Ultrasound Imaging for Risk Assessment in Atherosclerosis
Abstract
:1. Introduction
2. Atherosclerosis
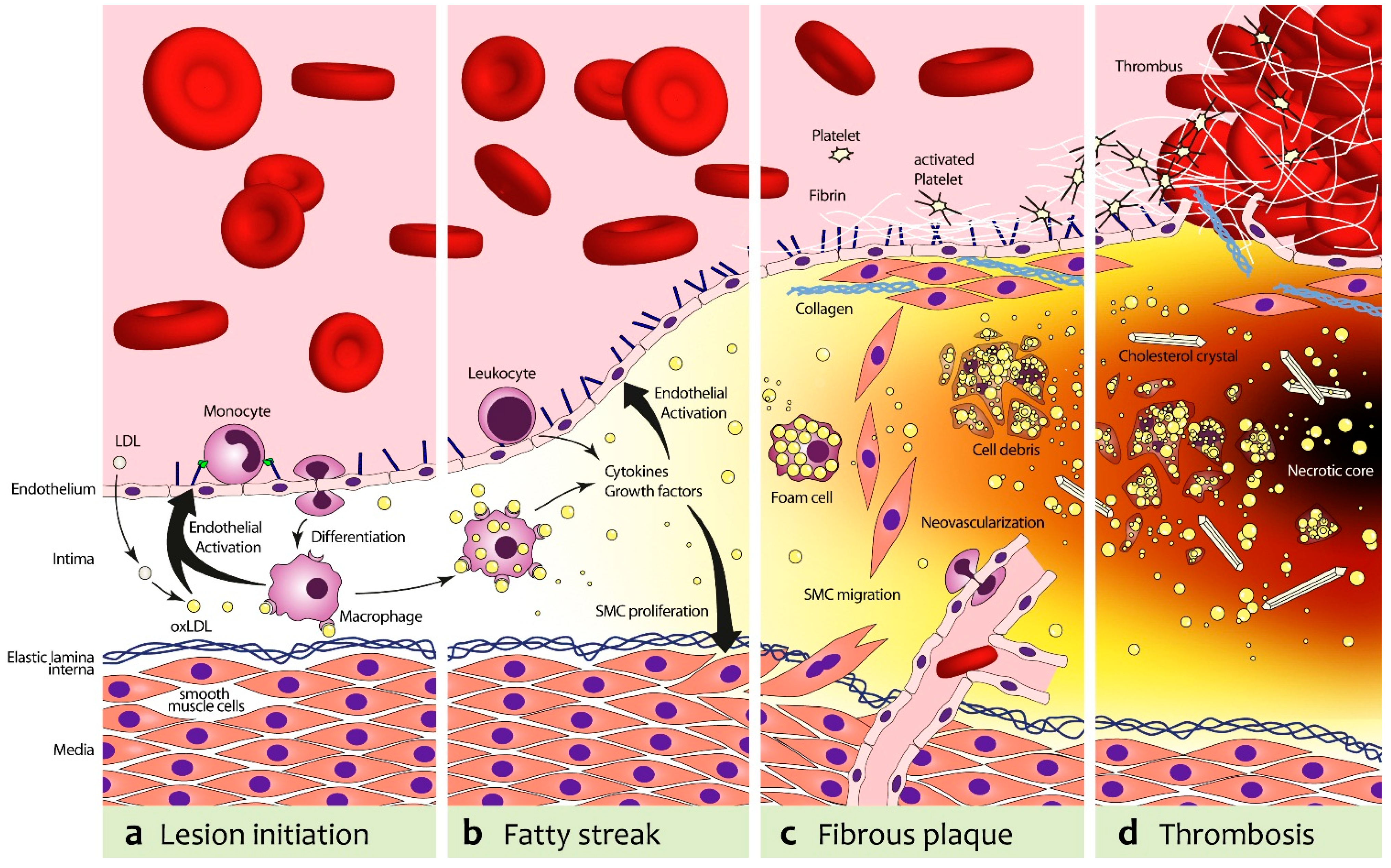
3. Anatomical Imaging of Atherosclerosis with Ultrasound
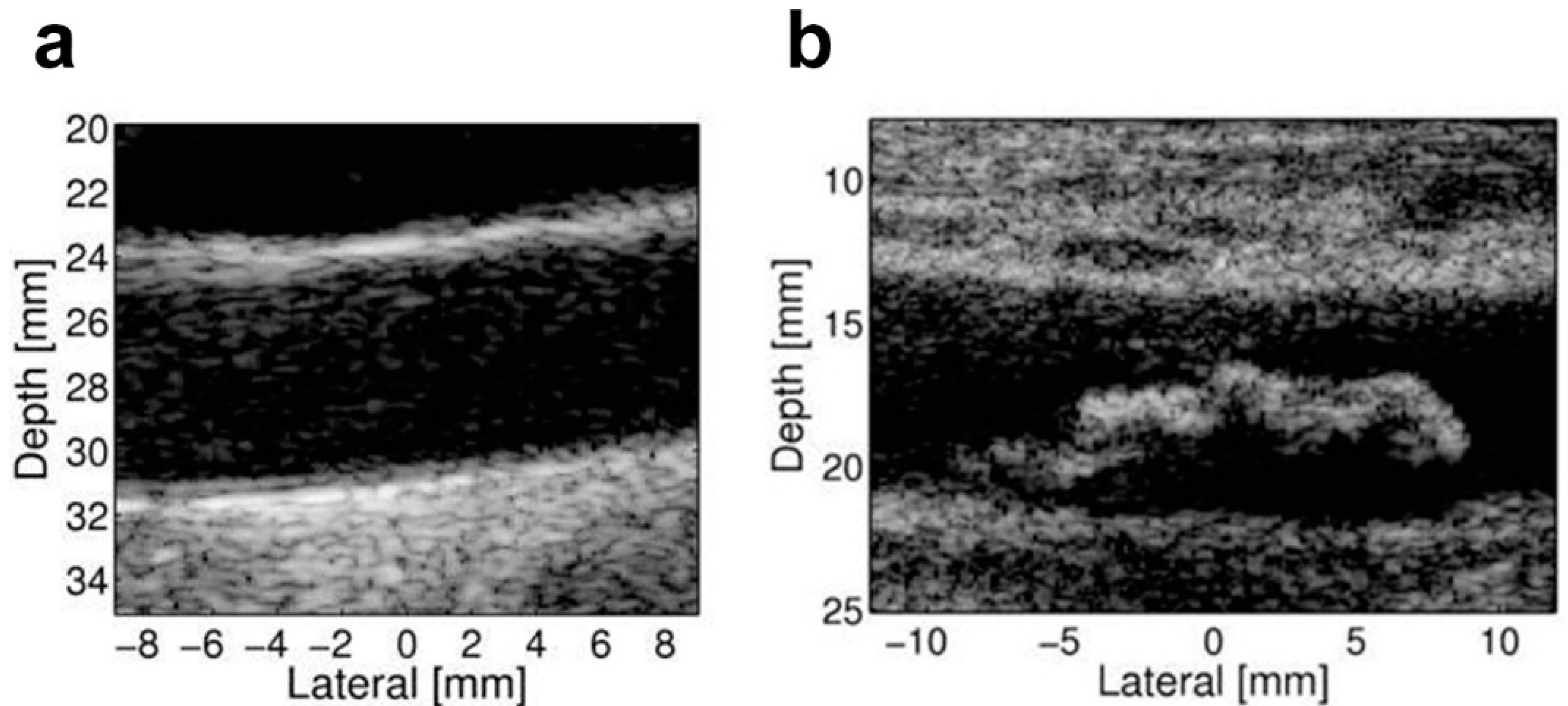
4. Biomechanical Imaging
5. Contrast Enhanced Ultrasound Imaging of Atherosclerosis
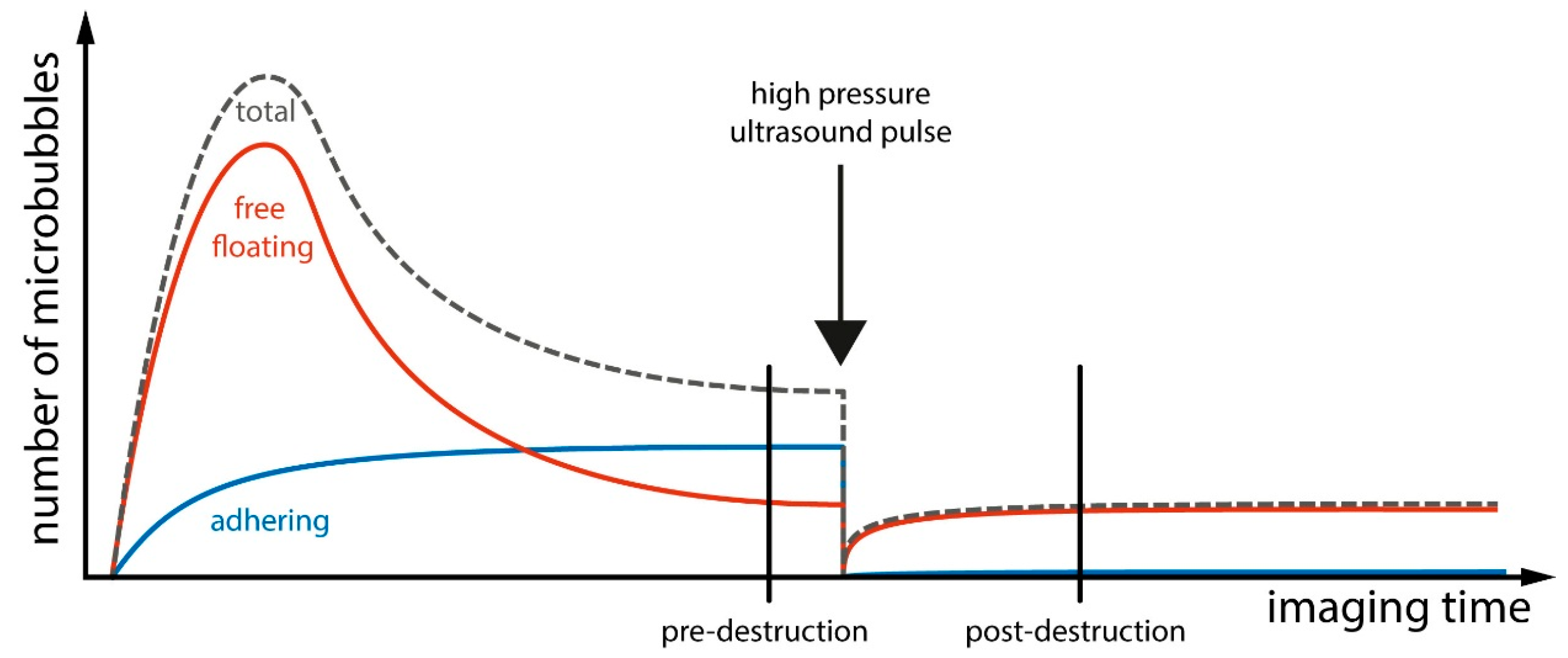
5.1. Physical Aspects of Contrast Enhanced Ultrasound Imaging
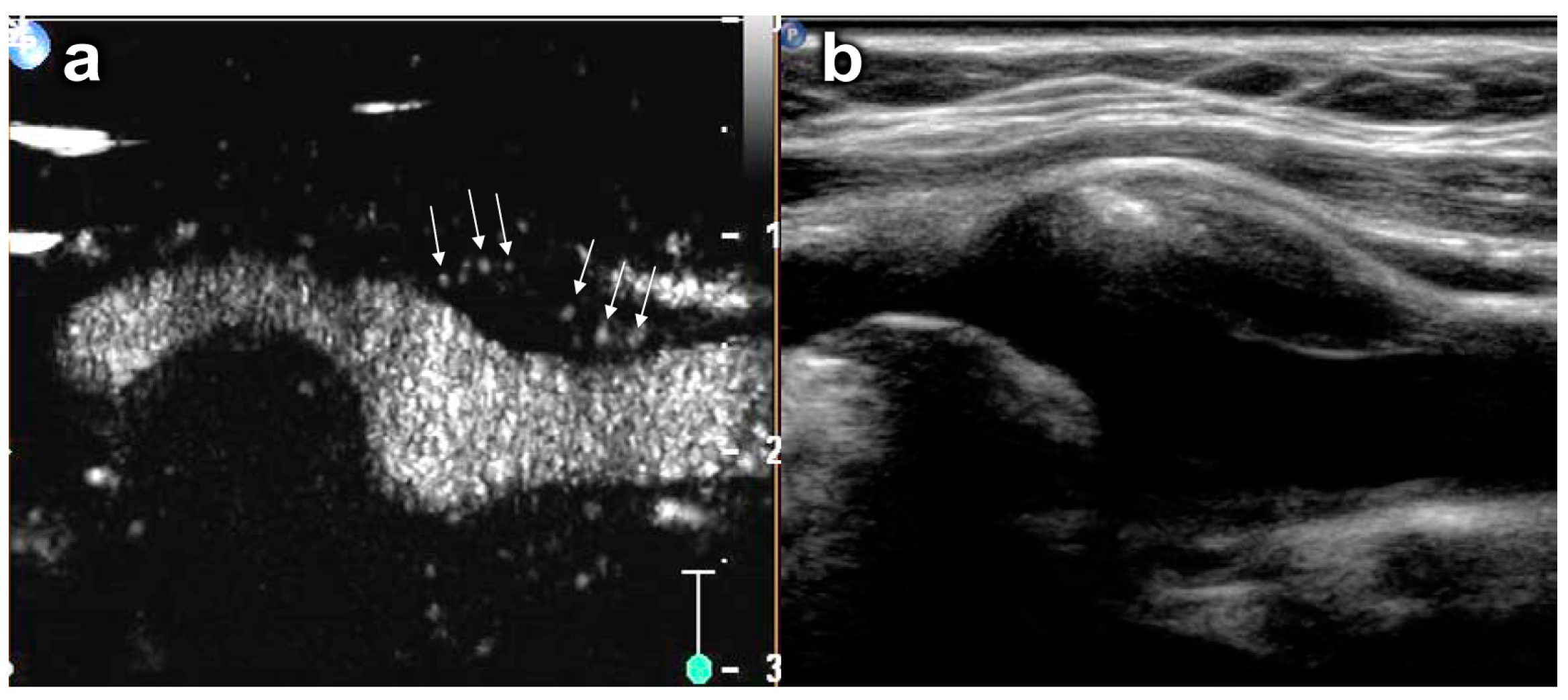
5.2. Plaque Neovascularization
5.3. Basic Principles of Molecular Imaging with Ultrasound Contrast Agents
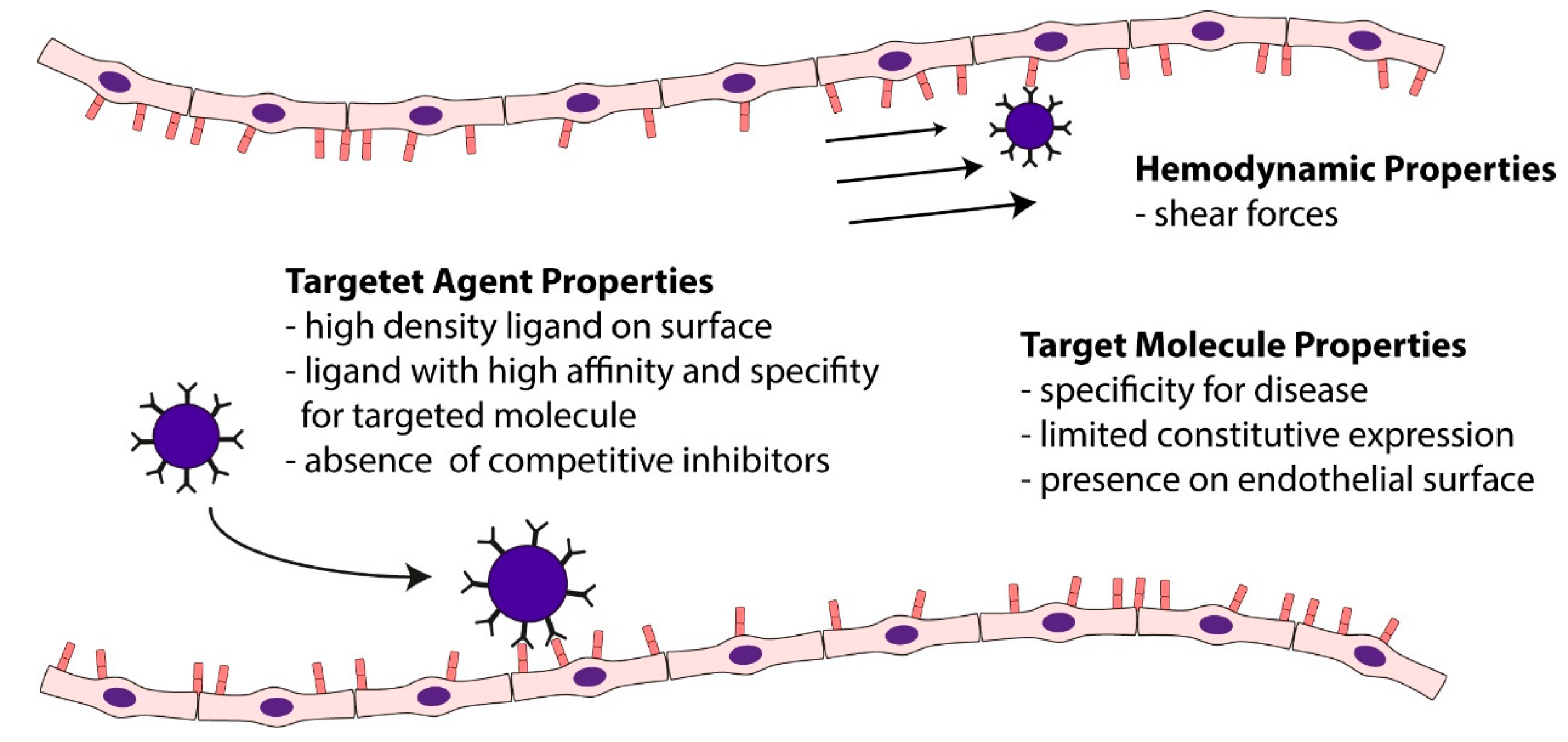
5.4. Ultrasound Molecular Imaging of Vascular Inflammation in Atherosclerosis

5.5. Ultrasound Molecular Imaging of Thrombus Formation and Vascular Thrombogenic Potential in Atherosclerosis
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ross, R. Atherosclerosis—An inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Wildgruber, M.; Swirski, F.K.; Zernecke, A. Molecular imaging of inflammation in atherosclerosis. Theranostics 2013, 3, 865–884. [Google Scholar] [CrossRef] [PubMed]
- Enos, W.F.; Holmes, R.H.; Beyer, J. Coronary disease among United-States soldiers killed in action in korea. JAMA J. Am. Med. Assoc. 1953, 152, 1090–1093. [Google Scholar] [CrossRef]
- Strong, J.P.; Malcom, G.T.; McMahan, C.A.; Tracy, R.E.; Newman, W.P., 3rd; Herderick, E.E.; Cornhill, J.F. Prevalence and extent of atherosclerosis in adolescents and young adults: Implications for prevention from the pathobiological determinants of atherosclerosis in youth study. JAMA 1999, 281, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Health Status, OECD Database 2014. Available online: https://stats.oecd.org/index.aspx?DataSetCode=HEALTH_STAT (accessed on 20 April 2015).
- Murray, C.J.L.; Vos, T.; Lozano, R.; Naghavi, M.; Flaxman, A.D.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; et al. Disability-adjusted life years (dalys) for 291 diseases and injuries in 21 regions, 1990–2010: A systematic analysis for the global burden of disease study 2010. Lancet 2012, 380, 2197–2223. [Google Scholar] [CrossRef] [PubMed]
- Ginghina, C.; Bejan, I.; Ceck, C.D. Modern risk stratification in coronary heart disease. J. Med. Life 2011, 4, 377–386. [Google Scholar] [PubMed]
- Witztum, J.L.; Steinberg, D. Role of oxidized low-density-lipoprotein in atherogenesis. J. Clin. Investig. 1991, 88, 1785–1792. [Google Scholar] [CrossRef] [PubMed]
- Kunsch, C.; Medford, R.M. Oxidative stress as a regulator of gene expression in the vasculature. Circ. Res. 1999, 85, 753–766. [Google Scholar] [CrossRef] [PubMed]
- Stocker, R.; Keaney, J.F. Role of oxidative modifications in atherosclerosis. Physiol. Rev. 2004, 84, 1381–1478. [Google Scholar] [CrossRef] [PubMed]
- Marui, N.; Offermann, M.K.; Swerlick, R.; Kunsch, C.; Rosen, C.A.; Ahmad, M.; Alexander, R.W.; Medford, R.M. Vascular cell-adhesion molecule-1 (VCAM-1) gene-transcription and expression are regulated through an antioxidant sensitive mechanism in human vascular endothelial-cells. J. Clin. Investig. 1993, 92, 1866–1874. [Google Scholar] [CrossRef] [PubMed]
- Bevilacqua, M.P. Endothelial-leukocyte adhesion molecules. Annu. Rev. Immunol. 1993, 11, 767–804. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.Q.; Hafezi-Moghadam, A.; Ley, K. Role of vascular cell adhesion molecule-1 and fibronectin connecting segment-1 in monocyte rolling and adhesion on early atherosclerotic lesions. Circ. Res. 2000, 87, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.J.; Sheedy, F.J.; Fisher, E.A. Macrophages in atherosclerosis: A dynamic balance. Nat. Rev. Immunol. 2013, 13, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Falk, E.; Shah, P.K.; Fuster, V. Coronary plaque disruption. Circulation 1995, 92, 657–671. [Google Scholar] [CrossRef] [PubMed]
- Theilmeier, G.; Michiels, C.; Spaepen, E.; Vreys, I.; Collen, D.; Vermylen, J.; Hoylaerts, M.F. Endothelial von willebrand factor recruits platelets to atherosclerosis-prone sites in response to hypercholesterolemia. Blood 2002, 99, 4486–4493. [Google Scholar] [CrossRef] [PubMed]
- Steffel, J.; Hermann, M.; Greutert, H.; Gay, S.; Luscher, T.F.; Ruschitzka, F.; Tanner, F.C. Celecoxib decreases endothelial tissue factor expression through inhibition of c-Jun terminal NH2 kinase phosphorylation. Circulation 2005, 111, 1685–1689. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, P.M.; Boutouyrie, P.; Cunha, P.; Kotsis, V.; Narkiewicz, K.; Parati, G.; Rietzschel, E.; Scuteri, A.; Laurent, S. Early vascular ageing in translation: From laboratory investigations to clinical applications in cardiovascular prevention. J. Hypertens. 2013, 31, 1517–1526. [Google Scholar] [CrossRef] [PubMed]
- Glagov, S.; Weisenberg, E.; Zarins, C.K.; Stankunavicius, R.; Kolettis, G.J. Compensatory enlargement of human atherosclerotic coronary arteries. N. Engl. J. Med. 1987, 316, 1371–1375. [Google Scholar] [CrossRef] [PubMed]
- Aggoun, Y.; Bonnet, D.; Sidi, D.; Girardet, J.P.; Brucker, E.; Polak, M.; Safar, M.E.; Levy, B.I. Arterial mechanical changes in children with familial hypercholesterolemia. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 2070–2075. [Google Scholar] [CrossRef] [PubMed]
- Doyle, B.; Caplice, N. Plaque neovascularization and antiangiogenic therapy for atherosclerosis. J. Am. Coll. Cardiol. 2007, 49, 2073–2080. [Google Scholar] [CrossRef] [PubMed]
- Amato, M.; Montorsi, P.; Ravani, A.; Oldani, E.; Galli, S.; Ravagnani, P.M.; Tremoli, E.; Baldassarre, D. Carotid intima-media thickness by B-mode ultrasound as surrogate of coronary atherosclerosis: Correlation with quantitative coronary angiography and coronary intravascular ultrasound findings. Eur. Heart J. 2007, 28, 2094–2101. [Google Scholar] [CrossRef] [PubMed]
- Stork, S.; Feelders, R.A.; van den Beld, A.W.; Steyerberg, E.W.; Savelkoul, H.F.J.; Lamberts, S.W.J.; Grobbee, D.E.; Bots, M.L. Prediction of mortality risk in the elderly. Am. J. Med. 2006, 119, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Mathiesen, E.B.; Johnsen, S.H.; Wilsgaard, T.; Bonaa, K.H.; Lochen, M.L.; Njolstad, I. Carotid plaque area and intima-media thickness in prediction of first-ever ischemic stroke a 10-year follow-up of 6584 men and women: The tromso study. Stroke 2011, 42, 972–978. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.D.; Ham, K.L.; Dumont, D.M.; Sileshi, B.; Trahey, G.E.; Dahl, J.J. The development and potential of acoustic radiation force impulse (ARFI) imaging for carotid artery plaque characterization. Vasc. Med. 2011, 16, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Gray-Weale, A.C.; Graham, J.C.; Burnett, J.R.; Byrne, K.; Lusby, R.J. Carotid artery atheroma: Comparison of preoperative B-mode ultrasound appearance with carotid endarterectomy specimen pathology. J. Cardiovasc. Surg. 1988, 29, 676–681. [Google Scholar]
- Mathiesen, E.B.; Bonaa, K.H.; Joakimsen, O. Echolucent plaques are associated with high risk of ischemic cerebrovascular events in carotid stenosis: The tromso study. Circulation 2001, 103, 2171–2175. [Google Scholar] [CrossRef] [PubMed]
- Reiter, M.; Effenberger, I.; Sabeti, S.; Mlekusch, W.; Schlager, O.; Dick, P.; Puchner, S.; Amighi, J.; Bucek, R.A.; Minar, E.; et al. Increasing carotid plaque echolucency is predictive of cardiovascular events in high-risk patients. Radiology 2008, 248, 1050–1055. [Google Scholar] [CrossRef] [PubMed]
- Nambi, V.; Chambless, L.; Folsom, A.R.; He, M.; Hu, Y.J.; Mosley, T.; Volcik, K.; Boerwinkle, E.; Ballantyne, C.M. Carotid intima-media thickness and presence or absence of plaque improves prediction of coronary heart disease risk the aric (atherosclerosis risk in communities) study. J. Am. Coll. Cardiol. 2010, 55, 1600–1607. [Google Scholar] [CrossRef] [PubMed]
- Bots, M.L.; Hoes, A.W.; Koudstaal, P.J.; Hofman, A.; Grobbee, D.E. Common carotid intima-media thickness and risk of stroke and myocardial infarction—The rotterdam study. Circulation 1997, 96, 1432–1437. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.A.; den Ruijter, H.M.; Grobbee, D.E.; Bots, M.L. Results from a carotid intima-media thickness trial as a decision tool for launching a large-scale morbidity and mortality trial. Circ. Cardiovasc. Imaging 2013, 6, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Den Ruijter, H.M.; Peters, S.A.; Anderson, T.J.; Britton, A.R.; Dekker, J.M.; Eijkemans, M.J.; Engstrom, G.; Evans, G.W.; de Graaf, J.; Grobbee, D.E.; et al. Common carotid intima-media thickness measurements in cardiovascular risk prediction: A meta-analysis. JAMA 2012, 308, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Finn, A.V.; Kolodgie, F.D.; Virmani, R. Correlation between carotid intimal/medial thickness and atherosclerosis: A point of view from pathology. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Molinari, F.; Zeng, G.; Suri, J.S. An integrated approach to computer-based automated tracing and its validation for 200 common carotid arterial wall ultrasound images: A new technique. J. Ultrasound Med. 2010, 29, 399–418. [Google Scholar] [PubMed]
- Sillesen, H.; Muntendam, P.; Adourian, A.; Entrekin, R.; Garcia, M.; Falk, E.; Fuster, V. Carotid plaque burden as a measure of subclinical atherosclerosis: Comparison with other tests for subclinical arterial disease in the high risk plaque bioimage study. JACC Cardiovasc. Imaging 2012, 5, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Baber, U.; Mehran, R.; Sartori, S.; Schoos, M.M.; Sillesen, H.; Muntendam, P.; Garcia, M.J.; Gregson, J.; Pocock, S.; Falk, E.; et al. Prevalence, impact, and predictive value of detecting subclinical coronary and carotid atherosclerosis in asymptomatic adults: The bioimage study. J. Am. Coll. Cardiol. 2015, 65, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, M.; Terashima, M.; Awano, K.; Kijima, M.; Nakatani, S.; Daikoku, S.; Ito, K.; Yasumura, Y.; Miyatake, K. Morphology of vulnerable coronary plaque: Insights from follow-up of patients examined by intravascular ultrasound before an acute coronary syndrome. J. Am. Coll. Cardiol. 2000, 35, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, R.A.; Edwards, W.D.; Warnes, C.A.; Reeder, G.S.; Holmes, D.R.; Tajik, A.J.; Yock, P.G. Intravascular ultrasound imaging-in vitro validation and pathological correlation. J. Am. Coll. Cardiol. 1990, 16, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Prati, F.; Arbustini, E.; Labellarte, A.; Dal Bello, B.; Sommariva, L.; Mallus, M.T.; Pagano, A.; Boccanelli, A. Correlation between high frequency intravascular ultrasound and histomorphology in human coronary arteries. Heart (Br. Card. Soc.) 2001, 85, 567–570. [Google Scholar] [CrossRef]
- Palmer, N.D.; Northridge, D.; Lessells, A.; McDicken, W.N.; Fox, K.A.A. In vitro analysis of coronary atheromatous lesions by intravascular ultrasound-reproducibility and histological correlation of lesion morphology. Eur. Heart J. 1999, 20, 1701–1706. [Google Scholar] [CrossRef] [PubMed]
- Potkin, B.N.; Bartorelli, A.L.; Gessert, J.M.; Neville, R.F.; Almagor, Y.; Roberts, W.C.; Leon, M.B. Coronary-artery imaging with intravascular high-frequency ultrasound. Circulation 1990, 81, 1575–1585. [Google Scholar] [CrossRef] [PubMed]
- Mintz, G.S.; Nissen, S.E.; Anderson, W.D.; Bailey, S.R.; Erbel, R.; Fitzgerald, P.J.; Pinto, F.J.; Rosenfield, K.; Siegel, R.J.; Tuzcu, E.M.; et al. American college of cardiology clinical expert consensus document on standards for acquisition, measurement and reporting of intravascular ultrasound studies (ivus). A report of the american college of cardiology task force on clinical expert consensus documents. J. Am. Coll. Cardiol. 2001, 37, 1478–1492. [Google Scholar] [CrossRef] [PubMed]
- Schoenhagen, P.; Stone, G.W.; Nissen, S.E.; Grines, C.L.; Griffin, J.; Clemson, B.S.; Vince, D.G.; Ziada, K.; Crowe, T.; Apperson-Hanson, C.; et al. Coronary plaque morphology and frequency of ulceration distant from culprit lesions in patients with unstable and stable presentation. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1895–1900. [Google Scholar] [CrossRef] [PubMed]
- Wuyts, F.L.; Vanhuyse, V.J.; Langewouters, G.J.; Decraemer, W.F.; Raman, E.R.; Buyle, S. Elastic properties of human aortas in relation to age and atherosclerosis—A structural model. Phys. Med. Biol. 1995, 40, 1577–1597. [Google Scholar] [CrossRef] [PubMed]
- Van Sloten, T.T.; Schram, M.T.; van den Hurk, K.; Dekker, J.M.; Nijpels, G.; Henry, R.M.; Stehouwer, C.D. Local stiffness of the carotid and femoral artery is associated with incident cardiovascular events and all-cause mortality: The hoorn study. J. Am. Coll. Cardiol. 2014, 63, 1739–1747. [Google Scholar] [CrossRef] [PubMed]
- Cecelja, M.; Chowienczyk, P. Dissociation of aortic pulse wave velocity with risk factors for cardiovascular disease other than hypertension: A systematic review. Hypertension 2009, 54, 1328–1336. [Google Scholar] [CrossRef] [PubMed]
- De Korte, C.L.; Pasterkamp, G.; van der Steen, A.F.; Woutman, H.A.; Bom, N. Characterization of plaque components with intravascular ultrasound elastography in human femoral and coronary arteries in vitro. Circulation 2000, 102, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Schaar, J.A.; van der Steen, A.F.; Mastik, F.; Baldewsing, R.A.; Serruys, P.W. Intravascular palpography for vulnerable plaque assessment. J. Am. Coll. Cardiol. 2006, 47, C86–C91. [Google Scholar] [CrossRef] [PubMed]
- De Korte, C.L.; Sierevogel, M.J.; Mastik, F.; Strijder, C.; Schaar, J.A.; Velema, E.; Pasterkamp, G.; Serruys, P.W.; van der Steen, A.F. Identification of atherosclerotic plaque components with intravascular ultrasound elastography in vivo: A yucatan pig study. Circulation 2002, 105, 1627–1630. [Google Scholar] [CrossRef] [PubMed]
- Schaar, J.A.; Regar, E.; Mastik, F.; McFadden, E.P.; Saia, F.; Disco, C.; de Korte, C.L.; de Feyter, P.J.; van der Steen, A.F.; Serruys, P.W. Incidence of high-strain patterns in human coronary arteries: Assessment with three-dimensional intravascular palpography and correlation with clinical presentation. Circulation 2004, 109, 2716–2719. [Google Scholar] [CrossRef] [PubMed]
- Kruizinga, P.; Mastik, F.; van den Oord, S.C.; Schinkel, A.F.; Bosch, J.G.; de Jong, N.; van Soest, G.; van der Steen, A.F. High-definition imaging of carotid artery wall dynamics. Ultrasound Med. Biol. 2014, 40, 2392–2403. [Google Scholar] [CrossRef] [PubMed]
- Svedlund, S.; Gan, L.M. Longitudinal common carotid artery wall motion is associated with plaque burden in man and mouse. Atherosclerosis 2011, 217, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Widman, E.; Caidahl, K.; Heyde, B.; D'Hooge, J.; Larsson, M. Ultrasound speckle tracking strain estimation of in vivo carotid artery plaque with in vitro sonomicrometry validation. Ultrasound Med. Biol. 2015, 41, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.C.; Sun, Y.T.; Liu, Y.W.; Ho, C.S.; Chen, J.Y.; Wang, M.C.; Tsai, L.M. Usefulness of vascular wall deformation for assessment of carotid arterial stiffness and association with previous stroke in elderly. Am. J. Hypertens. 2013, 26, 770–777. [Google Scholar] [CrossRef] [PubMed]
- Gramiak, R.; Shah, P.M. Echocardiography of the aortic root. Investig. Radiol. 1968, 3, 356–366. [Google Scholar] [CrossRef]
- Jayaweera, A.R.; Edwards, N.; Glasheen, W.P.; Villanueva, F.S.; Abbott, R.D.; Kaul, S. In vivo myocardial kinetics of air-filled albumin microbubbles during myocardial contrast echocardiography. Comparison with radiolabeled red blood cells. Circ. Res. 1994, 74, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Demos, S.M.; Onyuksel, H.; Gilbert, J.; Roth, S.I.; Kane, B.; Jungblut, P.; Pinto, J.V.; McPherson, D.D.; Klegerman, M.E. In vitro targeting of antibody-conjugated echogenic liposomes for site-specific ultrasonic image enhancement. J. Pharm. Sci. 1997, 86, 167–171. [Google Scholar] [CrossRef] [PubMed]
- de Jong, N.; Frinking, P.J.; Bouakaz, A.; Goorden, M.; Schourmans, T.; Jingping, X.; Mastik, F. Optical imaging of contrast agent microbubbles in an ultrasound field with a 100-MHz camera. Ultrasound Med. Biol. 2000, 26, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Dayton, P.A.; Morgan, K.E.; Klibanov, A.L.; Brandenburger, G.H.; Ferrara, K.W. Optical and acoustical observations of the effects of ultrasound on contrast agents. IEEE Trans. Ultrasonics Ferroelectr. Freq. Control 1999, 46, 220–232. [Google Scholar] [CrossRef]
- Kaufmann, B.A.; Wei, K.; Lindner, J.R. Contrast echocardiography. Curr. Probl. Cardiol. 2007, 32, 51–96. [Google Scholar] [CrossRef] [PubMed]
- Barger, A.C.; Beeuwkes, R., 3rd; Lainey, L.L.; Silverman, K.J. Hypothesis: Vasa vasorum and neovascularization of human coronary arteries. A possible role in the pathophysiology of atherosclerosis. N. Engl. J. Med. 1984, 310, 175–177. [Google Scholar] [CrossRef] [PubMed]
- Sluimer, J.C.; Kolodgie, F.D.; Bijnens, A.; Maxfield, K.; Pacheco, E.; Kutys, B.; Duimel, H.; Frederik, P.M.; van Hinsbergh, V.W.M.; Virmani, R.; et al. Thin-walled microvessels in human coronary atherosclerotic plaques show incomplete endothelial junctions relevance of compromised structural integrity for intraplaque microvascular leakage. J. Am. Coll. Cardiol. 2009, 53, 1517–1527. [Google Scholar] [CrossRef] [PubMed]
- Kolodgie, F.D.; Gold, H.K.; Burke, A.P.; Fowler, D.R.; Kruth, H.S.; Weber, D.K.; Farb, A.; Guerrero, L.J.; Hayase, M.; Kutys, R.; et al. Intraplaque hemorrhage and progression of coronary atheroma. N. Engl. J. Med. 2003, 349, 2316–2325. [Google Scholar] [CrossRef] [PubMed]
- Coli, S.; Magnoni, M.; Sangiorgi, G.; Marrocco-Trischitta, M.M.; Melisurgo, G.; Mauriello, A.; Spagnoli, L.; Chiesa, R.; Cianflone, D.; Maseri, A. Contrast-enhanced ultrasound imaging of intraplaque neovascularization in carotid arteries: Correlation with histology and plaque echogenicity. J. Am. Coll. Cardiol. 2008, 52, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Staub, D.; Partovi, S.; Schinkel, A.F.; Coll, B.; Uthoff, H.; Aschwanden, M.; Jaeger, K.A.; Feinstein, S.B. Correlation of carotid artery atherosclerotic lesion echogenicity and severity at standard us with intraplaque neovascularization detected at contrast-enhanced us. Radiology 2011, 258, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Hoogi, A.; Adam, D.; Hoffman, A.; Kerner, H.; Reisner, S.; Gaitini, D. Carotid plaque vulnerability: Quantification of neovascularization on contrast-enhanced ultrasound with histopathologic correlation. AJR Am. J. Roentgenol. 2011, 196, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Staub, D.; Patel, M.B.; Tibrewala, A.; Ludden, D.; Johnson, M.; Espinosa, P.; Coll, B.; Jaeger, K.A.; Feinstein, S.B. Vasa vasorum and plaque neovascularization on contrast-enhanced carotid ultrasound imaging correlates with cardiovascular disease and past cardiovascular events. Stroke 2010, 41, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Nagatsuka, K.; Ishibashi-Ueda, H.; Watanabe, A.; Kannki, H.; Iihara, K. Contrast-enhanced ultrasound for the evaluation of neovascularization in atherosclerotic carotid artery plaques. Stroke 2014, 45, 3073–3075. [Google Scholar] [CrossRef] [PubMed]
- Ten Kate, G.L.; Renaud, G.G.; Akkus, Z.; van den Oord, S.C.; ten Cate, F.J.; Shamdasani, V.; Entrekin, R.R.; Sijbrands, E.J.; de Jong, N.; Bosch, J.G.; et al. Far-wall pseudoenhancement during contrast-enhanced ultrasound of the carotid arteries: Clinical description and in vitro reproduction. Ultrasound Med. Biol. 2012, 38, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Renaud, G.; Bosch, J.G.; Ten Kate, G.L.; Shamdasani, V.; Entrekin, R.; de Jong, N.; van der Steen, A.F. Counter-propagating wave interaction for contrast-enhanced ultrasound imaging. Phys. Med. Biol. 2012, 57, L9–L18. [Google Scholar] [CrossRef] [PubMed]
- Staub, D.; Schinkel, A.F.L.; Coll, B.; Coli, S.; van der Steen, A.F.W.; Reed, J.D.; Krueger, C.; Thomenius, K.E.; Adam, D.; Sijbrands, E.J.; et al. Contrast-enhanced ultrasound imaging of the vasa vasorum from early atherosclerosis to the identification of unstable plaques. JACC Cardiovasc. Imaging 2010, 3, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Lindner, J.R.; Song, J.; Xu, F.; Klibanov, A.L.; Singbartl, K.; Ley, K.; Kaul, S. Noninvasive ultrasound imaging of inflammation using microbubbles targeted to activated leukocytes. Circulation 2000, 102, 2745–2750. [Google Scholar] [CrossRef] [PubMed]
- Lindner, J.R.; Coggins, M.P.; Kaul, S.; Klibanov, A.L.; Brandenburger, G.H.; Ley, K. Microbubble persistence in the microcirculation during ischemia/reperfusion and inflammation is caused by integrin- and complement-mediated adherence to activated leukocytes. Circulation 2000, 101, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Khanicheh, E.; Mitterhuber, M.; Kinslechner, K.; Xu, L.; Lindner, J.R.; Kaufmann, B.A. Factors affecting the endothelial retention of targeted microbubbles: Influence of microbubble shell design and cell surface projection of the endothelial target molecule. J. Am. Soc. Echocardiogr. 2012, 25, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Takalkar, A.M.; Klibanov, A.L.; Rychak, J.J.; Lindner, J.R.; Ley, K. Binding and detachment dynamics of microbubbles targeted to P-selectin under controlled shear flow. J. Control. Release 2004, 96, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Lindner, J.R. Molecular imaging with contrast ultrasound and targeted microbubbles. J. Nucl. Cardiol. 2004, 11, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Lankford, M.; Behm, C.Z.; Yeh, J.; Klibanov, A.L.; Robinson, P.; Lindner, J.R. Effect of microbubble ligation to cells on ultrasound signal enhancement: Implications for targeted imaging. Investig. Radiol. 2006, 41, 721–728. [Google Scholar] [CrossRef]
- Lindner, J.R. Molecular imaging of vascular phenotype in cardiovascular disease: New diagnostic opportunities on the horizon. J. Am. Soc. Echocardiogr. 2010, 23, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Fenyo, I.M.; Gafencu, A.V. The involvement of the monocytes/macrophages in chronic inflammation associated with atherosclerosis. Immunobiology 2013, 218, 1376–1384. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, J.M.; Xie, F.; Cano, M.; Chomas, J.; Phillips, P.; Radio, S.J.; Lof, J.; Porter, T.R. Detection of retained microbubbles in carotid arteries with real-time low mechanical index imaging in the setting of endothelial dysfunction. J. Am. Coll. Cardiol. 2004, 44, 1036–1046. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.R.; Tsutsui, J.M.; Xie, F.; Radio, S.J.; Porter, T.R. The role of complement in the adherence of microbubbles to dysfunctional arterial endothelium and atherosclerotic plaque. Cardiovasc. Res. 2007, 73, 597–606. [Google Scholar] [CrossRef] [PubMed]
- McEver, R.P.; Cummings, R.D. Perspectives series: Cell adhesion in vascular biology. Role of PSGL-1 binding to selectins in leukocyte recruitment. J. Clin. Investig. 1997, 100, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Ramos, C.L.; Huo, Y.; Jung, U.; Ghosh, S.; Manka, D.R.; Sarembock, I.J.; Ley, K. Direct demonstration of P-selectin- and VCAM-1-dependent mononuclear cell rolling in early atherosclerotic lesions of apolipoprotein E-deficient mice. Circ. Res. 1999, 84, 1237–1244. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, B.A.; Carr, C.L.; Belcik, J.T.; Xie, A.; Yue, Q.; Chadderdon, S.; Caplan, E.S.; Khangura, J.; Bullens, S.; Bunting, S.; et al. Molecular imaging of the initial inflammatory response in atherosclerosis: Implications for early detection of disease. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, F.S.; Lu, E.; Bowry, S.; Kilic, S.; Tom, E.; Wang, J.; Gretton, J.; Pacella, J.J.; Wagner, W.R. Myocardial ischemic memory imaging with molecular echocardiography. Circulation 2007, 115, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, B.A.; Lewis, C.; Xie, A.; Mirza-Mohd, A.; Lindner, J.R. Detection of recent myocardial ischaemia by molecular imaging of P-selectin with targeted contrast echocardiography. Eur. Heart J. 2007, 28, 2011–2017. [Google Scholar] [CrossRef] [PubMed]
- Walpola, P.L.; Gotlieb, A.I.; Cybulsky, M.I.; Langille, B.L. Expression of ICAM-1 and VCAM-1 and monocyte adherence in arteries exposed to altered shear stress. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Dansky, H.M.; Barlow, C.B.; Lominska, C.; Sikes, J.L.; Kao, C.; Weinsaft, J.; Cybulsky, M.I.; Smith, J.D. Adhesion of monocytes to arterial endothelium and initiation of atherosclerosis are critically dependent on vascular cell adhesion molecule-1 gene dosage. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1662–1667. [Google Scholar] [CrossRef] [PubMed]
- Iiyama, K.; Hajra, L.; Iiyama, M.; Li, H.; DiChiara, M.; Medoff, B.D.; Cybulsky, M.I. Patterns of vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 expression in rabbit and mouse atherosclerotic lesions and at sites predisposed to lesion formation. Circ. Res. 1999, 85, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, F.S.; Jankowski, R.J.; Klibanov, S.; Pina, M.L.; Alber, S.M.; Watkins, S.C.; Brandenburger, G.H.; Wagner, W.R. Microbubbles targeted to intercellular adhesion molecule-1 bind to activated coronary artery endothelial cells. Circulation 1998, 98, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, A.J.; Huang, S.L.; Warnick, D.; Rabbat, M.; Kane, B.; Nagaraj, A.; Klegerman, M.; McPherson, D.D. Intravascular ultrasound molecular imaging of atheroma components in vivo. J. Am. Coll. Cardiol. 2004, 43, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, B.A.; Sanders, J.M.; Davis, C.; Xie, A.; Aldred, P.; Sarembock, I.J.; Lindner, J.R. Molecular imaging of inflammation in atherosclerosis with targeted ultrasound detection of vascular cell adhesion molecule-1. Circulation 2007, 116, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Leong-Poi, H.; Bin, J.; Yang, L.; Liao, Y.; Liu, Y.; Cai, J.; Xie, J.; Liu, Y. Efficacy of contrast-enhanced us and magnetic microbubbles targeted to vascular cell adhesion molecule-1 for molecular imaging of atherosclerosis. Radiology 2011, 260, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Davidson, B.P.; Yue, Q.; Belcik, T.; Xie, A.; Inaba, Y.; McCarty, O.J.; Tormoen, G.W.; Zhao, Y.; Ruggeri, Z.M.; et al. Molecular imaging of inflammation and platelet adhesion in advanced atherosclerosis effects of antioxidant therapy with nadph oxidase inhibition. Circ. Cardiovasc. Imaging 2013, 6, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Khanicheh, E.; Qi, Y.; Xie, A.; Mitterhuber, M.; Xu, L.; Mochizuki, M.; Daali, Y.; Jaquet, V.; Krause, K.H.; Ruggeri, Z.M.; et al. Molecular imaging reveals rapid reduction of endothelial activation in early atherosclerosis with apocynin independent of antioxidative properties. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2187–2192. [Google Scholar] [CrossRef] [PubMed]
- Khanicheh, E.; Mitterhuber, M.; Xu, L.; Haeuselmann, S.P.; Kuster, G.M.; Kaufmann, B.A. Noninvasive ultrasound molecular imaging of the effect of statins on endothelial inflammatory phenotype in early atherosclerosis. PLoS ONE 2013, 8, e58761. [Google Scholar] [CrossRef] [PubMed]
- Unger, E.C.; McCreery, T.P.; Sweitzer, R.H.; Shen, D.; Wu, G. In vitro studies of a new thrombus-specific ultrasound contrast agent. Am. J. Cardiol. 1998, 81, 58g–61g. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Wang, Y.; Shen, S.; Wu, J.; Guo, S.; Su, L.; Hou, F.; Wang, Z.; Liao, Y.; Bin, J. In vivo ultrasound molecular imaging of inflammatory thrombosis in arteries with cyclic Arg-Gly-Asp-modified microbubbles targeted to glycoprotein IIB/IIIA. Investig. Radiol. 2013, 48, 803–812. [Google Scholar] [CrossRef]
- Wang, X.; Hagemeyer, C.E.; Hohmann, J.D.; Leitner, E.; Armstrong, P.C.; Jia, F.; Olschewski, M.; Needles, A.; Peter, K.; Ahrens, I. Novel single-chain antibody-targeted microbubbles for molecular ultrasound imaging of thrombosis: Validation of a unique noninvasive method for rapid and sensitive detection of thrombi and monitoring of success or failure of thrombolysis in mice. Circulation 2012, 125, 3117–3126. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.; Della Martina, A.; Stroick, M.; Fatar, M.; Griebe, M.; Pochon, S.; Schneider, M.; Hennerici, M.; Allemann, E.; Meairs, S. Molecular imaging of human thrombus with novel abciximab immunobubbles and ultrasound. Stroke 2007, 38, 1508–1514. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Palasubramaniam, J.; Gkanatsas, Y.; Hohmann, J.D.; Westein, E.; Kanojia, R.; Alt, K.; Huang, D.; Jia, F.; Ahrens, I.; et al. Towards effective and safe thrombolysis and thromboprophylaxis: Preclinical testing of a novel antibody-targeted recombinant plasminogen activator directed against activated platelets. Circ. Res. 2014, 114, 1083–1093. [Google Scholar] [CrossRef] [PubMed]
- Burger, P.C.; Wagner, D.D. Platelet P-selectin facilitates atherosclerotic lesion development. Blood 2003, 101, 2661–2666. [Google Scholar] [CrossRef] [PubMed]
- Massberg, S.; Brand, K.; Gruner, S.; Page, S.; Muller, E.; Muller, I.; Bergmeier, W.; Richter, T.; Lorenz, M.; Konrad, I.; et al. A critical role of platelet adhesion in the initiation of atherosclerotic lesion formation. J. Exp. Med. 2002, 196, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Henn, V.; Slupsky, J.R.; Grafe, M.; Anagnostopoulos, I.; Forster, R.; Muller-Berghaus, G.; Kroczek, R.A. Cd40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature 1998, 391, 591–594. [Google Scholar] [CrossRef] [PubMed]
- McCarty, O.J.; Conley, R.B.; Shentu, W.; Tormoen, G.W.; Zha, D.; Xie, A.; Qi, Y.; Zhao, Y.; Carr, C.; Belcik, T.; et al. Molecular imaging of activated von willebrand factor to detect high-risk atherosclerotic phenotype. JACC Cardiovasc. Imaging 2010, 3, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Couture, O.; Bannouf, S.; Montaldo, G.; Aubry, J.F.; Fink, M.; Tanter, M. Ultrafast imaging of ultrasound contrast agents. Ultrasound Med. Biol. 2009, 35, 1908–1916. [Google Scholar] [CrossRef] [PubMed]
- Mauldin, F.W., Jr.; Dhanaliwala, A.H.; Patil, A.V.; Hossack, J.A. Real-time targeted molecular imaging using singular value spectra properties to isolate the adherent microbubble signal. Phys. Med. Biol. 2012, 57, 5275–5293. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steinl, D.C.; Kaufmann, B.A. Ultrasound Imaging for Risk Assessment in Atherosclerosis. Int. J. Mol. Sci. 2015, 16, 9749-9769. https://doi.org/10.3390/ijms16059749
Steinl DC, Kaufmann BA. Ultrasound Imaging for Risk Assessment in Atherosclerosis. International Journal of Molecular Sciences. 2015; 16(5):9749-9769. https://doi.org/10.3390/ijms16059749
Chicago/Turabian StyleSteinl, David C., and Beat A. Kaufmann. 2015. "Ultrasound Imaging for Risk Assessment in Atherosclerosis" International Journal of Molecular Sciences 16, no. 5: 9749-9769. https://doi.org/10.3390/ijms16059749




