Carotenoid Profile of Tomato Sauces: Effect of Cooking Time and Content of Extra Virgin Olive Oil
Abstract
:1. Introduction
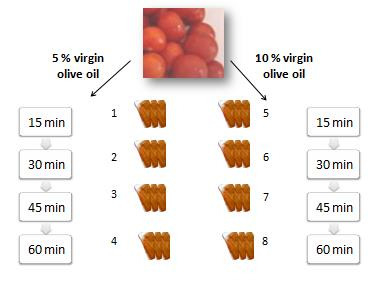
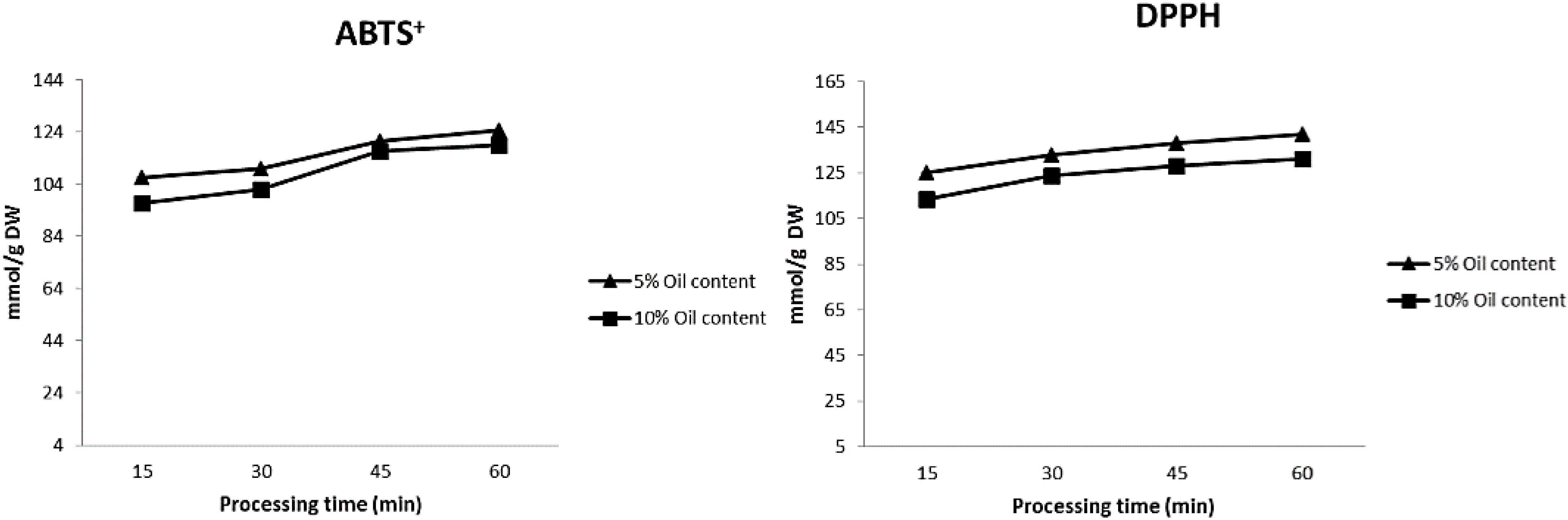
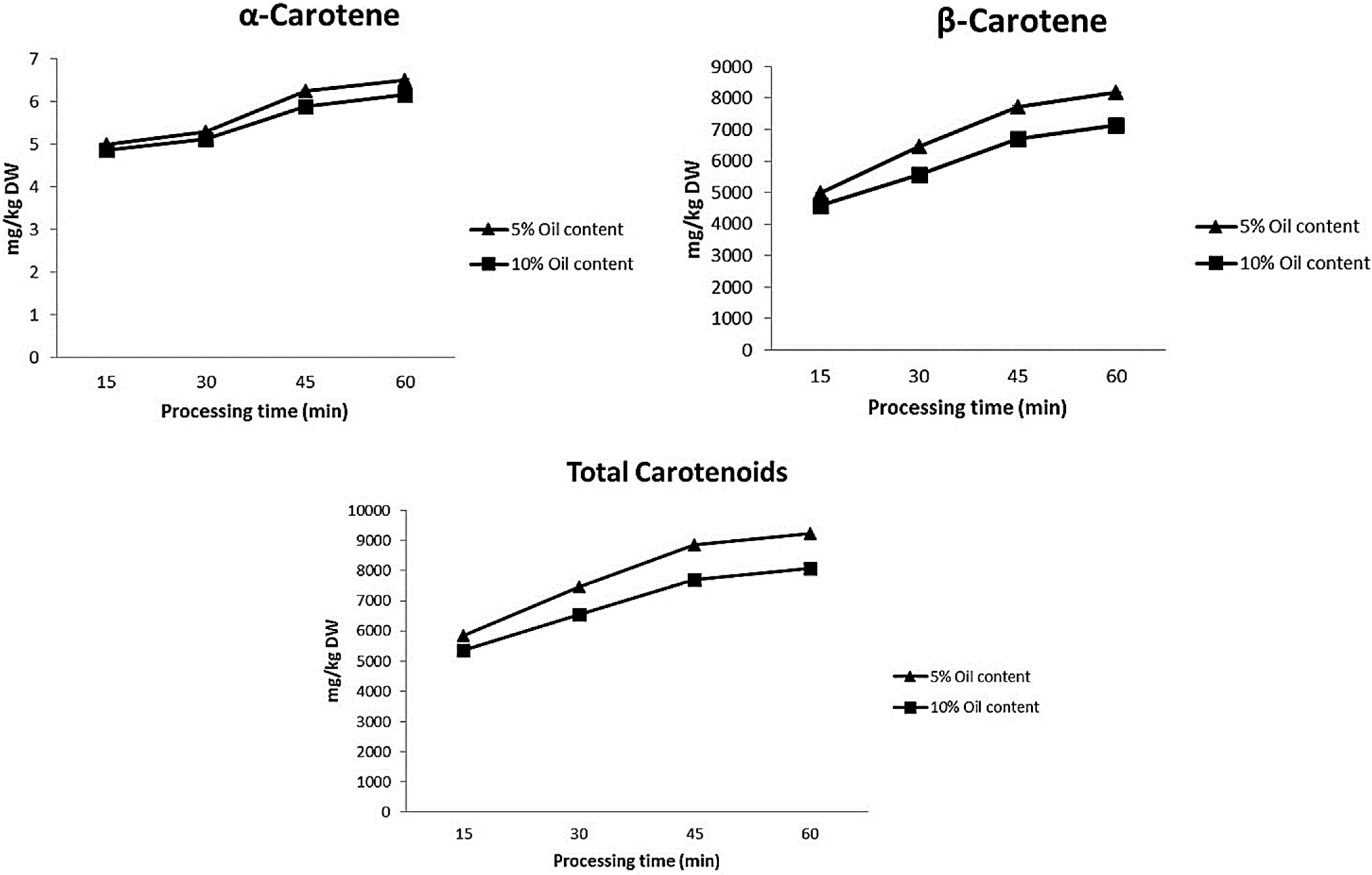
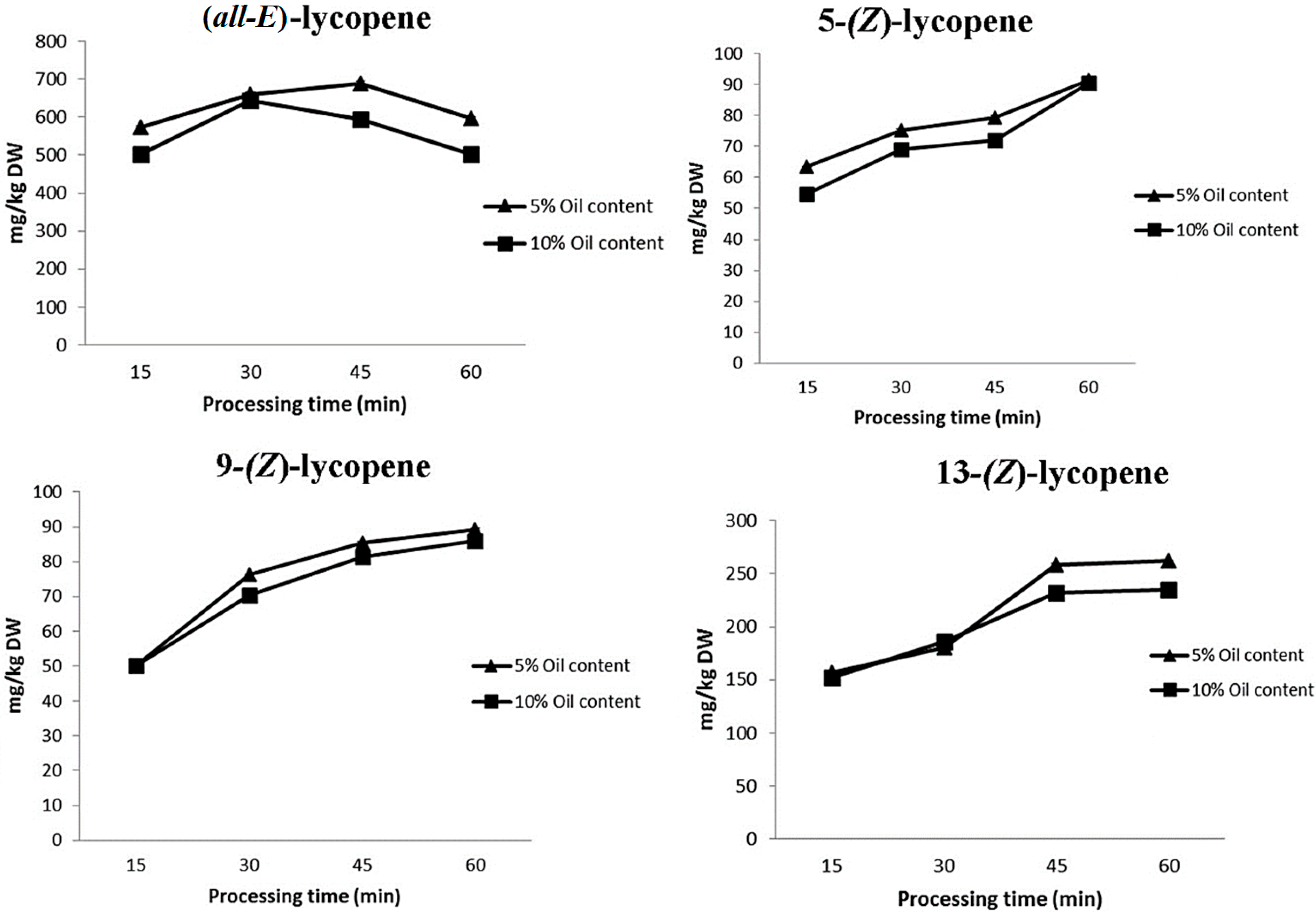
2. Results and Discussion
3. Experimental Section
3.1. Standards
3.2. Tomato Sauce Material
| Compounds | Tomato Sauce Control (mg/kg DW) |
|---|---|
| α-carotene | 4.3 ± 0.3 |
| β-carotene | 3266.9 ± 53.1 |
| (all-E)-Lycopene | 178.9 ± 10.1 |
| 5-(Z)-Lycopene | 20.3 ± 1.1 |
| 9-(Z)-Lycopene | 26.2 ± 0.9 |
| 13-(Z)-Lycopene | 33.6 ± 2.7 |
| Total Carotenoids | 3530.1 |
3.3. Extraction and Analysis of Carotenoids
3.3.1. Extraction of Carotenoid Compounds
3.3.2. Analysis of Carotenoid Compounds
3.4. Identification and Quantification of Carotenoids
3.4.1. Diode Array Detector
3.4.2. Mass Spectrometry
3.5. Antioxidant Capacity
3.5.1. ABTS+ Assay
3.5.2. DPPH Assay
3.6. Statistical Treatments
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Estruch, R.; Martinez-Gonzalez, M.A.; Corella, D.; Salas-Salvado, J.; Ruiz-Gutierrez, V.; Covas, M.I.; Fiol, M.; Gomez-Gracia, E.; Lopez-Sabater, M.C.; Vinyoles, E.; et al. Effects of a mediterranean-style diet on cardiovascular risk factorsa randomized trial. Ann. Intern. Med. 2006, 145, 1–11. [Google Scholar]
- Estruch, R.; Ros, E.; Salas-Salvado, J.; Covas, M.I.; Pharm, D.; Corella, D.; Aros, F.; Gomez-Gracia, E.; Ruiz-Gutierrez, V.; Fiol, M.; et al. Primary prevention of cardiovascular disease with a mediterranean diet. N. Engl. J. Med. 2013, 368, 1279–1290. [Google Scholar]
- Grieb, S.M.D.; Theis, R.P.; Burr, D.; Benardot, D.; Siddiqui, T.; Asal, N.R. Food groups and renal cell carcinoma: Results from a case-control study. J. Am. Diet. Assoc. 2009, 109, 656–667. [Google Scholar] [CrossRef] [PubMed]
- Silaste, M.L.; Alfthan, G.; Aro, A.; Kesaniemi, Y.A.; Horkko, S. Tomato juice decreases LDL cholesterol levels and increases LDL resistance to oxidation. Br. J. Nutr. 2007, 98, 1251–1258. [Google Scholar] [CrossRef] [PubMed]
- Olson, J.A. Biological actions of carotenoids—Introduction. J. Nutr. 1989, 119, 94–95. [Google Scholar] [PubMed]
- Agarwal, S.; Rao, A.V. Tomato lycopene and its role in human health and chronic diseases. CMAJ 2000, 163, 739–744. [Google Scholar] [PubMed]
- Torre-Carbot, K.; Jauregui, O.; Gimeno, E.; Castellote, A.; Lamuela-Raventos, R.M.; Lopez-Sabater, M.C. Characterization and quantification of phenolic compounds in olive oils by solid-phase extraction, HPLC-DAD, and HPLC-MS/MS. J. Agric. Food Chem. 2005, 53, 4331–4340. [Google Scholar] [CrossRef] [PubMed]
- Visioli, F.; Galli, C. Olive oil phenols and their potential effects on human health. J. Agric. Food Chem. 1998, 46, 4292–4296. [Google Scholar] [CrossRef]
- Vallverdu-Queralt, A.; Odriozola-Serrano, I.; Oms-Oliu, G.; Lamuela-Raventos, R.M.; Elez-Martinez, P.; Martin-Belloso, O. Impact of high-intensity pulsed electric fields on carotenoids profile of tomato juice made of moderate-intensity pulsed electric field-treated tomatoes. Food Chem. 2013, 141, 3131–3138. [Google Scholar] [CrossRef] [PubMed]
- Gärtner, C.; Stahl, W.; Sies, H. Lycopene is more bioavailable from tomato paste than from fresh tomatoes. Am. J. Clin. Nutr. 1997, 66, 116–122. [Google Scholar] [PubMed]
- Aguilo-Aguayo, I.; Soliva-Fortuny, R.; Martin-Belloso, O. Volatile compounds and changes in flavour-related enzymes during cold storage of high-intensity pulsed electric field- and heat-processed tomato juices. J. Sci. Food Agric. 2010, 90, 1597–1604. [Google Scholar] [CrossRef] [PubMed]
- Odriozola-Serrano, I.; Soliva-Fortuny, R.; Martin-Belloso, O. Changes of health-related compounds throughout cold storage of tomato juice stabilized by thermal or high intensity pulsed electric field treatments. Innov. Food Sci. Emerg. Technol. 2008, 9, 272–279. [Google Scholar] [CrossRef]
- Bonnie, T.Y.P.; Choo, Y.M. Oxidation and thermal degradation of carotenoids. J. Oil Palm Res. 1999, 2, 62–78. [Google Scholar]
- Boileau, A.C.; Merchen, N.R.; Wasson, K.; Atkinson, C.A.; Erdman, J.W. (cis)-Lycopene is more bioavailable than (trans)-lycopene in vitro and in vivo in lymph-cannulated ferrets. J. Nutr. 1999, 129, 1176–1181. [Google Scholar] [PubMed]
- Shi, J.; Le Maguer, M. Lycopene in tomatoes: Chemical and physical properties affected by food processing. Crit. Rev. Biotechnol. 2000, 20, 293–334. [Google Scholar] [CrossRef] [PubMed]
- Abushita, A.A.; Daood, H.G.; Biacs, P.A. Change in carotenoids and antioxidant vitamins in tomato as a function of varietal and technological factors. J. Agric. Food Chem. 2000, 48, 2075–2081. [Google Scholar] [CrossRef] [PubMed]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef] [PubMed]
- Piga, A.; Agabbio, M.; Gambella, F.; Nicoli, M.C. Retention of antioxidant activity in minimally processed mandarin and satsuma fruits. LWT Food Sci. Technol. 2002, 35, 344–347. [Google Scholar] [CrossRef]
- Lin, C.H.; Chen, B.H. Stability of carotenoids in tomato juice during storage. Food Chem. 2005, 90, 837–846. [Google Scholar] [CrossRef]
- Takeoka, G.R.; Dao, L.; Flessa, S.; Gillespie, D.M.; Jewell, W.T.; Huebner, B.; Bertow, D.; Ebeler, S.E. Processing effects on lycopene content and antioxidant activity of tomatoes. J. Agric. Food Chem. 2001, 49, 3713–3717. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, K.; Conrad, J.; Schmid, A.; Breithaupt, D.E.; Böhm, V. Isolation and structural elucidation of different geometrical isomers of lycopene. Int. J. Vitam. Nutr. Res. 2007, 77, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Schierle, J.; Bretzel, W.; Bühler, I.; Faccin, N.; Hess, D.; Steiner, K.; Schuep, W. Content and isomeric ratio of lycopene in food and human blood plasma. Food Chem. 1997, 59, 459–465. [Google Scholar] [CrossRef]
- Vallverdu-Queralt, A.; Arranz, S.; Casals-Ribes, I.; Lamuela-Raventos, R.M. Stability of the phenolic and carotenoid profile of gazpachos during storage. J. Agric. Food Chem. 2012, 60, 1981–1988. [Google Scholar] [CrossRef] [PubMed]
- Giovanelli, G.; Paradiso, A. Stability of dried and intermediate moisture tomato pulp during storage. J. Agric. Food Chem. 2002, 50, 7277–7281. [Google Scholar] [CrossRef] [PubMed]
- Bartley, G.E.; Scolnik, P.A. Plant carotenoids: Pigments for photoprotection, visual attraction, and human health. Plant Cell 1995, 7, 1027–1038. [Google Scholar] [CrossRef] [PubMed]
- DellaPenna, D.; Pogson, B.J. Vitamin synthesis in plants: Tocopherols and carotenoids. Annu. Rev. Plant Biol. 2006, 57, 711–738. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Alonso, F.J.; Bravo, S.; Casas, J.; Perez-Conesa, D.; Jacob, K.; Periago, M.J. Changes in antioxidant compounds during the shelf life of commercial tomato juices in different packaging materials. J. Agric. Food Chem. 2009, 57, 6815–6822. [Google Scholar] [CrossRef] [PubMed]
- Zu, K.; Mucci, L.; Rosner, B.A.; Clinton, S.K.; Loda, M.; Stampfer, M.J.; Giovannucci, E. Dietary lycopene, angiogenesis, and prostate cancer: A prospective study in the prostate-specific antigen era. J. Natl. Cancer Inst. 2014, 106, djt430. [Google Scholar] [CrossRef] [PubMed]
- Edinger, M.S.; Koff, W.J. Effect of the consumption of tomato paste on plasma prostate-specific antigen levels in patients with benign prostate hyperplasia. Braz. J. Med. Biol. Res. 2006, 39, 1115–1119. [Google Scholar] [CrossRef] [PubMed]
- Leenders, M.; Leufkens, A.M.; Siersema, P.D.; van Duijnhoven, F.J.; Vrieling, A.; Hulshof, P.J.; van Gils, C.H.; Overvad, K.; Roswall, N.; Kyrø, C.; et al. Plasma and dietary carotenoids and vitamins A, C and E and risk of colon and rectal cancer in the European Prospective Investigation into Cancer and Nutrition. Int. J. Cancer 2014, 135, 2930–2939. [Google Scholar]
- Wei, S.; Wang, Y.; Chai, Q.; Fang, Q.; Zhang, Y.; Wang, J. In vivo and in vitro effects of heme oxygenase-1 silencing on the survival of acute myelocytic leukemia-M2 cells. Exp. Ther. Med. 2015, 9, 931–940. [Google Scholar] [PubMed]
- Linnewiel-Hermoni, K.; Khanin, M.; Danilenko, M.; Zango, G.; Amosi, Y.; Levy, J.; Sharoni, Y. The anti-cancer effects of carotenoids and other phytonutrients resides in their combined activity. Arch. Biochem. Biophys. 2015, 572, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Vallverdu-Queralt, A.; Martinez-Huelamo, M.; Arranz-Martinez, S.; Miralles, E.; Lamuela-Raventos, R.M. Differences in the carotenoid content of ketchups and gazpachos through HPLC/ESI(Li+)-MS/MS correlated with their antioxidant capacity. J. Sci. Food Agric. 2012, 92, 2043–2049. [Google Scholar] [CrossRef] [PubMed]
- Matea, C.; Soran, M.; Pintea, A.; Bele, C. Analytical determination of carotenoids in inland Ocimum basilicum L. Bull. UASVM Agric. 2010, 67, 298–302. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vallverdú-Queralt, A.; Regueiro, J.; De Alvarenga, J.F.R.; Torrado, X.; Lamuela-Raventos, R.M. Carotenoid Profile of Tomato Sauces: Effect of Cooking Time and Content of Extra Virgin Olive Oil. Int. J. Mol. Sci. 2015, 16, 9588-9599. https://doi.org/10.3390/ijms16059588
Vallverdú-Queralt A, Regueiro J, De Alvarenga JFR, Torrado X, Lamuela-Raventos RM. Carotenoid Profile of Tomato Sauces: Effect of Cooking Time and Content of Extra Virgin Olive Oil. International Journal of Molecular Sciences. 2015; 16(5):9588-9599. https://doi.org/10.3390/ijms16059588
Chicago/Turabian StyleVallverdú-Queralt, Anna, Jorge Regueiro, José Fernando Rinaldi De Alvarenga, Xavier Torrado, and Rosa M. Lamuela-Raventos. 2015. "Carotenoid Profile of Tomato Sauces: Effect of Cooking Time and Content of Extra Virgin Olive Oil" International Journal of Molecular Sciences 16, no. 5: 9588-9599. https://doi.org/10.3390/ijms16059588