Hemipteran Mitochondrial Genomes: Features, Structures and Implications for Phylogeny
Abstract
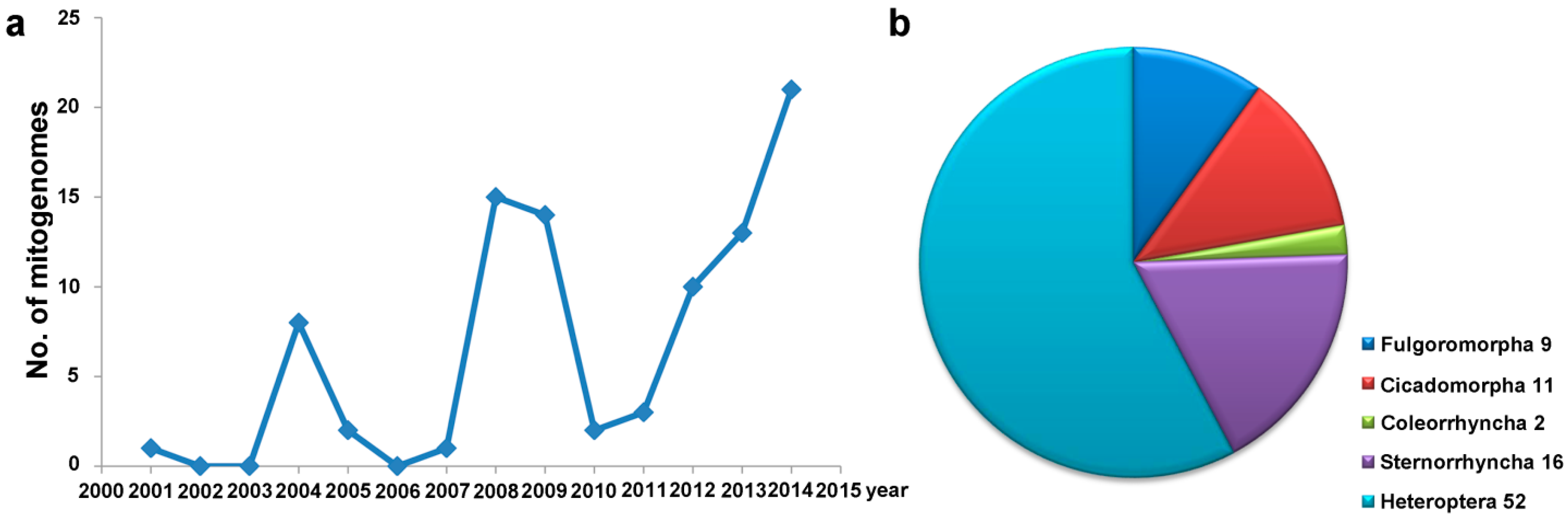
:1. Introduction
2. Mitogenomes of Hemiptera
| Suborder | Family | Species | GenBank No. | Reference |
|---|---|---|---|---|
| Cicadomorpha | Aphrophoridae | Philaenus spumarius | NC_005944 | [34] |
| Cercopidae | Abidama producta | NC_015799 | [35] | |
| Cercopidae | Aeneolamia contigua | NC_025495 | [35] | |
| Cercopidae | Callitetix braconoides | NC_025497 | [35] | |
| Cercopidae | Callitetix versicolor | EU725832 | [35] | |
| Cercopidae | Callitettix biformis | NC_025496 | [35] | |
| Cercopidae | Paphnutius ruficeps | NC_021100 | [36] | |
| Cicadellidae | Empoasca vitis | NC_024838 | [37] | |
| Cicadellidae | Homalodisca coagulata | AY875213 | - | |
| Cicadellidae | Homalodisca vitripennis | NC_006899 | * | |
| Membracidae | Leptobelus gazella | NC_023219 | * | |
| Coleorrhyncha | Peloridiidae | Hackeriella veitchi | GQ884145 | [12] |
| Peloridiidae | Hemiodoecus leai | NC_025329 | [32] | |
| Fulgoromorpha | Delphacidae | Laodelphax striatella | JX880068 | [27] |
| Delphacidae | Laodelphax striatellus | NC_013706 | [38] | |
| Delphacidae | Nilaparvata lugens | NC_021748 | [27] | |
| Delphacidae | Nilaparvata muiri | NC_024627 | - | |
| Flatidae | Geisha distinctissima | NC_012617 | [39] | |
| Fulgoridae | Laternaria candelaria | NC_019576 | [40] | |
| Fulgoridae | Lycorma delicatula | NC_012835 | [19] | |
| Issidae | Sivaloka damnosa | NC_014286 | [41] | |
| Ricaniidae | Ricania marginalis | JN242415 | [40] | |
| Heteroptera | Alydidae | Riptortus pedestris | NC_012462 | [23] |
| Anthocoridae | Orius niger | NC_012429 | [23] | |
| Anthocoridae | Orius sauteri | NC_024583 | [42] | |
| Aradidae | Aradacanthia heissi | HQ441233 | [43] | |
| Aradidae | Brachyrhynchus hsiaoi | NC_022670 | [44] | |
| Aradidae | Neuroctenus parus | NC_012459 | [23] | |
| Berytidae | Yemmalysus parallelus | NC_012464 | [23] | |
| Colobathristidae | Phaenacantha marcida | NC_012460 | [23] | |
| Coreidae | Hydaropsis longirostris | NC_012456 | [23] | |
| Cydnidae | Macroscytus gibbulus | EU427338 | [23] | |
| Enicocephalidae | Stenopirates sp. | NC_016017 | [45] | |
| Gelastocoridae | Nerthra indica | NC_012838 | [19] | |
| Geocoridae | Geocoris pallidipennis | NC_012424 | [23] | |
| Gerridae | Aquarius paludum | NC_012841 | [19] | |
| Hydrometridae | Hydrometra greeni | NC_012842 | [19] | |
| Largidae | Physopelta gutta | NC_012432 | [23] | |
| Lygaeidae | Kleidocerys resedae | KJ584365 | [46] | |
| Heteroptera | Malcidae | Chauliops fallax | NC_020772 | [47] |
| Malcidae | Malcus inconspicuus | NC_012458 | [23] | |
| Miridae | Adelphocoris fasciaticollis | NC_023796 | [48] | |
| Miridae | Apolygus lucorum | NC_023083 | [49] | |
| Miridae | Lygus lineolaris | EU401991 | - | |
| Miridae | Nesidiocoris tenuis | NC_022677 | [50] | |
| Nabidae | Alloeorhynchus bakeri | HM235722 | [51] | |
| Nabidae | Gorpis annulatus | NC_019595 | [24] | |
| Nabidae | Gorpis humeralis | NC_019593 | [24] | |
| Nabidae | Nabis apicalis | NC_019594 | [24] | |
| Naucoridae | Ilyocoris cimicoides | NC_012845 | [19] | |
| Nepidae | Laccotrephes robustus | NC_012817 | [19] | |
| Notonectidae | Enithares tibialis | NC_012819 | [19] | |
| Ochteridae | Ochterus marginatus | NC_012820 | [19] | |
| Pentatomidae | Dolycoris baccarum | NC_020373 | [52] | |
| Pentatomidae | Halyomorpha halys | NC_013272 | [53] | |
| Pentatomidae | Nezara viridula | NC_011755 | [23] | |
| Plataspidae | Coptosoma bifaria | NC_012449 | [23] | |
| Plataspidae | megacopta cribraria | NC_015342 | * | |
| Pleidae | Paraplea frontalis | NC_012822 | [19] | |
| Pyrrhocoridae | Dysdercus cingulatus | NC_012421 | [23] | |
| Reduviidae | Agriosphodrus dohrni | NC_015842 | [54] | |
| Reduviidae | Brontostoma colossus | NC_024745 | [28] | |
| Reduviidae | Oncocephalus breviscutum | NC_022816 | [55] | |
| Reduviidae | Peirates arcuatus | NC_024264 | [56] | |
| Reduviidae | Sirthenea flavipes | NC_020143 | [57] | |
| Reduviidae | Triatoma dimidiata | NC_002609 | [9] | |
| Reduviidae | Valentia hoffmanni | NC_012823 | [19] | |
| Rhopalidae | Aeschyntelus notatus | NC_012446 | [23] | |
| Rhopalidae | Stictopleurus subviridis | NC_012888 | - | |
| Saldidae | Saldula arsenjevi | NC_012463 | [23] | |
| Tessaratomidae | Eusthenes cupreus | NC_022449 | [58] | |
| Tingidae | Corythucha ciliata | NC_022922 | [59] | |
| Tingidae | Pseudacysta perseae | NC_025299 | * | |
| Urostylididae | Urochela quadrinotata | NC_020144 | [60] | |
| Sternorrhyncha | Aleyrodidae | Aleurochiton aceris | NC_006160 | [22] |
| Aleyrodidae | Aleurodicus dugesii | NC_005939 | [22] | |
| Aleyrodidae | Bemisia afer | NC_024056 | [25] | |
| Aleyrodidae | Bemisia tabaci | NC_006279 | [22] | |
| Aleyrodidae | Neomaskellia andropogonis | NC_006159 | [22] | |
| Aleyrodidae | Tetraleurodes acaciae | NC_006292 | [22] | |
| Aleyrodidae | Trialeurodes vaporariorum | NC_006280 | [22] | |
| Aphididae | Acyrthosiphon pisum | NC_011594 | * | |
| Aphididae | Aphis gossypii | NC_024581 | [61] | |
| Aphididae | Cavariella salicicola | NC_022682 | [62] | |
| Aphididae | Cervaphis quercus | NC_024926 | [33] | |
| Aphididae | Diuraphis noxia | NC_022727 | [63] | |
| Aphididae | Schizaphis graminum | NC_006158 | [22] | |
| Aphididae | Sitobion avenae | NC_024683 | [64] | |
| Psyllidae | Pachypsylla venusta | NC_006157 | [22] | |
| Psyllidae | Paratrioza sinica | NC_024577 | [65] |
3. Features of Hemipteran Mitogenomes
3.1. Genome Organization
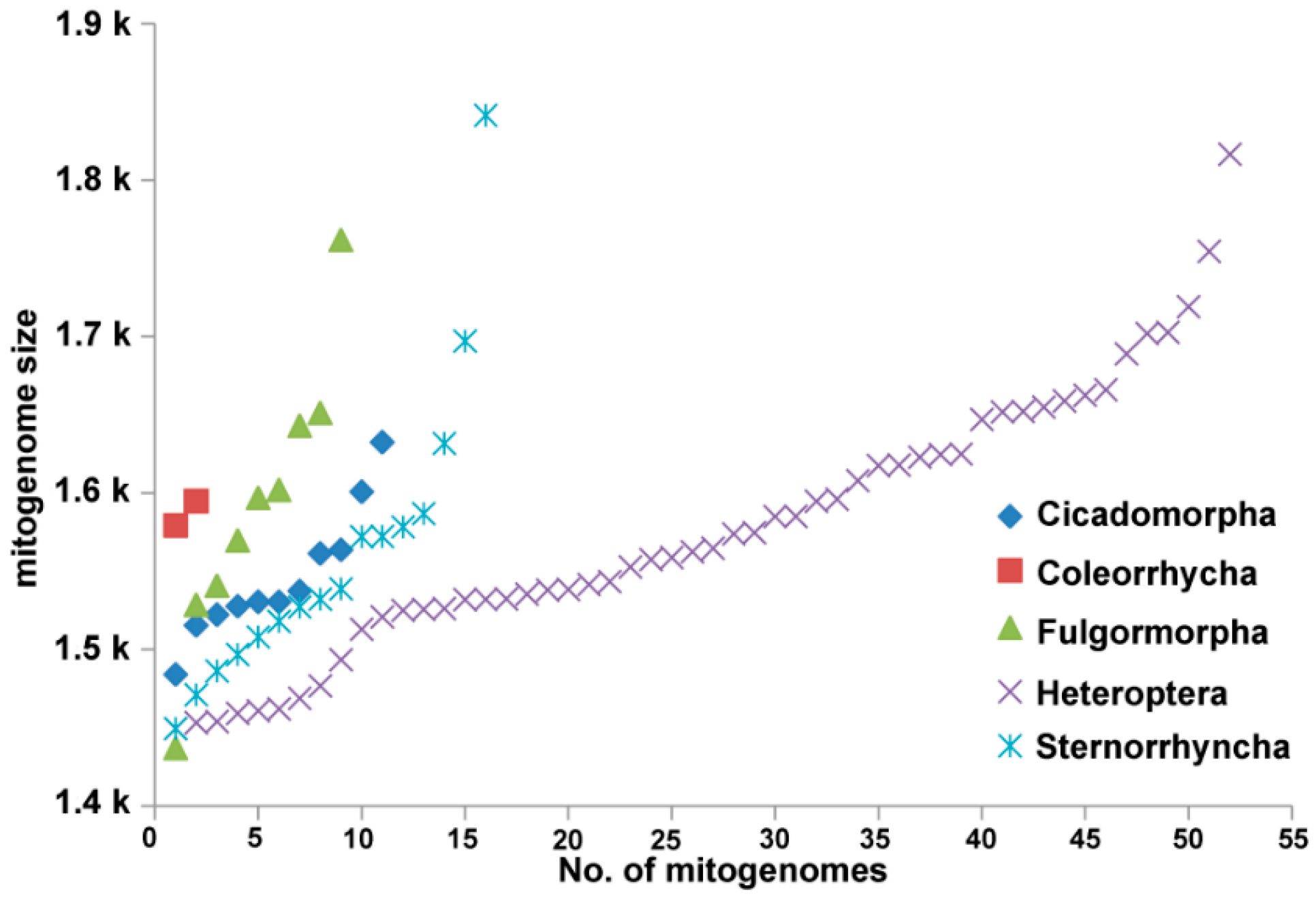
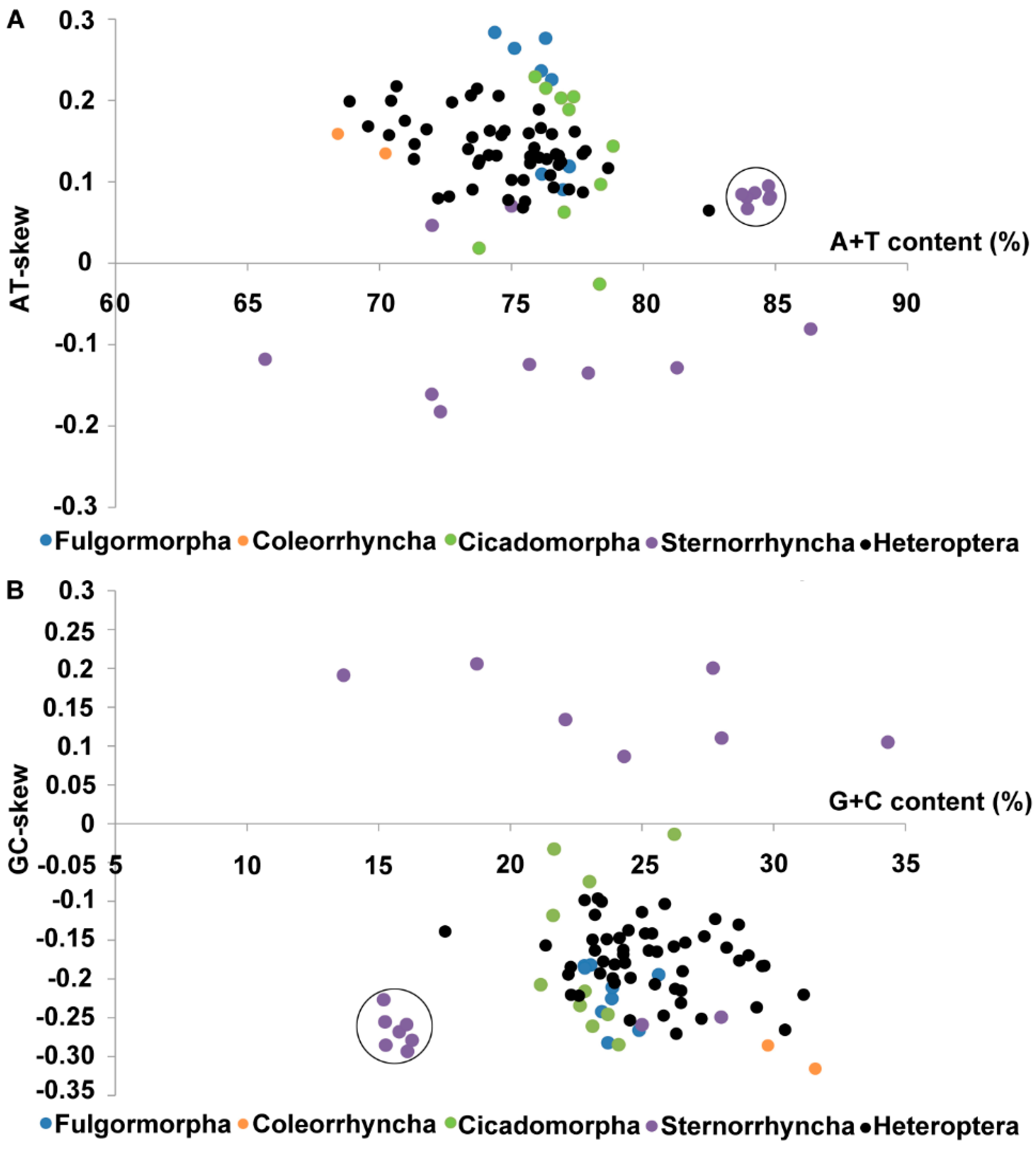
3.2. Nucleotide Composition
3.3. Protein-Coding Genes

3.4. tRNAs and rRNAs
3.5. Non-Coding Regions
3.5.1. Control Region
3.5.2. Repeat Region
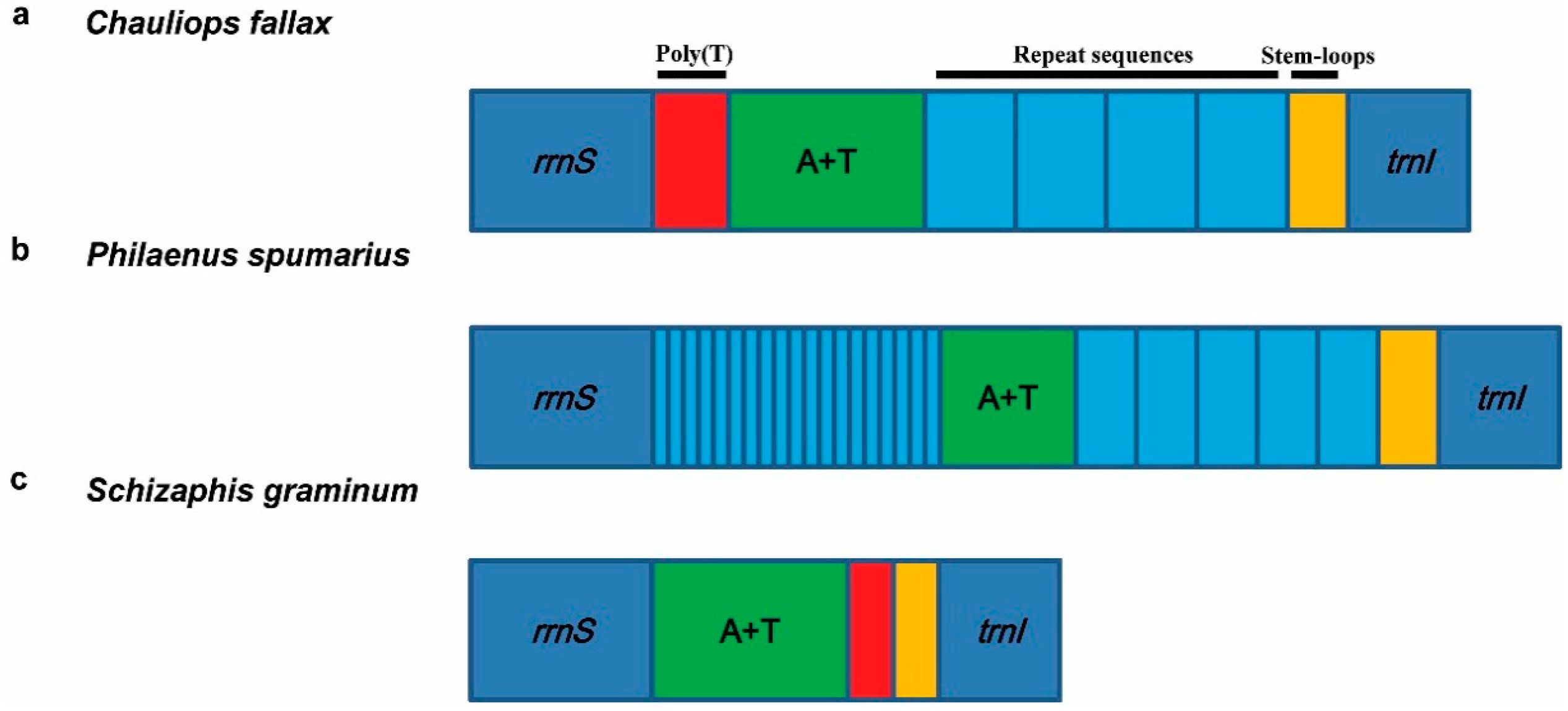
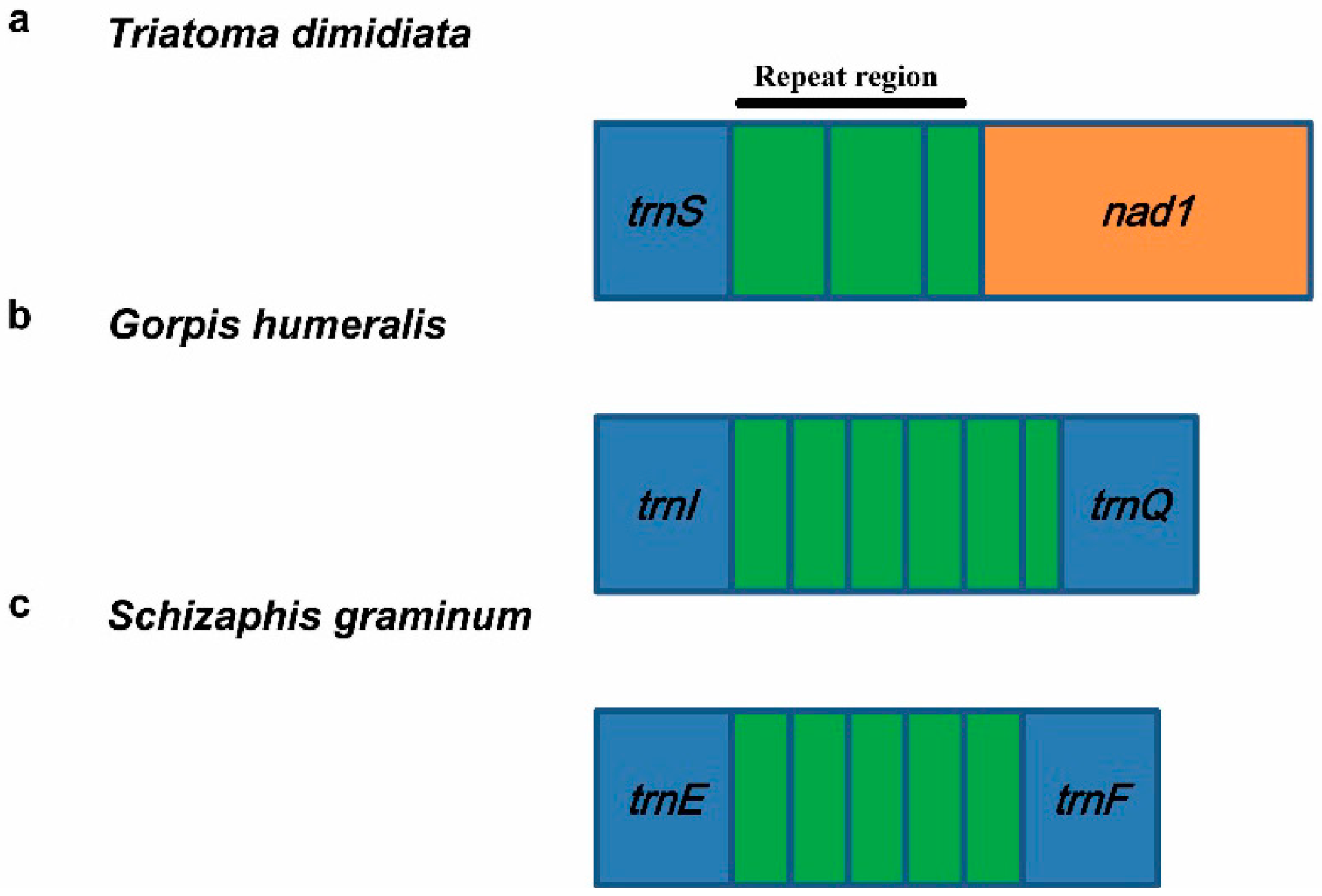
4. Hemipteran Mitogenome Arrangements and Evolution
| Species | Classification | Location | Repeat Number | Repeat Unit Size | Reference |
|---|---|---|---|---|---|
| Agriosphodrus dohrni | Heteroptera: Reduviidae | trnS-nad1 | two and a partial | 58 bp | [54] |
| Triatoma dimidiata | Heteroptera: Reduviidae | trnS-nad1 | two and a partial | 135 bp | [9] |
| Gorpis annulatus | Heteroptera: Nabidae | trnS-nad1 | three and a partial | 179 bp | [24] |
| Gorpis humeralis | Heteroptera: Nabidae | trnS-nad1 | two and a partial | 188 bp | [24] |
| Gorpis humeralis | Heteroptera: Nabidae | trnI-trnQ | five and a partial | 244 bp | [24] |
| Himacerus nodipes | Heteroptera: Nabidae | trnI-trnQ | four | 135 bp | [24] |
| Acyrthosiphon pisum | Sternorrhyncha: Aphididae | trnE-trnF | seven and a partial | 203–206 bp | * |
| Aphis gossypii | Sternorrhyncha: Aphididae | trnE-trnF | four and a partial | 196 bp | [61] |
| Cavariella salicicola | Sternorrhyncha: Aphididae | trnE-trnF | three | 199 bp | [62] |
| Diuraphis noxia | Sternorrhyncha: Aphididae | trnE-trnF | three and a partial | 194–195 bp | [63] |
| Schizaphis graminum | Sternorrhyncha: Aphididae | trnE-trnF | four and a partial | 151–153 bp | [22] |
| Sitobion avenae | Sternorrhyncha: Aphididae | trnE-trnF | one and a partial | 202 bp | [64] |
| Classification | Species | Level | Rearrangement | Reference |
|---|---|---|---|---|
| Fulgoromorpha: Delphacidae | Laodelphax striatella | family | Inversion of trnC and trnW, inverse transposition: trnT-trnP-nad6 → nad6-trnP-trnT | [27] |
| Fulgoromorpha: Delphacidae | Laodelphax striatellus | family | Inversion of trnC and trnW, transposition of trnH, and inverse transposition: trnT-trnP-nad6 → nad6-trnP-trnT | [38] |
| Fulgoromorpha: Delphacidae | Nilaparvata lugens | family | Inversion of trnC and trnW, inverse transposition: trnT-trnP-nad6 → nad6-trnP-trnT, and insertion two trnC | [27] |
| Heteroptera: Aradidae | Aradacanthia heissi | species | Inversion of trnI and trnQ, inversion of trnC and trnW | [43] |
| Heteroptera: Aradidae | Brachyrhynchus hsiaoi | genus | Inversion of trnI and trnQ | [44] |
| Heteroptera: Aradidae | Neuroctenus parus | genus | Inversion of trnI and trnQ | [23] |
| Heteroptera: Enicocephalidae | Stenopirates sp. | species | Inversion of trnT and trnP, inverse transposition: trnT-trnP-nad6-cytB-trnS-nad1-trnL-rrnL-trnV-rrnS-control region → cytB-trnS-control region-rrnL-trnV-rrnS-nad1-trnL-trnP-trnT-nad6 | [45] |
| Heteroptera: Largidae | Physopelta gutta | superfamily | Inversion of trnT and trnP | [23] |
| Heteroptera: Pyrrhocoridae | Dysdercus cingulatus | superfamily | Inversion of trnT and trnP | [23] |
| Sternorrhyncha: Aleyrodidae | Aleurochiton aceris | genus | Inversion of trnC and trnY, inverse transposition: cox3-trnG-nad3 → insertion the location cob-nad1 | [22] |
| Sternorrhyncha: Aleyrodidae | Aleurodicus dugesii | genus | Inversion of trnC and trnY | [22] |
| Sternorrhyncha: Aleyrodidae | Bemisia afer | genus | Inversion of trnC and trnY, transposition of trnQ, and inverse transposition: cox3-trnG-nad3 → insertion the location control region-rrnS | [25] |
| Sternorrhyncha: Aleyrodidae | Bemisia tabaci | genus | Inversion of trnC and trnY, transposition of trnQ, and inverse transposition: cox3-trnG-nad3 → insertion the location control region-rrnS | [22] |
| Sternorrhyncha: Aleyrodidae | Neomaskellia andropogonis | genus | Transposition of trnH and trnK, and inverse transposition: cox3-trnG-nad3 → insertion the location rrnL-rrnS | [22] |
| Sternorrhyncha: Aleyrodidae | Tetraleurodes acaciae | genus | Inversion of trnC and trnY, transposition of trnQ and trnA, and inverse transposition: cox3-trnG-nad3 → insertion the location control region-rrnS | [22] |
| Sternorrhyncha: Aleyrodidae | Trialeurodes vaporariorum | genus | Inversion of trnI and trnQ, inversion of trnC and trnY, and transposition of trnG | [22] |
5. Phylogenetic Inferences by Hemipteran Mitogenomes
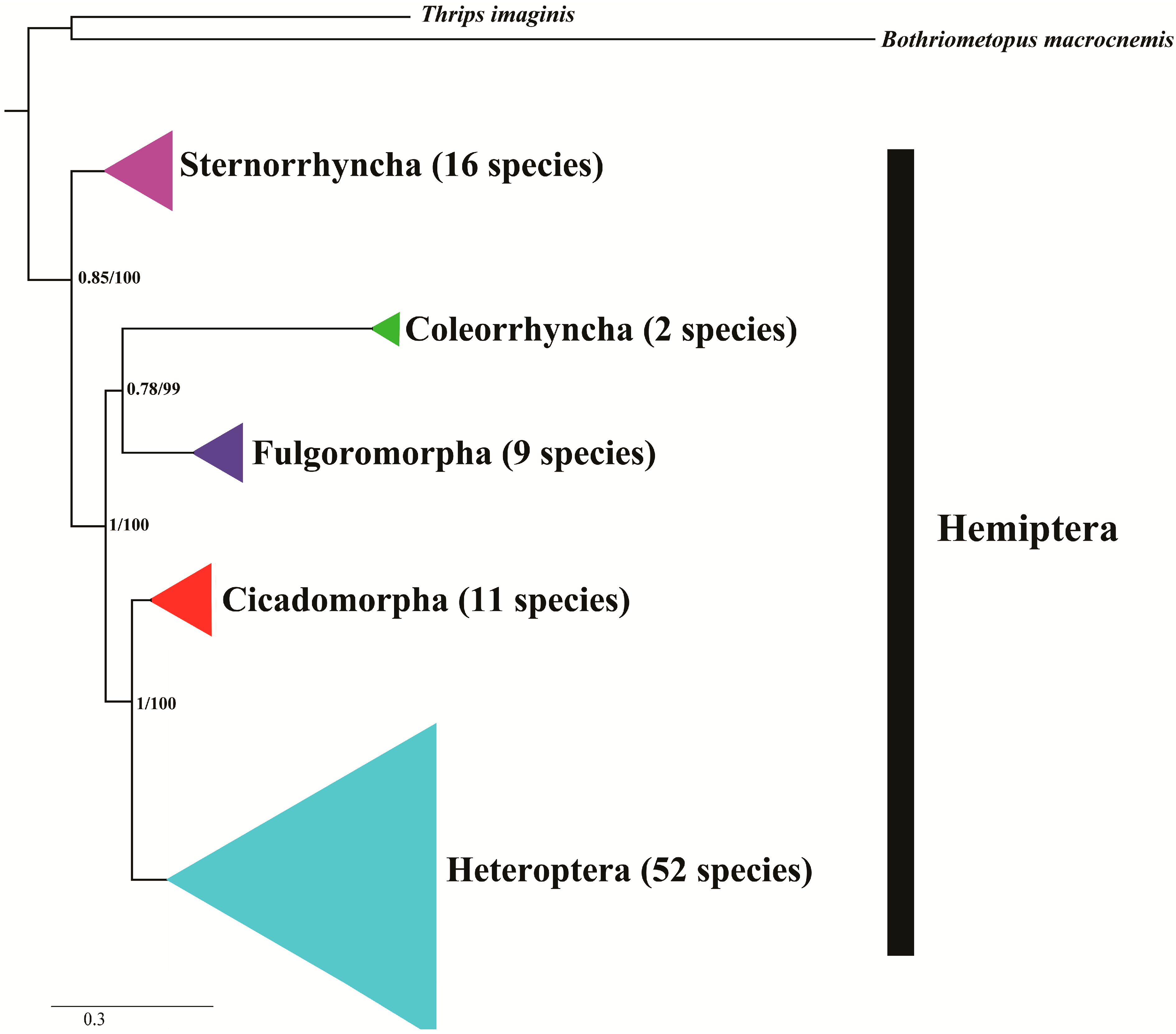
| Classification | Level | Viewpoint | Reference |
|---|---|---|---|
| Cicadomorpha: Cercopidae and Aphrophoridae | family | The monophyly of five Callitettixini species. | [35] |
| Sternorrhyncha: Aleyrodidae, whiteflies | genus | Four types of the mitochondrial gene rearrangements among whiteflies were corresponding to the branches of phylogenetic tree. | [22] |
| Sternorrhyncha: Aphididae, aphids | subfamily | Treat pterocommatines as members of Macrosiphini. | [62] |
| Heteroptera: Reduviidae | subfamily | The monophyly of Reduviidae and the Peiratinae presents a sister position to the Triatominae + (Salyavatinae + Harpactorinae). | [57] |
| Heteroptera: Pentatomomorpha | superfamily | The monophyly of Pentatomoidea, Pyrrhocoroidea, Lygaeoidea, and Coreoidea; Aradoidea and the Trichophora are sister groups. | [23] |
| Heteroptera: Nepomorpha | superfamily | Pleoidea is not a member of the Nepomorpha and Aphelocheiroidea should be grouped back into Naucoroidea. | [19] |
| Heteroptera: Nabidae | subfamily | Three tribes from two subfamilies of Nabidae. | [24] |
| Heteroptera | intraorder | The paraphyly of Cimicomorpha, and within Reduviidae, Harpactorinae is a sister group to the Salyavatinae + Triatominae. | [54] |
| Heteroptera | intraorder | The paraphyly of Cimicomorpha, and Reduviidae was paraphyletic with respect to Anthocoridae and Miridae. | [60] |
| Heteroptera | intraorder | The sister-relationship within the individual infraorders are supported for the Pentatomomorpha, Nepomorpha, Leptopodomorpha and Gerromorpha; Stenopirates sp. (Enicocephalomorpha) is the sister group to all the remaining Heteroptera. | [45] |
| Heteroptera | intraorder | Two Gerromorpha superfamilies were monophyletic in the basal position of these five infraorders. Within Cimicomorpha, Reduviidae was paraphyletic with respect to Anthocoridae and Miridae. | [47] |
| Heteroptera | intraorder | Stenopirates sp. was the sister group to all the remaining Heteroptera; the sister relationships within Nepomorpha and Gerromorpha. | [49] |
6. Experimental Section
6.1. Sampling
6.2. Analysis of Sequence Data
6.3. Phylogenetic Analysis
7. Conclusions and Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Linnaeus, C. Systema naturae per regna tria naturae secundum classes ordines genera species, cum characteribus differentiis synonymis locis. In Proceedings of the 10th edn Reformata, Salviae Holmiae, Stockholm, Sweden; 1758. [Google Scholar]
- Latreille, P.A. Considérations Generales sur l’ordre naturel des Animaux Composant les Classes des Crustaces, des Arachnides, et des Insectes; avec un Tableau Methodique de Leurs Genres, disposes en Familles; Chez F. Schoell: Paris, France, 1810. [Google Scholar]
- Hennig, W. Die Stammesgeschichte der Insekten; Waldemar Kramer: Frankfurt am Main, Germany, 1969. [Google Scholar]
- Kristensen, N.P. Phylogeny of Extant Hexapods. In Insects of Australia, 2nd ed.; Cornell University Press: Ithaca, NY, USA, 1991; pp. 125–140. [Google Scholar]
- Yoshizawa, K.; Saigusa, T. Phylogenetic analysis of paraneopteran orders (Insecta: Neoptera) based on forewing base structure, with comments on monophyly of Auchenorrhyncha (Hemiptera). Syst. Entomol. 2001, 26, 1–13. [Google Scholar] [CrossRef]
- Schuh, R.T.; Slater, J.A. True Bugs of the World (Hemiptera: Heteroptera) Classifiation and Natural History; Cornell University Press: Ithaca, NY, USA, 1995. [Google Scholar]
- Jon, M.; Mick, W. Hemiptera It’s A Bug’s Life; Natural History Museum: Visitado em, Germany, 2010. [Google Scholar]
- Dyck, V.A.; Thomas, B. The brown planthopper problem. IRRI. Los. Banos. Philippines. 1979, 369, 3–17. [Google Scholar]
- Dotson, E.M.; Beard, C.B. Sequence and organization of the mitochondrial genome of the Chagas disease vector, Triatoma dimidiata. Insect Mol. Biol. 2001, 10, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Carver, M.; Gross, G.F.; Woodward, T.E. Hemipter (Bugs, Leafhoppers, Cicadas, Aphids, Scale Insects, etc.); Melbourne University Press: Victoria, Australia, 1991; pp. 429–509. [Google Scholar]
- Evans, J.W. The phylogeny of the Homoptera. Annu. Rev. Entomol. 1963, 8, 77–94. [Google Scholar] [CrossRef]
- Cui, Y.; Xie, Q.; Hua, J.M.; Dang, K.; Zhou, J.; Liu, X.; Wang, G.; Yu, X.; Bu, W.J. Phylogenomics of Hemiptera (Insecta: Paraneoptera) based on mitochondrial genomes. Syst. Entomol. 2013, 38, 233–245. [Google Scholar] [CrossRef]
- Cobben, R.H. Evolutionary Trends in Heteroptera II Mouthpartstructures and Feeding Strategies; Mededelingen Landbouwhogeschool: Wageningen, The Netherlands, 1978. [Google Scholar]
- Hamilton, K.G.A. Morphology and evolution of the rhynchotan head (Insecta: Hemiptera, Homoptera). Can. Entomol. 1981, 113, 953–974. [Google Scholar] [CrossRef]
- Popov, Y.A.; Shcherbakov, D.E. Mesozoic Peloridioidea and their ancestors (Insecta: Hemiptera, Coleorrhyncha). Geol. Palaeontol. 1991, 25, 215–235. [Google Scholar]
- Clary, D.O.; Wolstenholme, D.R. The mitochondrial DNA molecular of Drosophila yakuba: Nucleotide sequence, gene organization and genetic code. J. Mol. Evol. 1985, 22, 252–271. [Google Scholar] [CrossRef] [PubMed]
- Cameron, S.L. Insect mitochondrial genomics: Implications for evolution and phylogeny. Annu. Rev. Entomol. 2014, 59, 95–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boore, J.L. Animal mitochondrial genomes. Nucleic Acids Res. 1999, 27, 1767–1780. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.M.; Li, M.; Dong, P.Z.; Cui, Y.; Xie, Q.; Bu, W.J. Phylogenetic analysis of the true water bugs (Insecta: Hemiptera: Heteroptera: Nepomorpha): Evidence from mitochondrial genomes. BMC Evol. Biol. 2009, 9, 134. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Yang, P.C.; Jiang, F.; Chapuis, M.-P.; Shall, Y.; Sword, G.A.; Kang, L. Mitochondrial genomes reveal the global phylogeography and dispersal routes of the migratory locust. Mol. Ecol. 2012, 21, 4344–4458. [Google Scholar] [CrossRef] [PubMed]
- Nelson, L.A.; Lambkin, C.L.; Batterham, P.; Wallman, J.F.; Dowton, M.; Whiting, M.F.; Yeates, D.K.; Cameron, S.L. Beyond barcoding: a mitochondrial genomics approach to molecular phylogenetics and diagnostics of blowflies (Diptera: Calliphoridae). Gene 2012, 511, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Thao, M.L.; Baumann, L.; Baumann, P. Organization of the mitochondrial genomes of whiteflies, aphids, and psyllids (Hemiptera, Sternorrhyncha). BMC Evol. Biol. 2004, 4, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hua, J.M.; Li, M.; Dong, P.Z.; Cui, Y.; Xie, Q.; Bu, W.J. Comparative and phylogenomic studies on the mitochondrial genomes of Pentatomomorpha (Insecta: Hemiptera: Heteroptera). BMC Genomics 2008, 9, 610. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, H.Y.; Song, F.; Shi, A.M.; Zhou, X.G.; Cai, W.Z. Comparative mitogenomic analysis of damsel bugs representing three tribes in the family Nabidae (Insecta: Hemiptera). PLoS ONE 2012, 7, e45925. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Xiao, N.; Yang, J.; Wang, X.W.; Colvin, J.; Liu, S.S. The complete mitochondrial genome of Bemisia afer (Hemiptera: Aleyrodidae). Mitochondrial DNA 2014. [Google Scholar] [CrossRef]
- The International Aphid Genomics Consortium. Genome sequence of the pea aphid Acyrthosiphon pisum. PLoS Biol. 2010, 8, e1000313. [Google Scholar]
- Zhang, K.J.; Zhu, W.C.; Rong, X.; Zhang, Y.K.; Ding, X.L.; Liu, J.; Chen, D.S.; Du, Y.; Hong, X.Y. The complete mitochondrial genomes of two rice planthoppers, Nilaparvata. lugens and Laodelphax striatellus: Conserved genome rearrangement in Delphacidae and discovery of new characteristics of atp8 and tRNA genes. BMC Genomics 2013, 14, 417. [Google Scholar] [CrossRef] [PubMed]
- Kocher, A.; Kamilari, M.; Lhuillier, E.; Coissac, E.; Péneau, J.; Chave, J.; Murienne, J. Shotgun assembly of the assassin bug Brontostoma colossus mitochondrial genome (Heteroptera, Reduviidae). Gene 2014, 552, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Weirauch, C.; Schuh, R.T. Systematics and evolution of Heteroptera: 25 years of progress. Annu. Rev. Entomol. 2011, 56, 487–510. [Google Scholar] [CrossRef] [PubMed]
- Bechly, G.; Szwedo, J. Coleorrhyncha: Moss bugs. In The Crato. Fossil Beds of Brazil: Window into an Ancient World; Martill, D.M., Bechly, G., Loveridge, R.F., Eds.; Cambridge University Press: Cambridge, UK, 2007; pp. 313–317. [Google Scholar]
- Burckhardt, D.; Bochud, E.; Damgaard, J.; W.gibbs, G.; Hartung, V.; Larivière, M.C.; Wyniger, D.; Zuercher, I. A review of the moss bug genus Xenophyes (Hemiptera: Coleorrhyncha: Peloridiidae) from New Zealand: Systematics and biogeography. Zootaxa 2011, 2923, 1–26. [Google Scholar]
- Gao, J.; Liang, A.P. The complete mitochondrial genome of Hemiodoecus leai (Hemiptera: Coleorrhyncha: Peloridiidae). Mitochondrial DNA 2014. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, X.L.; Qiao, G.X. The complete mitochondrial genome of Cervaphis quercus (Insecta: Hemiptera: Aphididae: Greenideinae). Insect Sci. 2014, 21, 278–290. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.B.; Beckenbach, A.T. Insect mitochondrial genomics: The complete mitochondrial genome sequence of the meadow spittlebug Philaenus spumarius (Hemiptera: Auchenorrhyncha: Cercopoidae). Genome 2005, 48, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Bu, C.P.; Wipfler, B.; Liang, A.P. Comparative analysis of the mitochondrial genomes of callitettixini spittlebugs (Hemiptera: Cercopidae) confirms the overall high evolutionary speed of the at-rich region but reveals the presence of short conservative elements at the tribal level. PLoS ONE 2014, 9, e109140. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liang, A.P. The complete mitochondrial genome of spittlebug Paphnutius ruficeps (Insecta: Hemiptera: Cercopidae) with a fairly short putative control region. Acta Biochim. Biophys. Sin. 2013, 45, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.N.; Wang, M.X.; Cui, L.; Chen, X.X.; Han, B.Y. Complete mitochondrial genome of Empoasca vitis (Hemiptera: Cicadellidae). Mitochondrial DNA 2014. [Google Scholar] [CrossRef]
- Song, N.; Liang, A.P. Complete mitochondrial genome of the small brown planthopper, Laodelphax striatellus (Delphacidae: Hemiptera), with a novel gene order. Zool. Sci. 2009, 26, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Liang, A.P. The complete mitochondrial genome sequence of Geisha distinctissima (Hemiptera: Flatidae) and comparison with other Hemipteran insects. Acta Biochim. Biophys. Sin. 2009, 41, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Liang, A.P.; Bu, C.P. A molecular phylogeny of Hemiptera inferred from mitochondrial genome sequences. PLoS ONE 2012, 7, e48778. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Liang, A.P.; Ma, C. The complete mitochondrial genome sequence of the planthopper, Sivaloka damnosus. J. Insect. Sci. 2010, 10, 76. [Google Scholar] [CrossRef] [PubMed]
- Du, B.Z.; Niu, F.F.; Wei, S.J. The complete mitochondrial genome of the predatory bug Orius sauteri (Poppius) (Hemiptera: Anthocoridae). Mitochondrial DNA 2014. [Google Scholar] [CrossRef]
- Shi, A.M.; Li, H.; Bai, X.S.; Dai, X.; Chang, J.; Guilbert, E.; Cai, W.Z. The complete mitochondrial genome of the flat bug Aradacanthia heissi (Hemiptera: Aradidae). Zootaxa 2012, 3238, 23–38. [Google Scholar]
- Li, H.; Shi, A.M.; Song, F.; Cai, W.Z. Complete mitochondrial genome of the flat bug Brachyrhynchus. hsiaoi (Hemiptera: Aradidae). Mitochondrial DNA 2014. [Google Scholar] [CrossRef]
- Li, H.; Liu, H.; Shi, A.M.; Stys, P.; Zhou, X.G.; Cai, W.Z. The complete mitochondrial genome and novel gene arrangement of the unique-headed bug Stenopirates sp. (Hemiptera: Enicocephalidae). PLoS ONE 2012, 7, e29419. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yi, W.B.; Zhang, H.G.; Xie, Q.; Bu, W.J. Complete mitochondrial genome of the birch catkin bug Kleidocerys resedae resedae, as the first representative from the family Lygaeidae (Hemiptera: Heteroptera: Lygaeoidea). Mitochondrial DNA 2014. [Google Scholar] [CrossRef]
- Li, T.; Gao, C.; Cui, Y.; Xie, Q.; Bu, W.J. The complete mitochondrial genome of the stalk-eyed bug Chauliops fallax scott, and the monophyly of malcidae (Hemiptera: Heteroptera). PLoS ONE 2013, 8, e55381. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, H.; Xun, H.Z.; Cai, W.Z. Complete mitochondrial genome sequence of the plant bug Adelphocoris fasciaticollis (Hemiptera: Heteroptera: Miridae). Mitochondrial DNA 2014. [Google Scholar] [CrossRef]
- Wang, P.; Li, H.; Wang, Y.; Zhang, J.H.; Dai, X.; Chang, J.; Hu, B.W.; Cai, W.Z. The mitochondrial genome of the plant bug Apolygus lucorum (Hemiptera: Miridae): Presently known as the smallest in Heteroptera. Insect Sci. 2014, 21, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Xun, H.Z.; Chang, J.; Zhang, J.H.; Hu, B.W.; Li, H.; Yuan, X.Q.; Cai, W.Z. The complete mitochondrial genome of the plant bug Nesidiocoris tenuis (Reuter) (Hemiptera: Miridae: Bryocorinae: Dicyphini). Zootaxa 2012, 3554, 30–44. [Google Scholar]
- Li, H.; Liu, H.Y.; Cao, L.M.; Shi, A.M.; Yang, H.L.; Cai, W.Z. The complete mitochondrial genome of the damsel bug Alloeorhynchus bakeri (Hemiptera: Nabidae). Int. J. Biol. Sci. 2012, 8, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.L.; Yuan, M.L.; Shen, Y.Y. The complete mitochondrial genome of Dolycoris baccarum (Insecta: Hemiptera: Pentatomidae). Mitochondrial DNA 2013. [Google Scholar] [CrossRef]
- Lee, W.; Kang, J.; Jung, C.; Hoelmer, K.; Lee, S.; Lee, S. Complete mitochondrial genome of brown marmorated stink bug Halyomorpha halys (Hemiptera: Pentatomidae), and phylogenetic relationships of hemipteran suborders. Mol. Cells 2009, 28, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Gao, J.Y.; Liu, H.Y.; Liu, H.; Liang, A.P.; Cai, W.Z. The architecture and complete sequence of mitochondrial genome of an assassin bug Agriosphodrus dohrni (Hemiptera: Reduviidae). Int. J. Biol. Sci. 2011, 7, 792–804. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Gao, J.Y.; Cai, W.Z. Complete mitochondrial genome of the assassin bug Oncocephalus breviscutum (Hemiptera: Reduviidae). Mitochondrial DNA 2013. [Google Scholar] [CrossRef]
- Zhao, G.Y.; Li, H.; Song, F.; Cai, W.Z. The complete mitochondrial genome of an assassin bug Peirates Arcuatus (Hemiptera: Reduviidae). Mitochondrial DNA 2014. [Google Scholar] [CrossRef]
- Gao, J.Y.; Li, H.; Truong, X.L.; Dai, X.; Chang, J.; Cai, W.Z. Complete nucleotide sequence and organization of the mitochondrial genome of Sirthenea flavipes (Hemiptera: Reduviidae: Peiratinae) and comparison with other assassin bugs. Zootaxa 2013, 3699, 1–16. [Google Scholar] [CrossRef]
- Song, W.; Li, H.; Song, F.; Liu, L.; Wang, P.; Xun, H.; Dai, X.; Chang, J.; Cai, W.Z. The complete mitochondrial genome of a tessaratomid bug, Eusthenes cupreus (Hemiptera: Heteroptera: Pentatomomorpha: Tessaratomidae). Zootaxa 2013, 3620, 260–272. [Google Scholar] [CrossRef]
- Yang, W.; Yu, W.W.; Du, Y.Z. The complete mitochondrial genome of the sycamore lace bug Corythucha ciliata (Hemiptera: Tingidae). Gene 2013, 532, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.T.; Li, H.; Jiang, P.; Song, F.; Ye, Z.; Yuang, X.; Dai, X.; Chang, J.; Cai, W.Z. Sequence and organization of the mitochondrial genome of an urostylidid bug, Urochela. quadrinotata Reuter (Hemiptera: Urostylididae). Entomotaxonomia 2012, 34, 613–623. [Google Scholar]
- Zhang, S.; Luo, J.; Wang, C.; Lv, L.; Li, C.; Jiang, W.; Cui, J.; Rajput, L.B. Complete mitochondrial genome of Aphis gossypii Glover (Hemiptera: Aphididae). Mitochondrial DNA 2014. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, X.L.; Qiao, G.X. Comparative analysis of mitochondrial genomes of five aphid species (Hemiptera: Aphididae) and phylogenetic implications. PLoS ONE 2013, 8, e77511. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Ma, C.; Edwards, O.; Fuller, S.; Kang, L. The mitochondrial genome of the Russian wheat aphid Diuraphis noxia: Large repetitive sequences between trnE and trnF in aphids. Gene 2014, 533, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zheng, J.; Liang, L.; Fuller, S.; Ma, C.S. The complete mitochondrial genome of Sitobion avenae (Hemiptera: Aphididae). Mitochondrial DNA 2014. [Google Scholar] [CrossRef]
- Zhang, Q.L.; Guo, Z.L.; Yuan, M.L. The complete mitochondrial genome of Poratrioza Sinica (Insecta: Hemiptera: Psyllidae). Mitochondrial DNA 2014. [Google Scholar] [CrossRef]
- Yukuhiro, K.; Sezutsu, H.; Itoh, M.; Shimizu, K.; Banno, Y. Significant levels of sequence divergence and gene rearrangements have occurred between the mitochondrial genomes of the wild mulberry silkmoth, Bombyx mandarina, and its close relative, the domesticated silkmoth, Bombyx mori. Mol. Biol. Evol. 2002, 19, 1385–1389. [Google Scholar] [CrossRef] [PubMed]
- Ojala, D.; Montoya, J.; Attardi, G. tRNA punctuation model of RNA processing in human mitochondria. Nature 1981, 290, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Nei, M.; Gojobori, T. Simple methods for estimating the numbers of synonymous and nonsynonymous nucleotide substitutions. Mol. Biol. Evol. 1986, 3, 418–426. [Google Scholar] [PubMed]
- Schmidt, T.R.; Wu, W.; Goodman, M.; Grossman, L.I. Evolution of nuclearand mitochondrial-encoded subunit interaction in cytochrome c oxidase. Mol. Biol. Evol. 2001, 18, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Hebert, P.D.N.; Ratnasingham, S.; de Waard, J.R. Barcoding animal life: Cytochrome c oxidase subunit 1 divergences among closely related species. Proc. R. Soc. Lond. B 2003, 270, S96–S99. [Google Scholar] [CrossRef] [PubMed]
- Castellana, S.; Vicario, S.; Saccone, C. Evolutionary patterns of the mitochondrial genome in Metazoa: Exploring the role of mutation and selection in mitochondrial protein coding genes. Genome Biol. Evol. 2011, 3, 1067–1079. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.J.; Shi, M.; Sharkey, M.J.; van Achterberg, C.; Chen, X.X. Comparative mitogenomics of Braconidae (Insecta: Hymenoptera) and the phylogenetic utility of mitochondrial genomes with special reference to holometabolous insects. BMC Genomics 2010, 11, 371. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Cha, S.Y.; Yoon, M.H.; Hwang, J.S.; Lee, S.M.; Sohn, H.D.; Jin, B.R. The complete nucleotide sequence and geneorganization of the mitochondrial genome of the oriental mole cricket, Gryllotalpa orientalis (Orthoptera: Gryllotalpidae). Gene 2005, 353, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.X.; Szymura, J.M.; Hewitt, G.M. Evolution and structural conservation of the control region of insect mitochondrial DNA. J. Mol. Evol. 1995, 40, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Fenn, J.D.; Cameron, S.L.; Whiting, M.F. The complete mitochondrial genome sequence of the Mormon cricket (Anabrus simplex: Tettigoniidae: Orthoptera) and an analysis of control region variability. Insect Mol. Biol. 2007, 16, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Cook, C.E.; Yue, Q.; Akam, M. Mitochondrial genomes suggest that hexapods and crustaceans are mutually paraphyletic. Proc. Biol. Sci. 2005, 272, 1295–1304. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, J.J.; Johnston, J.S.; Cannone, J.J.; Gutell, R.R. Characteristics of the nuclear (18S, 5.8S, 28S and 5S) and mitochondrial (12S and 16S) rRNA genes of Apis mellifera (Insecta: Hymenoptera): Structure, organization and retrotransposable elements. Insect Mol. Biol. 2006, 15, 657–686. [Google Scholar] [CrossRef] [PubMed]
- Dowton, M.; Castro, L.R.; Austin, A.D. Mitochondrial gene rearrangements as phylogenetic characters in the invertebrates: The examination of genome “morphology”. Invertebr. Syst. 2002, 16, 345–356. [Google Scholar] [CrossRef]
- Burckhardt, D. Taxonomy and phylogeny of the Gondwanan moss bugs or Peloridiidae (Hemiptera, Coleorrhyncha). Dtsch. Entomol. Z 2009, 56, 173–235. [Google Scholar] [CrossRef]
- Kuechler, S.M.; Gibbs, G.; Burckhardt, D.; Dettner, K.; Hartung, V. Diversity of bacterial endosymbionts and bacteria-Host co-evolution in Gondwanan relict moss bugs (Hemiptera: Coleorrhyncha: Peloridiidae). Environ. Microbiol. 2013, 15, 2031–2042. [Google Scholar] [CrossRef] [PubMed]
- Koga, R.; Bennett, G.; Cryan, J.R.; Moran, N.A. Evolutionary replacement of obligate symbionts in an ancient and diverse insect lineage. Environ. Microbiol. 2013, 5, 2073–2081. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Librado, P.; Rozas, J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef] [PubMed]
- Guindon, S.; Gascuel, O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 2003, 52, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef] [PubMed]
- Posada, D. jModelTest: Phylogenetic model averaging. Mol. Biol. Evol. 2008, 25, 1253–1256. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Chen, J.; Jiang, L.-Y.; Qiao, G.-X. Hemipteran Mitochondrial Genomes: Features, Structures and Implications for Phylogeny. Int. J. Mol. Sci. 2015, 16, 12382-12404. https://doi.org/10.3390/ijms160612382
Wang Y, Chen J, Jiang L-Y, Qiao G-X. Hemipteran Mitochondrial Genomes: Features, Structures and Implications for Phylogeny. International Journal of Molecular Sciences. 2015; 16(6):12382-12404. https://doi.org/10.3390/ijms160612382
Chicago/Turabian StyleWang, Yuan, Jing Chen, Li-Yun Jiang, and Ge-Xia Qiao. 2015. "Hemipteran Mitochondrial Genomes: Features, Structures and Implications for Phylogeny" International Journal of Molecular Sciences 16, no. 6: 12382-12404. https://doi.org/10.3390/ijms160612382





