.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, M.; Zeng, L.; Zhang, T.; Zhong, Y. Phylogeography of Thlaspi arvense (Brassicaceae) in China Inferred from Chloroplast and Nuclear DNA Sequences and Ecological Niche Modeling. Int. J. Mol. Sci. 2015, 16, 13339-13355. https://doi.org/10.3390/ijms160613339
An M, Zeng L, Zhang T, Zhong Y. Phylogeography of Thlaspi arvense (Brassicaceae) in China Inferred from Chloroplast and Nuclear DNA Sequences and Ecological Niche Modeling. International Journal of Molecular Sciences. 2015; 16(6):13339-13355. https://doi.org/10.3390/ijms160613339
Chicago/Turabian StyleAn, Miao, Liyan Zeng, Ticao Zhang, and Yang Zhong. 2015. "Phylogeography of Thlaspi arvense (Brassicaceae) in China Inferred from Chloroplast and Nuclear DNA Sequences and Ecological Niche Modeling" International Journal of Molecular Sciences 16, no. 6: 13339-13355. https://doi.org/10.3390/ijms160613339





