A New Treatment Strategy for Inactivating Algae in Ballast Water Based on Multi-Trial Injections of Chlorine
Abstract
:1. Introduction
2. Results and Discussion
2.1. One Trial Injection of Electrolytic Products
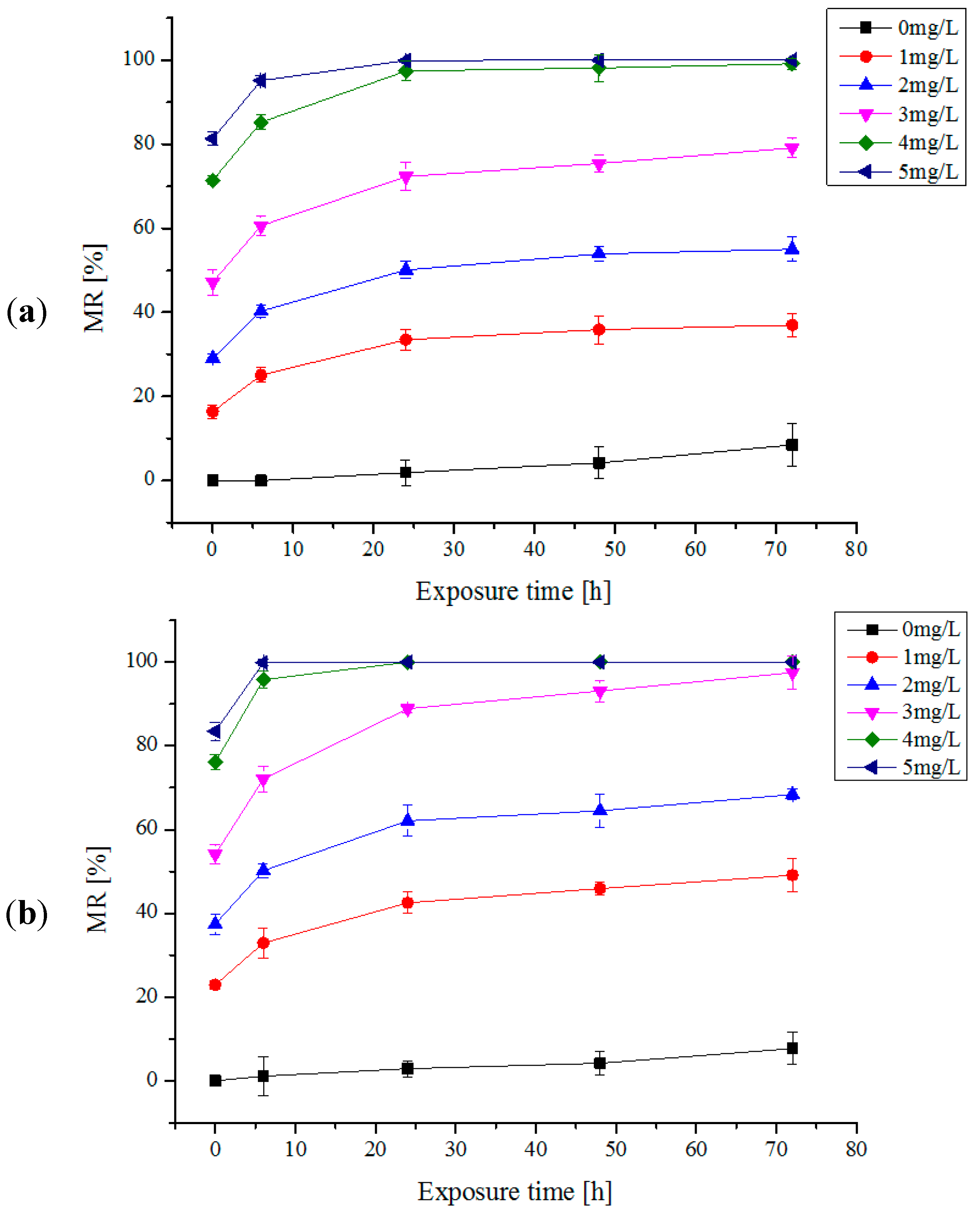

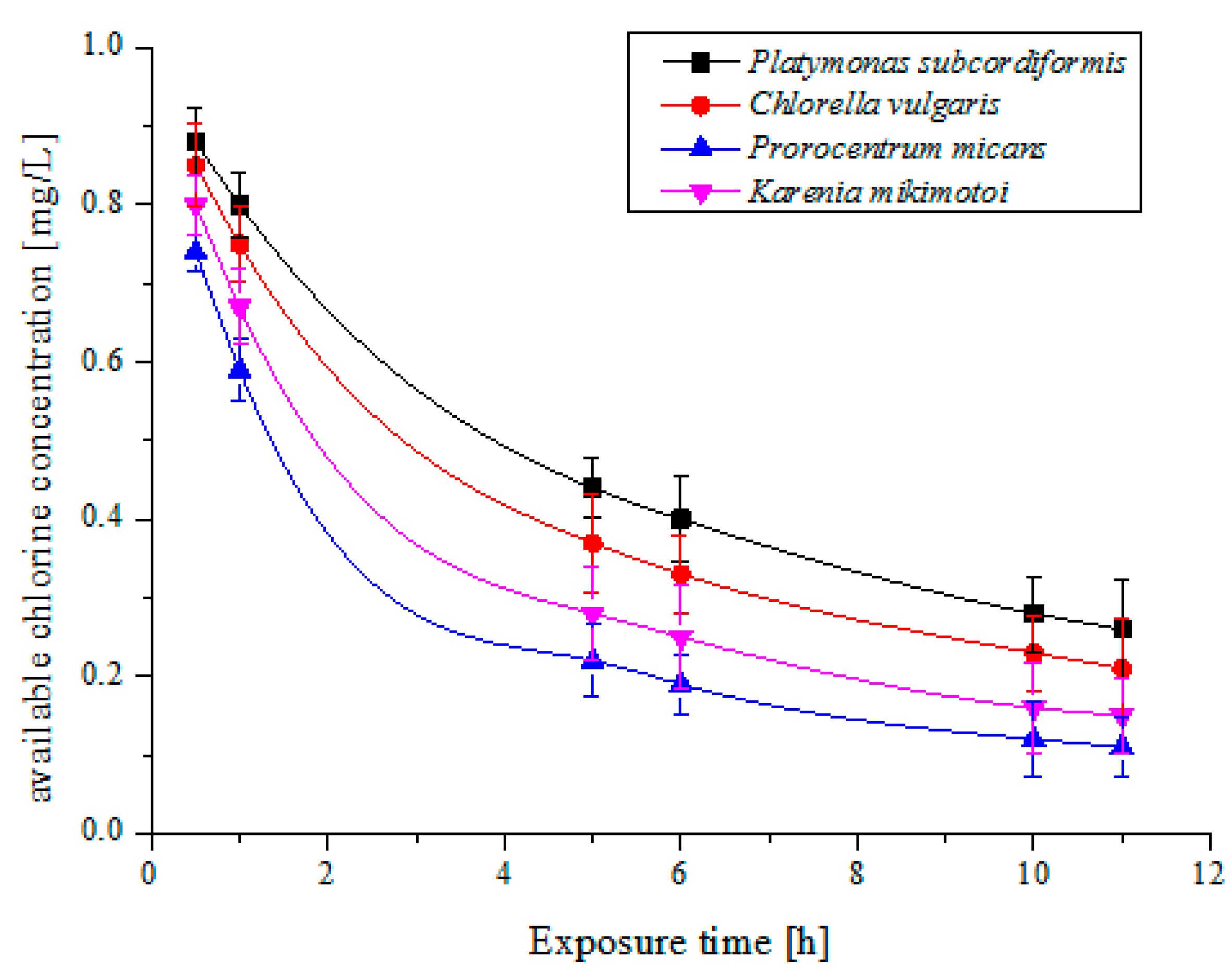
2.2. Multi-Trial Injection with a Small Dose of Electrolytic Products


| Species | Available Chlorine Concentration (mg/L) | Exposure Time (h) | Results | |||
|---|---|---|---|---|---|---|
| Conventional Experiment | Strategy Method | Conventional Experiment | Strategy Method | Saving Dose | Saving Time | |
| Platymonas subcordiformis | 5 | 3 | 48 | 11 | 40% | 77% |
| Chlorella vulgaris | 4 | 3 | 24 | 11 | 25% | 54% |
| Prorocentrum micans | 5 | 3 | 72 | 11 | 40% | 84% |
| Karenia mikimotoi | 4 | 3 | 24 | 11 | 25% | 54% |
2.3. Discussion

3. Experimental Section
3.1. Principle of Ion Exchange Membrane Electrolysis
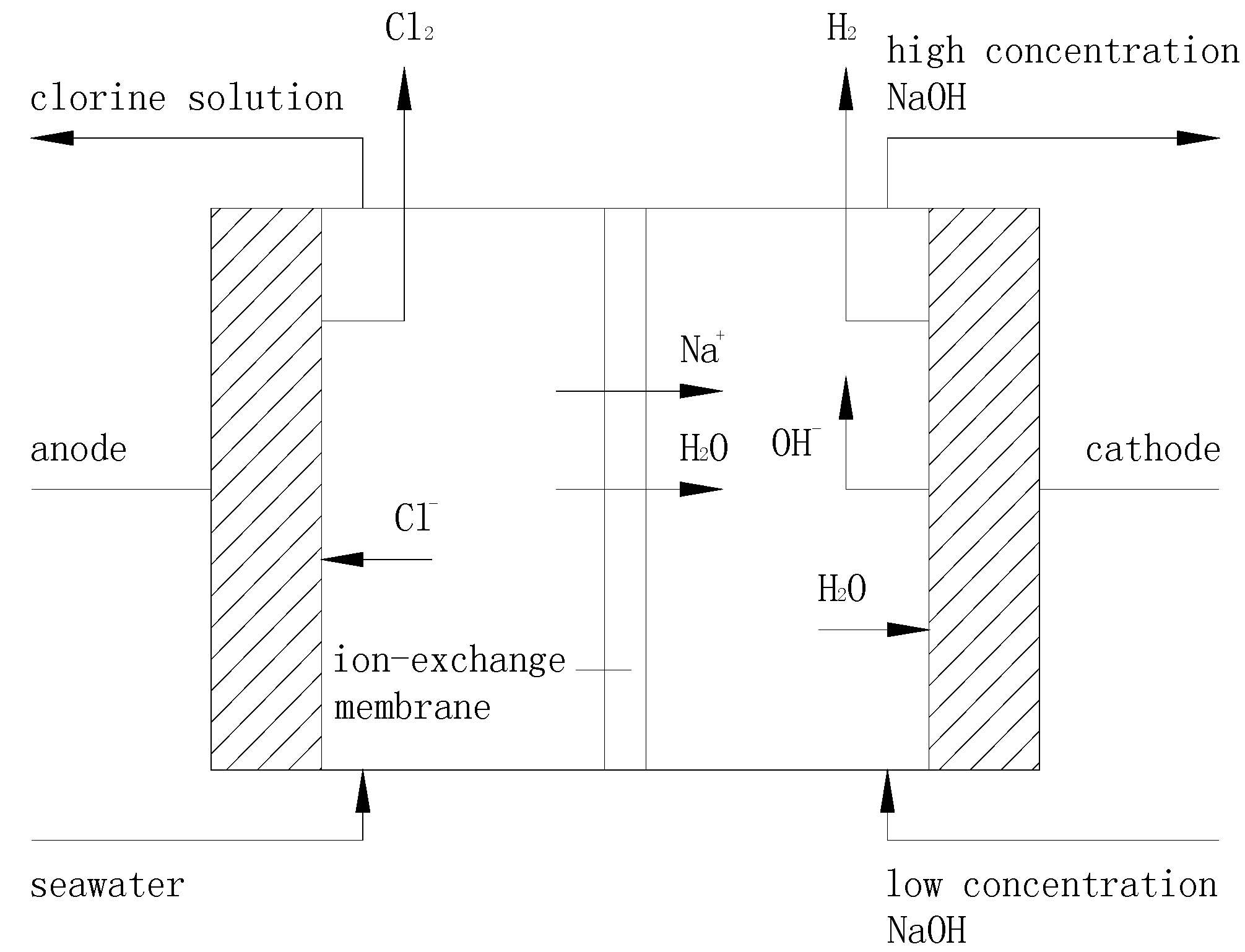
3.2. Setup and Procedures of the Ion Exchange Membrane Electrolysis System

| No. | Equipment Name | Size | Quantity |
|---|---|---|---|
| 1 | Electrolyzer | 400 × 500 × 110 (mm) | 1 |
| 2 | Magnetic drive pump | 3 m; 15 L/min | 2 |
| 3 | Magnetic circulation pump | 2.1 m; 5.5 L/min | 1 |
| 4 | Water storage tank | Stainless steel 720 × 500 × 600 (mm) | 1 |
| 5 | Cathode gas-liquid separator tank | Stainless steel 700 × 500 × 600 (mm) | 1 |
| 6 | Brine circulation tank | φ-600; h-600 (mm) | 1 |
| 7 | Anode gas-liquid separation tank | φ-600; h-600 (mm) | 1 |
| 8 | Glass rotor flowmeter | 6–60 L/h | 2 |
| 9 | U-shaped pressure gauge | 5000 Pa | 1 |
| 10 | Temperature control instrument | −49.9–149.9 °C | 2 |
| 11 | High frequency dc power supply | 380 V × 3 | 1 |
| 12 | Electric heater | 220 V; 2 kW | 2 |
| 13 | Pressure gauge | 0–0.25 Pa | 1 |
| 14 | Thermometer | 0–100 °C | 1 |
3.3. Algae Species Preparation
3.4. Algae Inactivation Evaluation and Treatments
3.4.1. Algae Inactivation Evaluation
3.4.2. Inactivation Algae Test by One Trial Injection of Electrolytic Products
3.4.3. Inactivation Algae Test by Multi-Trial Injection with Small Dose of Electrolytic Products
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nanayakkara, K.G.; Alam, A.K.; Zheng, Y.M.; Chen, P.J. Low-energy intensive electrochemical system for the eradication of Escherichia coli from ballast water: Process development, disinfection chemistry, and kinetics modeling. Mar. Pollut. Bull. 2012, 64, 1238–1245. [Google Scholar] [CrossRef] [PubMed]
- McCollin, T.; Shanks, A.M.; Dunn, J. Changes in zooplankton abundance and diversity after ballast water exchange in regional seas. Mar. Pollut. Bull. 2008, 56, 834–844. [Google Scholar] [CrossRef] [PubMed]
- Banerji, S.; Werschkun, B.; Höfer, T. Assessing the risk of ballast water treatment to human health. Regul. Toxicol. Pharmacol. 2012, 62, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.Z. Foreign about no ballast water ship exploration. Mar. Technol. 2005, 5, 64–66. [Google Scholar]
- Wang, J.S.; Sun, J.Y.; Song, Y.X.; Xu, Y.Y.; Pan, X.X.; Sun, Y.Q.; Li, D.Q. A label-free microfluidic biosensor for activity detection of single microalgae cells based on chlorophyll fluorescence. Sensors 2013, 13, 16075–16089. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.J.; Butkus, M.A.; Xie, Y.F. Enhanced performance of crumb rubber filtration for ballastwater treatment. Chemosphere 2009, 74, 1396–1399. [Google Scholar] [CrossRef] [PubMed]
- Nielson, R.J.; Moffitt, C.M.; Watten, B.J. Hydrocyclonic separation of invasive New Zealand mudsnails from an aquaculture water source. Aquaculture 2012, 326, 156–162. [Google Scholar] [CrossRef]
- Liebich, V.; Stehouwer, P.P.; Veldhuis, M. Re-growth of potential invasive phytoplankton following UV-based ballast water treatment. Aquat. Invasions 2012, 7, 29–36. [Google Scholar] [CrossRef]
- Wright, D.A.; Gensemer, R.W.; Mitchelmore, C.L.; Stubblefield, W.A.; Genderen, E.; Dawson, R.; Orano-Dawson, C.E.; Bearr, J.S.; Mueller, R.; Cooper, W.J. Shipboard trials of an ozone-based ballast water treatment system. Mar. Pollut. Bull. 2010, 60, 1571–1583. [Google Scholar] [CrossRef] [PubMed]
- Lacasa, E.; Tsolaki, E.; Sbokou, Z.; Rodrigo, M.A.; Mantzavinos, D.; Diamadopoulos, E. Electrochemical disinfection of simulated ballast water on conductive diamond electrodes. Chem. Eng. J. 2013, 223, 516–523. [Google Scholar]
- Carbona, S.; Viitasalo-Frösen, S.; Masson, D.; Sassi, J.; Pineau, S.; Lehtiniemi, M.; Corroler, D. Efficacy and environmental acceptability of two ballast water treatment chemicals and an alkylamine based-biocide. Sci. Total Environ. 2010, 409, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Holm, E.R.; Stampe, D.M.; Barnes, R.A.; Barnes, L.; Deamer, N.; Burkholder, J.M. Sonication of bacteria, phytoplankton and zooplankton: Application to treatment of ballast water. Mar. Pollut. Bull. 2008, 56, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Chatzisymeon, E.; Xekoukoulotakis, N.; Coz, A.; Kalogerakis, N.; Mantzavinos, D. Electrochemical treatment of textile dyes and dyehouse effluents. J. Hazard. Mater. 2006, 137, 998–1007. [Google Scholar] [CrossRef] [PubMed]
- Angala, A.; Urtiaga, A.; Ortiz, I. Pilot scale performance of the electro-oxidation of landfill leachate at boron-doped diamond anodes. Environ. Sci. Technol. 2009, 43, 2035–2040. [Google Scholar] [CrossRef]
- Kraft, A. Electrochemical water disinfection: A short review. Platin. Met. Rev. 2008, 52, 177–185. [Google Scholar] [CrossRef]
- Gómez-López, V.M.; Gobet, J.; Selma, M.V.; Gil, M.I.; Allende, A. Operating conditions for the electrolytic disinfection of process wash water from the fresh-cut industry contaminated with E. colio157: H7. Food Control 2013, 29, 42–48. [Google Scholar] [CrossRef]
- Sun, J.Y.; Wang, J.S.; Pan, X.X.; Yuan, H.C. An Investigation on a Novel Ballast Water Treatment Method and System Based on Ion-exchange Membrane Electrolysis. Indian J. Mar. Sci. 2015, in press. [Google Scholar]
- Pereira, N.N.; Brinati, H.L. Onshore ballast water treatment: A viable option for major ports. Mar. Pollut. Bull. 2012, 64, 2296–2304. [Google Scholar] [CrossRef] [PubMed]
- Christ, N.; Ferrantino, M.J. Land transport for export: The effects of cost, time, and uncertainty in sub-Saharan Africa. World Dev. 2011, 39, 1749–1759. [Google Scholar] [CrossRef]
- Qi, X.; Song, D.P. Minimizing fuel emissions by optimizing vessel schedules in liner shipping with uncertain port times. Transp. Res. E 2012, 48, 863–880. [Google Scholar] [CrossRef]
- Richardson, S.D.; Plewa, M.J.; Wagner, E.D.; Schoeny, R.; DeMarini, D.M. Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: A review and roadmap for research. Mutat. Res. 2007, 636, 178–242. [Google Scholar] [CrossRef] [PubMed]
- Gallard, H.; von Gunten, U. Chlorination of natural organic matter: Kinetics of chlorination and of THM formation. Water Res. 2002, 36, 65–74. [Google Scholar] [CrossRef]
- Krasner, S.W.; Weinberg, H.S.; Richardson, S.D.; Pastor, S.J.; Chinn, R.; Sclimenti, M.J.; Onstad, G.D.; Thruston, A.D. Occurrence of a new generation of disinfection byproducts. Environ. Sci. Technol. 2006, 40, 7175–7185. [Google Scholar] [CrossRef] [PubMed]
- Cantor, K.P.; Villanueva, C.M.; Silverman, D.T.; Figueroa, J.D.; Real, F.X.; Garcia-Closas, M.; Carrato, A.; Castaño-Vinyals, G.; Samanic, C.; Rothman, N.; et al. Polymorphisms in GST1, GSTZ1, and CYP2E1, disinfection by-products, and risk of bladder cancer in Spain. Environ. Health Perspect. 2010, 118, 1545–1550. [Google Scholar] [CrossRef] [PubMed]
- Fisher, I.; Kastl, G.; Sathasivan, A. A suitable model of combined effects of temperature and initial condition on chlorine bulk decay in water distribution systems. Water Res. 2012, 46, 3293–3303. [Google Scholar] [CrossRef] [PubMed]
- Miao, H.F.; Tao, W.Y. The mechanisms of ozonation on cyanobacteria and its toxins removal. Sep. Purif. Technol. 2009, 60, 187–193. [Google Scholar] [CrossRef]
- Peterson, H.G.; Hrudey, S.E.; Cantin, I.A.; Perley, T.R.; Kenefick, S.L. Physiological toxicity, cell membrane damage and the release of dissolved organic carbon and geosmin by Aphanizomenon flos-aquae after exposure to water treatment chemicals. Water Res. 1995, 29, 1515–1523. [Google Scholar] [CrossRef]
- Chen, J.J.; Yeh, H.H. The mechanisms of potassium permanganate on algae removal. Water Res. 2005, 39, 4420–4428. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Liu, W. Effectiveness and mechanism of potassium ferrate(VI) preoxidation for algae removal by coagulation. Water Res. 2002, 36, 871–878. [Google Scholar] [CrossRef]
- Liang, H.; Yang, Y.L.; Gong, W.J.; Li, X.; Li, G.B. Effect of pretreatment by permanganate/chlorine on algae fouling control for ultrafiltration (UF) membrane system. Desalination 2008, 222, 74–80. [Google Scholar]
- Hall, A.; Fielding, A.H.; Butler, M. Mechanisms of copper tolerance in the marine fouling alga Ectocarpus siliculosus—Evidence for an exclusion mechanism. Mar. Biol. 1979, 54, 195–199. [Google Scholar] [CrossRef]
- Strange, J.; Macnair, R. Evidence for a role for the cell membrane in copper tolerance of Mimulus guttatus Fischer ex DC. New Phytol. 1999, 119, 383–388. [Google Scholar] [CrossRef]
- Valenzuela-Espinoza, E.; Millán-Núñez, R.; Núñez-Cebrero, F. Protein, carbohydrate, lipid and chlorophyll a content in Isochrysis aff. Galbana (clone T-Iso) cultured with a low cost alternative to the f/2 medium. Aquac. Eng. 2002, 25, 207–216. [Google Scholar]
- Frankin, N.M.; Stauber, J.; Lim, R.P. Development of flow cytometry-based algal bioassays for assessing toxicity of copper in natural water. Environ. Toxicol. Chem. 2001, 20, 160–170. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Wang, J.; Pan, X.; Yuan, H. A New Treatment Strategy for Inactivating Algae in Ballast Water Based on Multi-Trial Injections of Chlorine. Int. J. Mol. Sci. 2015, 16, 13158-13171. https://doi.org/10.3390/ijms160613158
Sun J, Wang J, Pan X, Yuan H. A New Treatment Strategy for Inactivating Algae in Ballast Water Based on Multi-Trial Injections of Chlorine. International Journal of Molecular Sciences. 2015; 16(6):13158-13171. https://doi.org/10.3390/ijms160613158
Chicago/Turabian StyleSun, Jinyang, Junsheng Wang, Xinxiang Pan, and Haichao Yuan. 2015. "A New Treatment Strategy for Inactivating Algae in Ballast Water Based on Multi-Trial Injections of Chlorine" International Journal of Molecular Sciences 16, no. 6: 13158-13171. https://doi.org/10.3390/ijms160613158





