The Effect of Electrospun Gelatin Fibers Alignment on Schwann Cell and Axon Behavior and Organization in the Perspective of Artificial Nerve Design
Abstract
:1. Introduction
2. Results
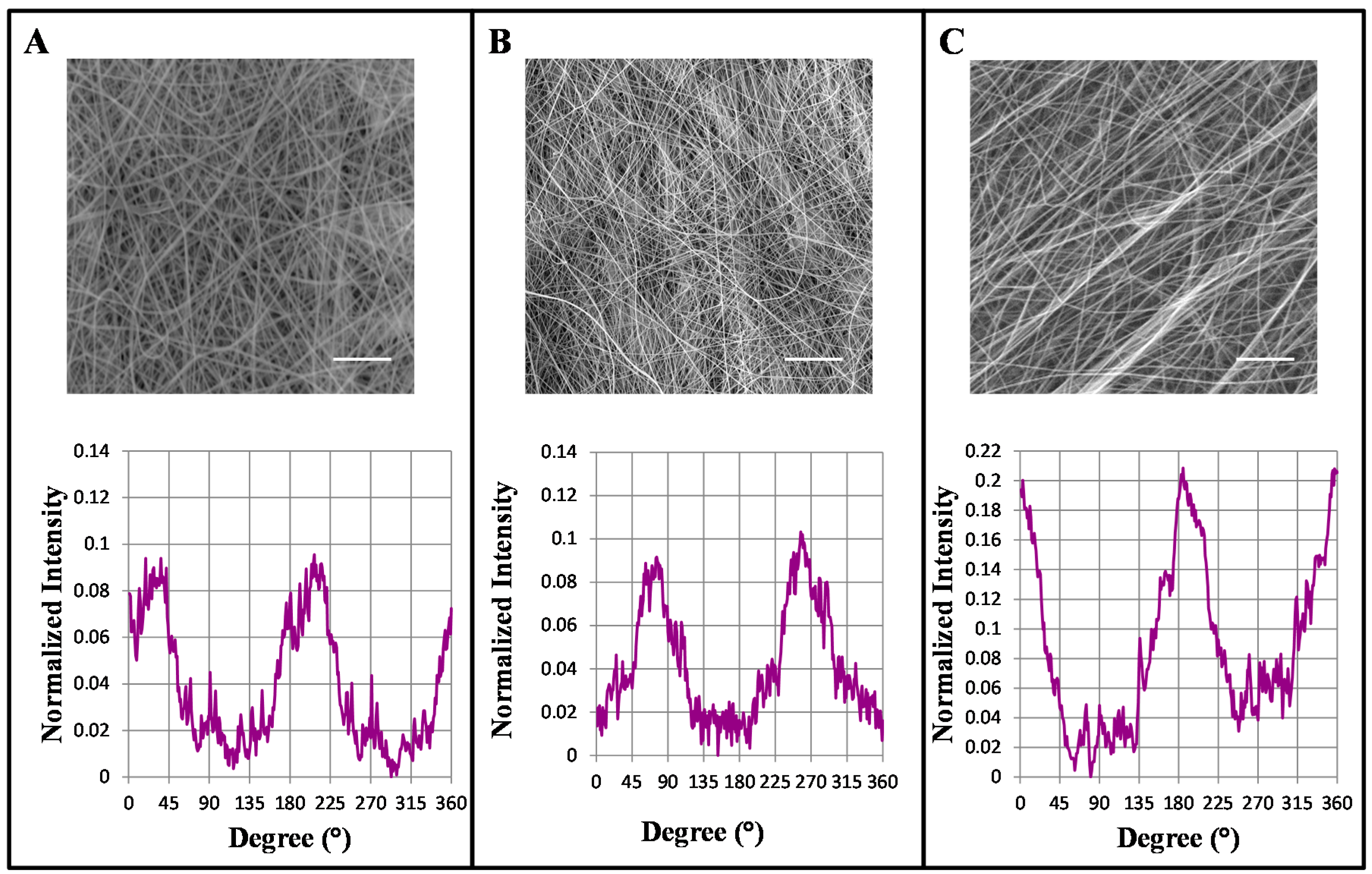
2.1. Influence of Mandrel Collector Speed on the Alignment of the Nano-Fibers
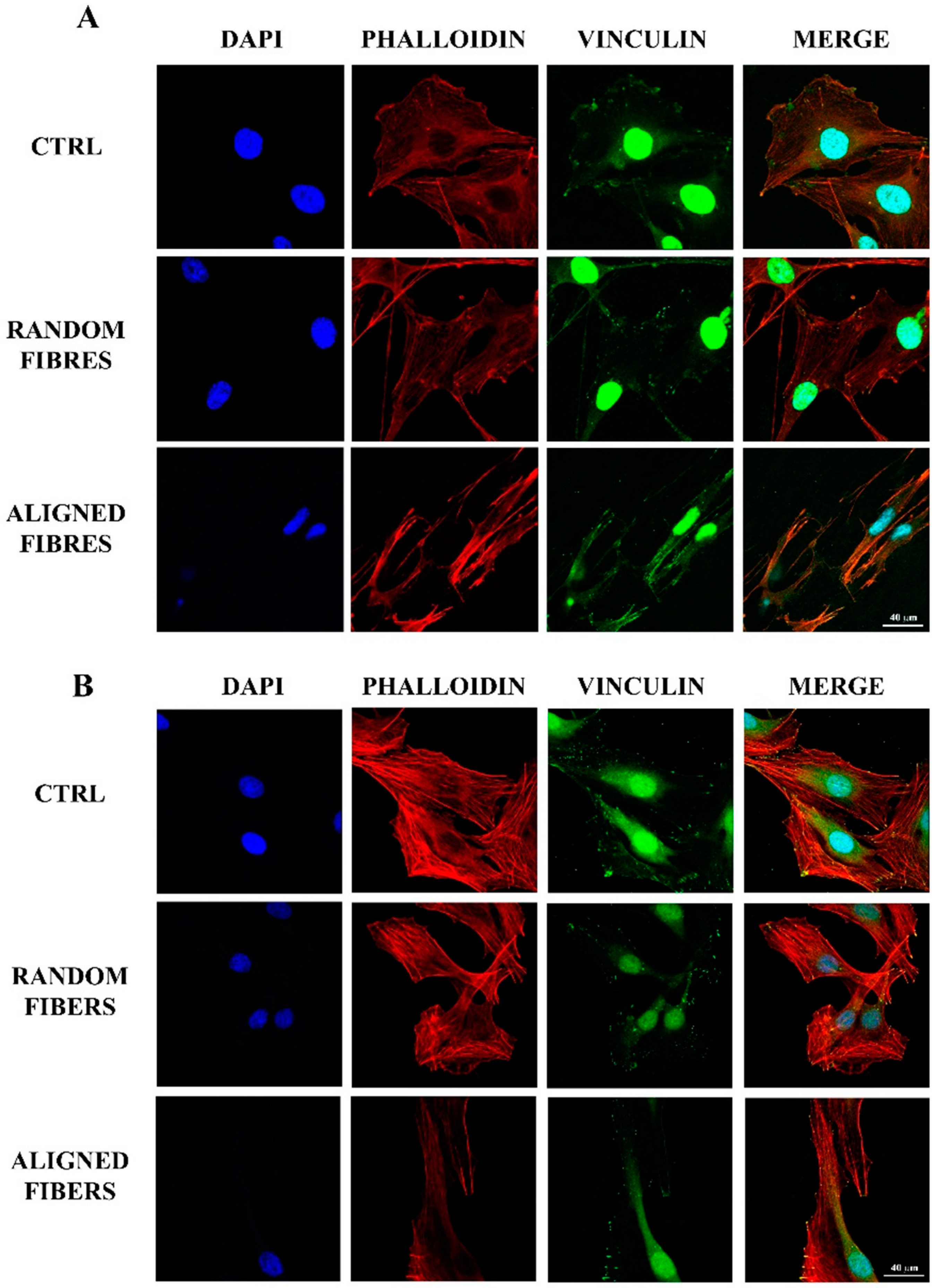
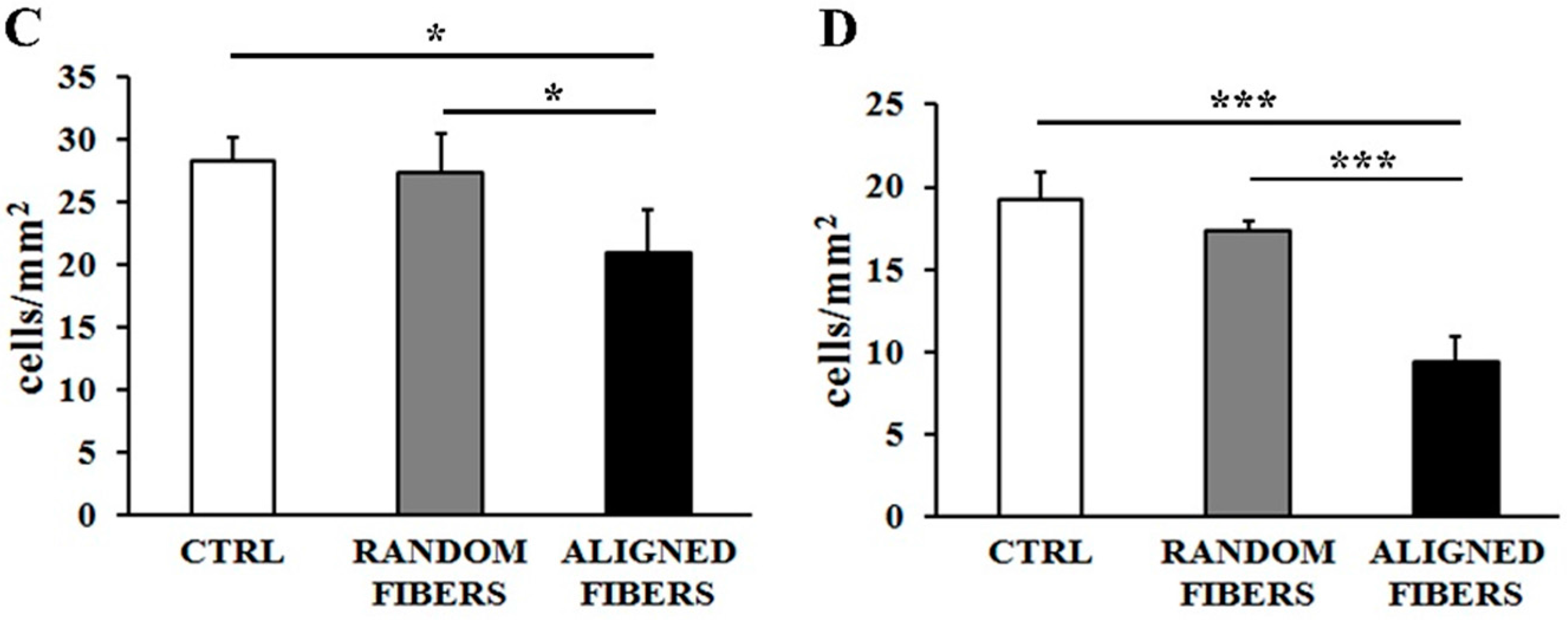
2.2. Aligning Gelatin Nano-Fibers Decreased the Number of Adherent Schwann Cells
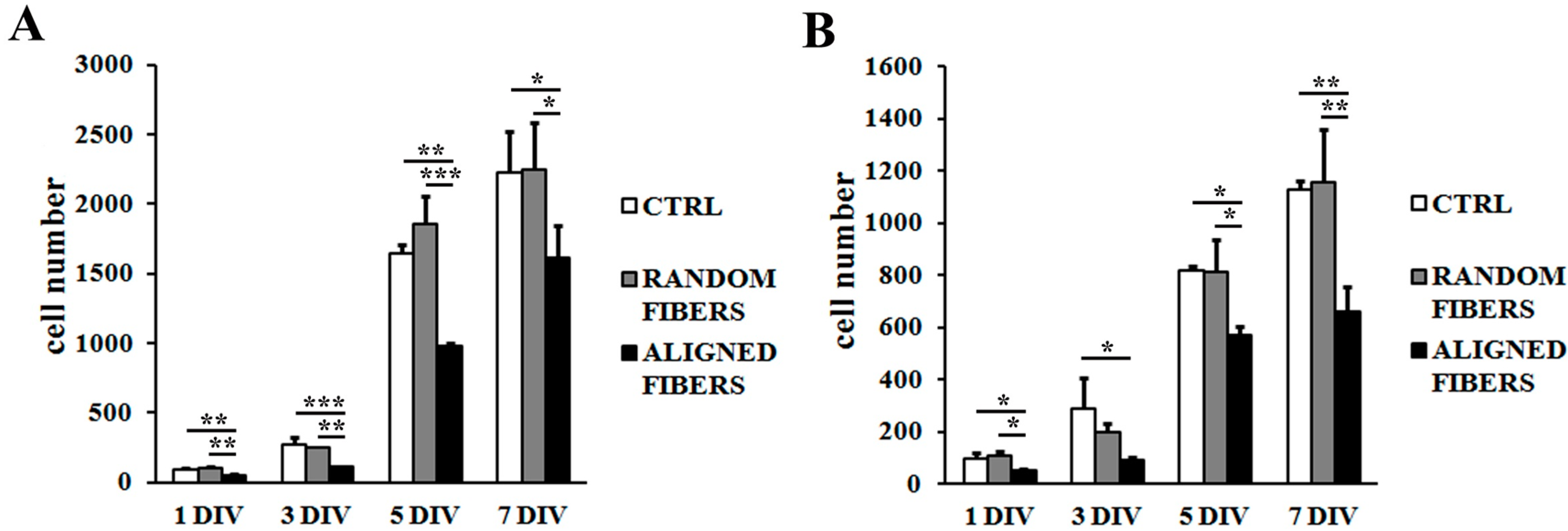
2.3. Aligning Gelatin Nano-Fibers Reduced Schwann Cell Proliferation Rate


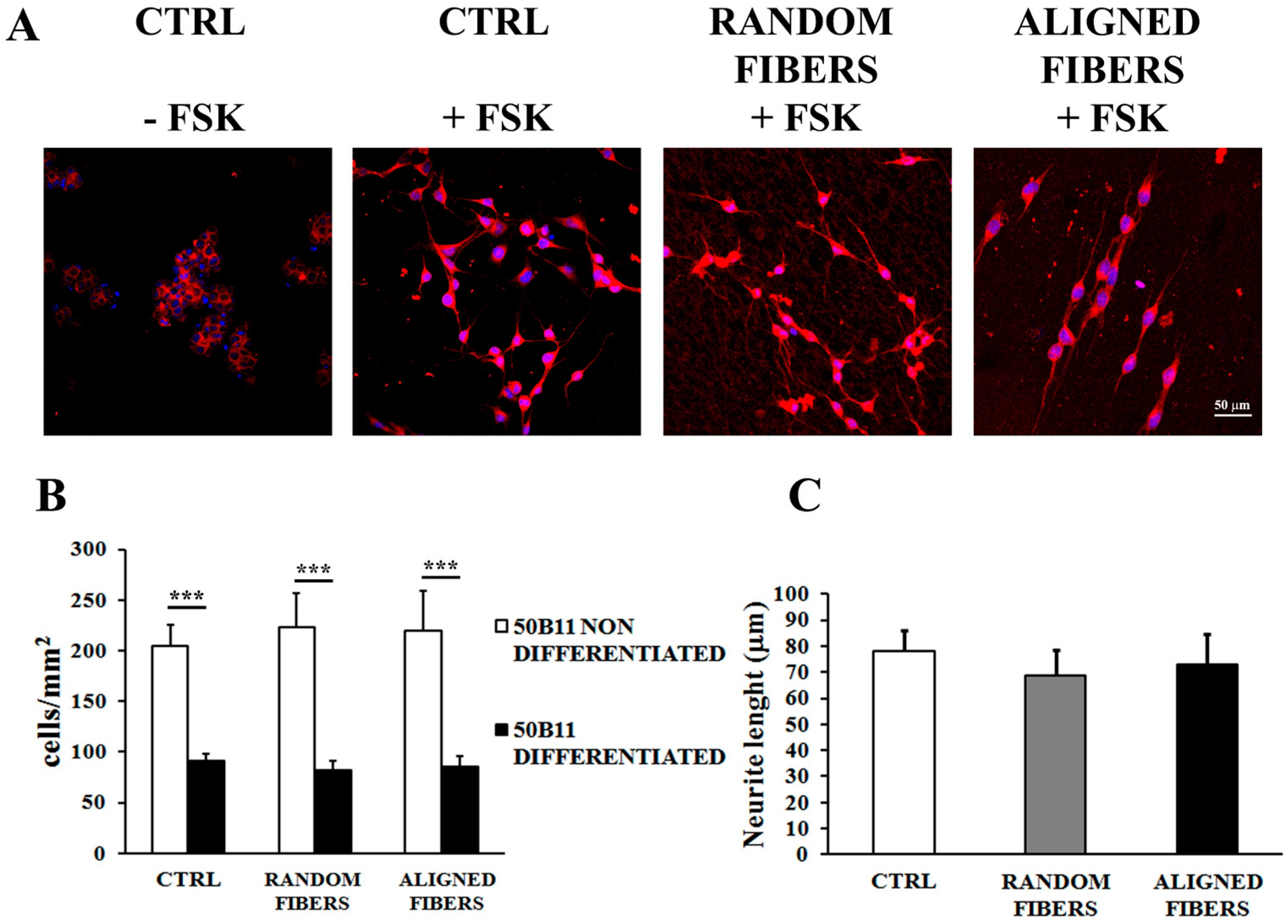
2.4. Aligning Gelatin Nano-Fibers Resulted in Neurites Alignment
3. Discussion
3.1. Increasing Mandrel Collector Speed Rotation Resulted in Fibers Alignment
3.2. Fibers Alignment Reduced Schwann Cells Adhesion and Proliferation but Enhanced the Alignment of Schwann Cells Actin Filaments
3.3. Alignment of Gelatin Electrospun Fibers Does Not Affect Neurite Length but Induce Neurites Alignment
4. Experimental Section
4.1. Preparation of Gelatin Solution and Nano-Fibers
4.2. Electrospinning of Randomly Oriented and Aligned GL Based Nano-Fibers
4.3. Scanning Electron Microscopy
4.4. Cell Culture
4.5. Adhesion Assay
4.6. Proliferation
4.7. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide (MTT) Assay
4.8. 50B11 Differentiation
4.9. Confocal Microscopy
4.10. Statistics
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Battiston, B.; Raimondo, S.; Tos, P.; Gaidano, V.; Audisio, C.; Scevola, A.; Perroteau, I.; Geuna, S. Chapter 11: Tissue engineering of peripheral nerves. Int. Rev. Neurobiol. 2009, 87, 227–249. [Google Scholar] [PubMed]
- De Ruiter, G.C.; Malessy, M.J.; Yaszemski, M.J.; Windebank, A.J.; Spinner, R.J. Designing ideal conduits for peripheral nerve repair. Neurosurg. Focus 2009, 26, E5. [Google Scholar] [CrossRef] [PubMed]
- Deumens, R.; Bozkurt, A.; Meek, M.F.; Marcus, M.A.; Joosten, E.A.; Weis, J.; Brook, G.A. Repairing injured peripheral nerves: Bridging the gap. Prog. Neurobiol. 2010, 92, 245–276. [Google Scholar] [CrossRef] [PubMed]
- Geuna, S.; Gnavi, S.; Perroteau, I.; Tos, P.; Battiston, B. Tissue engineering and peripheral nerve reconstruction: An overview. Int. Rev. Neurobiol. 2013, 108, 35–57. [Google Scholar] [PubMed]
- Biazar, E.; Khorasani, M.T.; Montazeri, N.; Pourshamsian, K.; Daliri, M.; Rezaei, M.; Jabarvand, M.; Khoshzaban, A.; Heidari, S.; Jafarpour, M.; et al. Types of neural guides and using nanotechnology for peripheral nerve reconstruction. Int. J. Nanomed. 2010, 5, 839–852. [Google Scholar] [CrossRef] [PubMed]
- Chiono, V.; Tonda-Turo, C.; Ciardelli, G. Chapter 9: Artificial scaffolds for peripheral nerve reconstruction. Int. Rev. Neurobiol. 2009, 87, 173–198. [Google Scholar] [PubMed]
- Ciardelli, G.; Chiono, V. Materials for peripheral nerve regeneration. Macromol. Biosci. 2006, 6, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Tabesh, H.; Amoabediny, G.; Nik, N.S.; Heydari, M.; Yosefifard, M.; Siadat, S.O.; Mottaghy, K. The role of biodegradable engineered scaffolds seeded with Schwann cells for spinal cord regeneration. Neurochem. Int. 2009, 54, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Willerth, S.M.; Sakiyama-Elbert, S.E. Approaches to neural tissue engineering using scaffolds for drug delivery. Adv. Drug Deliv. Rev. 2007, 59, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Dahlin, L.B.; Anagnostaki, L.; Lundborg, G. Tissue response to silicone tubes used to repair human median and ulnar nerves. Scand. J. Plast. Reconstr. Surg. Hand Surg. 2001, 35, 29–34. [Google Scholar] [PubMed]
- Lundborg, G.; Rosen, B.; Dahlin, L.; Holmberg, J.; Rosen, I. Tubular repair of the median or ulnar nerve in the human forearm: A 5-year follow-up. J. Hand Surg. Br. 2004, 29, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Pfister, L.A.; Papaloizos, M.; Merkle, H.P.; Gander, B. Nerve conduits and growth factor delivery in peripheral nerve repair. J. Peripher. Nerv. Syst. 2007, 12, 65–82. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Lim, S.H.; Mao, H.Q.; Chew, S.Y. Current applications and future perspectives of artificial nerve conduits. Exp. Neurol. 2010, 223, 86–101. [Google Scholar] [CrossRef] [PubMed]
- Cunha, C.; Panseri, S.; Antonini, S. Emerging nanotechnology approaches in tissue engineering for peripheral nerve regeneration. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yan, Y.; Li, S. PDLLA/chondroitin sulfate/chitosan/NGF conduits for peripheral nerve regeneration. Biomaterials 2011, 32, 4506–4516. [Google Scholar] [CrossRef] [PubMed]
- Bacakova, L.; Filova, E.; Parizek, M.; Ruml, T.; Svorcik, V. Modulation of cell adhesion, proliferation and differentiation on materials designed for body implants. Biotechnol. Adv. 2011, 29, 739–767. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.G.; Hill, E.W.; Bayat, A. Designing implant surface topography for improved biocompatibility. Exp. Rev. Med. Devices 2013, 10, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Rahmany, M.B.; van Dyke, M. Biomimetic approaches to modulate cellular adhesion in biomaterials: A review. Acta Biomater. 2013, 9, 5431–5437. [Google Scholar] [CrossRef] [PubMed]
- Deumens, R.; Bozkurt, A.; Brook, G.A. US Food and Drug Administration/Conformit Europe-approved absorbable nerve conduits for clinical repair of peripheral and cranial nerves. Commentary. Ann. Plast. Surg. 2010, 65, 371. [Google Scholar] [CrossRef] [PubMed]
- Kehoe, S.; Zhang, X.F.; Boyd, D. FDA approved guidance conduits and wraps for peripheral nerve injury: A review of materials and efficacy. Injury 2012, 43, 553–572. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Feng, Q.; Liu, X.; Dong, W.; Cui, F. Collagen-based implants reinforced by chitin fibres in a goat shank bone defect model. Biomaterials 2006, 27, 1917–1923. [Google Scholar] [CrossRef] [PubMed]
- Longo, F.M.; Hayman, E.G.; Davis, G.E.; Ruoslahti, E.; Engvall, E.; Manthorpe, M.; Varon, S. Neurite-promoting factors and extracellular matrix components accumulating in vivo within nerve regeneration chambers. Brain Res. 1984, 309, 105–117. [Google Scholar] [CrossRef]
- Parenteau-Bareil, R.; Gauvin, R.B.F. Collagen-based biomaterials for tissue engineering applications. Materials 2010, 3, 1863–1887. [Google Scholar] [CrossRef]
- Chevallay, B.; Herbage, D. Collagen-based biomaterials as 3D scaffold for cell cultures: Applications for tissue engineering and gene therapy. Med. Biol. Eng. Comput. 2000, 38, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Chiono, V.; Pulieri, E.; Vozzi, G.; Ciardelli, G.; Ahluwalia, A.; Giusti, P. Genipin-crosslinked chitosan/gelatin blends for biomedical applications. J. Mater. Sci. Mater. Med. 2008, 19, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Santoro, M.; Tatara, A.M.; Mikos, A.G. Gelatin carriers for drug and cell delivery in tissue engineering. J. Control. Release 2014, 190, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Gnavi, S.; Fornasari, B.E.; Tonda-Turo, C.; Ciardelli, G.; Zanetti, M.; Geuna, S.; Perroteau, I. The influence of electrospun fibre size on Schwann cell behaviour and axonal outgrowth. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 48, 620–631. [Google Scholar] [CrossRef] [PubMed]
- Tonda-Turo, C.; Cipriani, E.; Gnavi, S.; Chiono, V.; Mattu, C.; Gentile, P.; Perroteau, I.; Zanetti, M.; Ciardelli, G. Crosslinked gelatin nanofibres: Preparation, characterisation and in vitro studies using glial-like cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 2723–2735. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Huang, Y.; Yang, X.; Mei, F.; Ma, Q.; Chen, G.; Ryu, S.; Deng, X. Gelatin nanofibrous membrane fabricated by electrospinning of aqueous gelatin solution for guided tissue regeneration. J. Biomed. Mater. Res. A 2009, 90, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Ding, F.; Yang, Y.; Liu, J. Construction of tissue engineered nerve grafts and their application in peripheral nerve regeneration. Prog. Neurobiol. 2011, 93, 204–230. [Google Scholar] [CrossRef] [PubMed]
- Gnavi, S.; di Blasio, L.; Tonda-Turo, C.; Mancardi, A.; Primo, L.; Ciardelli, G.; Gambarotta, G.; Geuna, S.; Perroteau, I. Gelatin-based hydrogel for vascular endothelial growth factor release in peripheral nerve tissue engineering. J. Tissue Eng. Regen. Med. 2014. [Google Scholar] [CrossRef] [PubMed]
- Maturana, L.G.; Pierucci, A.; Simoes, G.F.; Vidigal, M.; Duek, E.A.; Vidal, B.C.; Oliveira, A.L. Enhanced peripheral nerve regeneration by the combination of a polycaprolactone tubular prosthesis and a scaffold of collagen with supramolecular organization. Brain Behav. 2013, 3, 417–430. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Li, R.; Liu, W.; Dai, J.; Du, Z.; Wang, X.; Ma, J.; Zhao, J. Dynamic culture of a thermosensitive collagen hydrogel as an extracellular matrix improves the construction of tissue-engineered peripheral nerve. Neural Regen. Res. 2014, 9, 1371–1378. [Google Scholar] [PubMed]
- Shakhbazau, A.; Archibald, S.J.; Shcharbin, D.; Bryszewska, M.; Midha, R. Aligned collagen-GAG matrix as a 3D substrate for Schwann cell migration and dendrimer-based gene delivery. J. Mater. Sci. Mater. Med. 2014, 25, 1979–1989. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Xiao, Z.; Meng, D.; Hou, X.; Zhu, J.; Dai, J.; Xu, R. Use of natural neural scaffolds consisting of engineered vascular endothelial growth factor immobilized on ordered collagen fibers filled in a collagen tube for peripheral nerve regeneration in rats. Int. J. Mol. Sci. 2014, 15, 18593–18609. [Google Scholar] [CrossRef] [PubMed]
- Arnaout, A.; Fontaine, C.; Chantelot, C. Sensory recovery after primary repair of palmar digital nerves using a Revolnerv® collagen conduit: A prospective series of 27 cases. Chir. Main 2014, 33, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, X.; Cui, L.; Brunson, C.; Zhao, W.; Bhat, N.R.; Zhang, N.; Wen, X. Engineering an in situ crosslinkable hydrogel for enhanced remyelination. FASEB J. 2013, 27, 1127–1136. [Google Scholar] [CrossRef] [PubMed]
- Hato, N.; Nota, J.; Komobuchi, H.; Teraoka, M.; Yamada, H.; Gyo, K.; Yanagihara, N.; Tabata, Y. Facial nerve decompression surgery using bFGF-impregnated biodegradable gelatin hydrogel in patients with Bell palsy. Otolaryngol. Head Neck Surg. 2012, 146, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Gamez Sazo, R.E.; Maenaka, K.; Gu, W.; Wood, P.M.; Bunge, M.B. Fabrication of growth factor- and extracellular matrix-loaded, gelatin-based scaffolds and their biocompatibility with Schwann cells and dorsal root ganglia. Biomaterials 2012, 33, 8529–8539. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Venugopal, J.; Prabhakaran, M.P.; Dev, V.R.; Low, S.; Choon, A.T.; Ramakrishna, S. Aligned and random nanofibrous substrate for the in vitro culture of Schwann cells for neural tissue engineering. Acta Biomater. 2009, 5, 2560–2569. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, J.; Low, S.; Choon, A.T.; Ramakrishna, S. Interaction of cells and nanofiber scaffolds in tissue engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 84, 34–48. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; MacEwan, M.R.; Schwartz, A.G.; Xia, Y. Electrospun nanofibers for neural tissue engineering. Nanoscale 2010, 2, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Bettahalli, N.M.; Arkesteijn, I.T.; Wessling, M.; Poot, A.A.; Stamatialis, D. Corrugated round fibers to improve cell adhesion and proliferation in tissue engineering scaffolds. Acta Biomater. 2013, 9, 6928–6935. [Google Scholar] [CrossRef] [PubMed]
- Mahairaki, V.; Lim, S.H.; Christopherson, G.T.; Xu, L.; Nasonkin, I.; Yu, C.; Mao, H.Q.; Koliatsos, V.E. Nanofiber matrices promote the neuronal differentiation of human embryonic stem cell-derived neural precursors in vitro. Tissue Eng. A 2011, 17, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; MacEwan, M.R.; Li, X.; Sakiyama-Elbert, S.E.; Xia, Y. Neurite outgrowth on nanofiber scaffolds with different orders, structures, and surface properties. ACS Nano 2009, 3, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Kontoveros, D.; Lin, F.; Hua, G.; Reneker, D.H.; Becker, M.L.; Willits, R.K. Enhanced Schwann cell attachment and alignment using one-pot “Dual Click” GRGDS and YIGSR derivatized nanofibers. Biomacromolecules 2015, 16, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.G.; Ciofani, G.; Polini, A.; Liakos, I.; Iandolo, D.; Athanassiou, A.; Pisignano, D.; Mattoli, V.; Menciassi, A. PC12 neuron-like cell response to electrospun poly(3-hydroxybutyrate) substrates. J. Tissue Eng. Regen. Med. 2015, 9, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Nectow, A.R.; Marra, K.G.; Kaplan, D.L. Biomaterials for the development of peripheral nerve guidance conduits. Tissue Eng. B Rev. 2012, 18, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Gnavi, S.; Barwig, C.; Freier, T.; Haastert-Talini, K.; Grothe, C.; Geuna, S. The use of chitosan-based scaffolds to enhance regeneration in the nervous system. Int. Rev. Neurobiol. 2013, 109, 1–62. [Google Scholar] [PubMed]
- Agarwal, S.W.H.; Greiner, A. Use of electrospinning technique for biomedical applications. Polymer 2008, 49, 5603–5621. [Google Scholar] [CrossRef]
- Christopherson, G.T.; Song, H.; Mao, H.Q. The influence of fiber diameter of electrospun substrates on neural stem cell differentiation and proliferation. Biomaterials 2009, 30, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Jha, B.S.; Colello, R.J.; Bowman, J.R.; Sell, S.A.; Lee, K.D.; Bigbee, J.W.; Bowlin, G.L.; Chow, W.N.; Mathern, B.E.; Simpson, D.G. Two pole air gap electrospinning: Fabrication of highly aligned, three-dimensional scaffolds for nerve reconstruction. Acta Biomater. 2011, 7, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.J.; Fan, J.T.; Chu, C.C.; Wu, J. Electrospinning of small diameter 3-D nanofibrous tubular scaffolds with controllable nanofiber orientations for vascular grafts. J. Mater. Sci. Mater. Med 2010, 21, 3207–3215. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Mi, R.; Haughey, N.; Oz, M.; Hoke, A. Immortalization and characterization of a nociceptive dorsal root ganglion sensory neuronal line. J. Peripher. Nerv. Syst. 2007, 12, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Bhattacherjee, A.; Liao, Z.; Smith, P.G. Trophic factor and hormonal regulation of neurite outgrowth in sensory neuron-like 50B11 cells. Neurosci. Lett. 2014, 558, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Gilardino, A.; Farcito, S.; Zamburlin, P.; Audisio, C.; Lovisolo, D. Specificity of the second messenger pathways involved in basic fibroblast growth factor-induced survival and neurite growth in chick ciliary ganglion neurons. J. Neurosci. Res. 2009, 87, 2951–2962. [Google Scholar] [CrossRef] [PubMed]
- Zamburlin, P.; Gilardino, A.; Dalmazzo, S.; Ariano, P.; Lovisolo, D. Temporal dynamics of neurite outgrowth promoted by basic fibroblast growth factor in chick ciliary ganglia. J. Neurosci. Res. 2006, 84, 505–514. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gnavi, S.; Fornasari, B.E.; Tonda-Turo, C.; Laurano, R.; Zanetti, M.; Ciardelli, G.; Geuna, S. The Effect of Electrospun Gelatin Fibers Alignment on Schwann Cell and Axon Behavior and Organization in the Perspective of Artificial Nerve Design. Int. J. Mol. Sci. 2015, 16, 12925-12942. https://doi.org/10.3390/ijms160612925
Gnavi S, Fornasari BE, Tonda-Turo C, Laurano R, Zanetti M, Ciardelli G, Geuna S. The Effect of Electrospun Gelatin Fibers Alignment on Schwann Cell and Axon Behavior and Organization in the Perspective of Artificial Nerve Design. International Journal of Molecular Sciences. 2015; 16(6):12925-12942. https://doi.org/10.3390/ijms160612925
Chicago/Turabian StyleGnavi, Sara, Benedetta Elena Fornasari, Chiara Tonda-Turo, Rossella Laurano, Marco Zanetti, Gianluca Ciardelli, and Stefano Geuna. 2015. "The Effect of Electrospun Gelatin Fibers Alignment on Schwann Cell and Axon Behavior and Organization in the Perspective of Artificial Nerve Design" International Journal of Molecular Sciences 16, no. 6: 12925-12942. https://doi.org/10.3390/ijms160612925






