Effects of Mechanical Stretching on the Morphology and Cytoskeleton of Vaginal Fibroblasts from Women with Pelvic Organ Prolapse
Abstract
:1. Introduction
2. Results
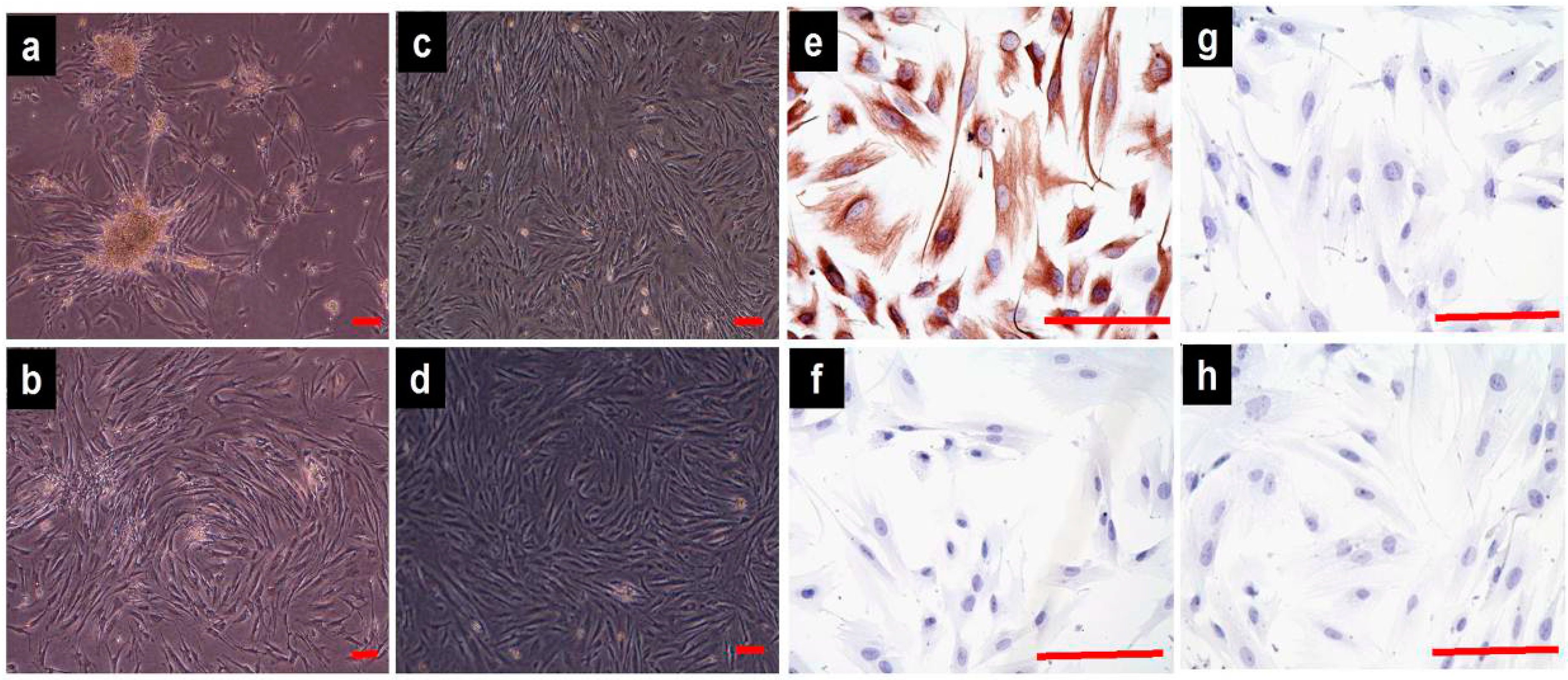
2.1. Cell Culture and Identification
2.2. Effects of Cyclic Mechanical Stretching and 17-β-Estradiol (E2) on Vaginal Fibroblast Proliferation
2.3. Effects of Cyclic Mechanical Stretching and E2 on Vaginal Fibroblast Morphology
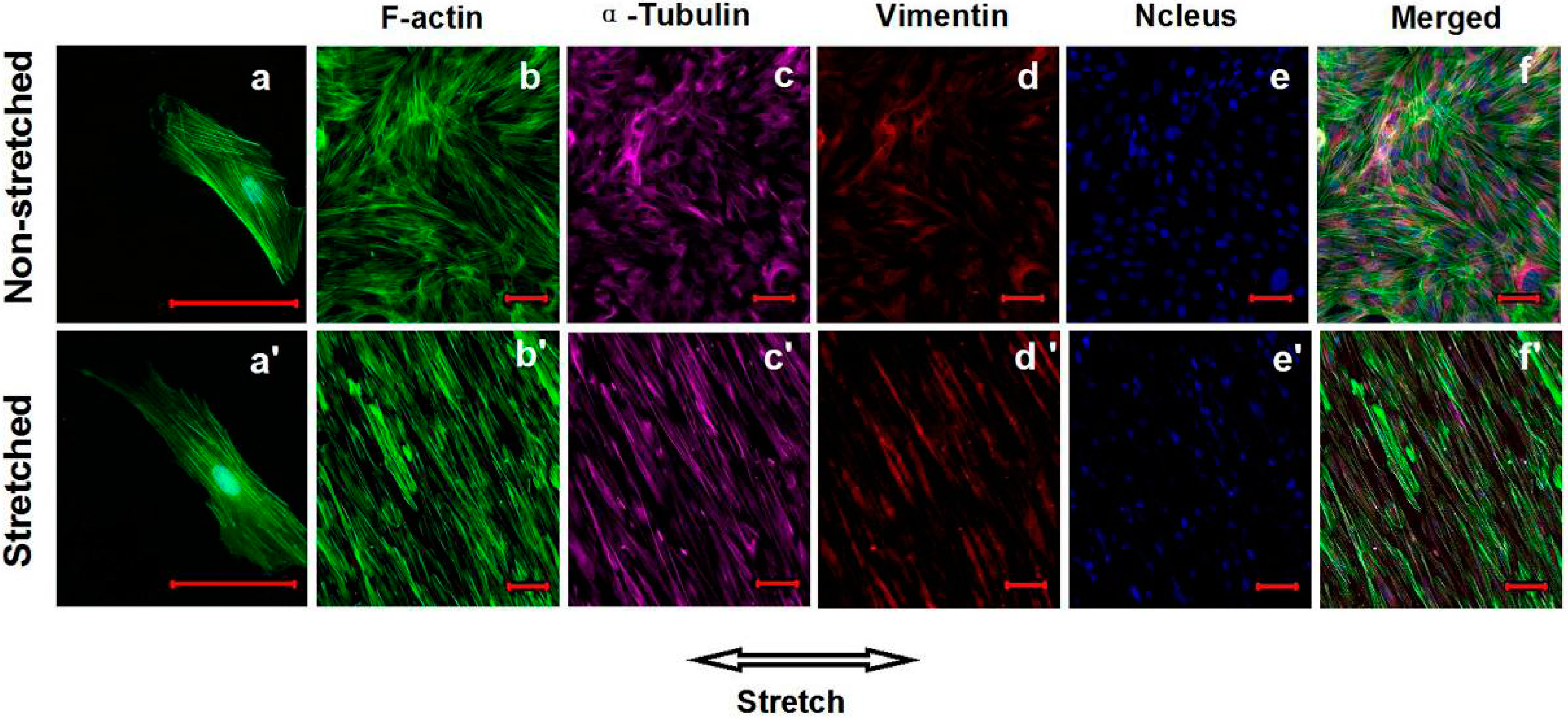
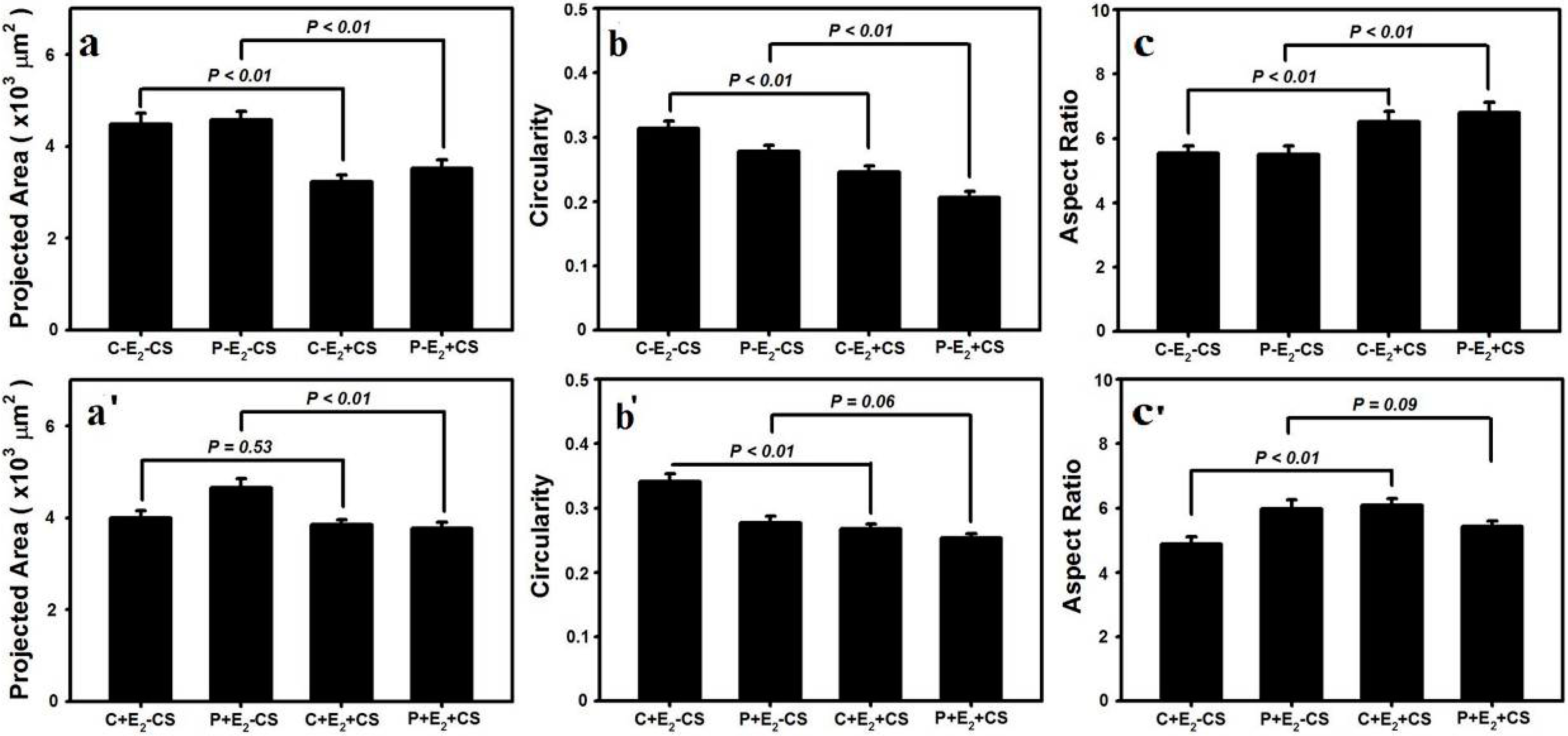
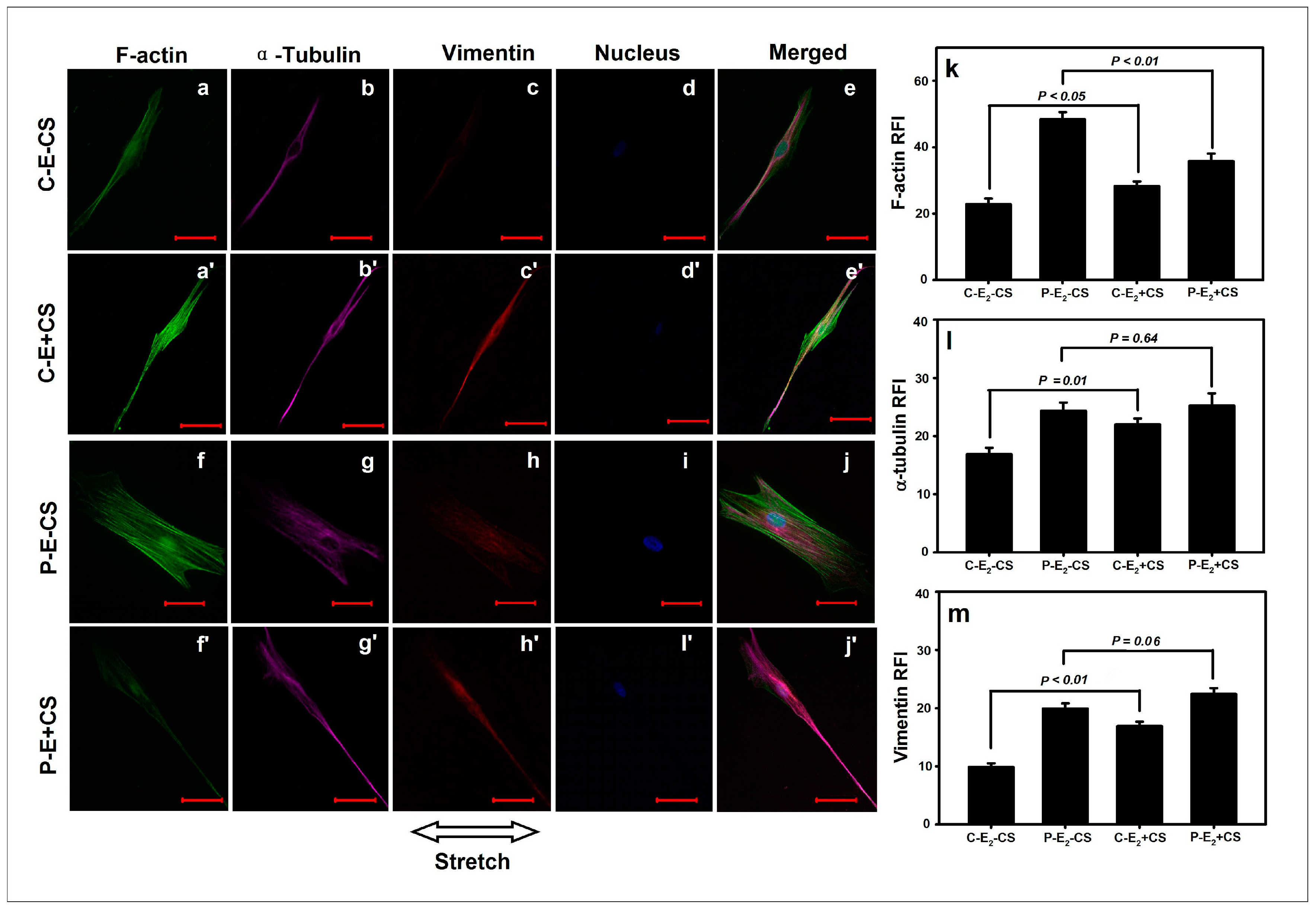
2.4. Effects of Cyclic Mechanical Stretching and E2 on Vaginal Fibroblast Cytoskeletons
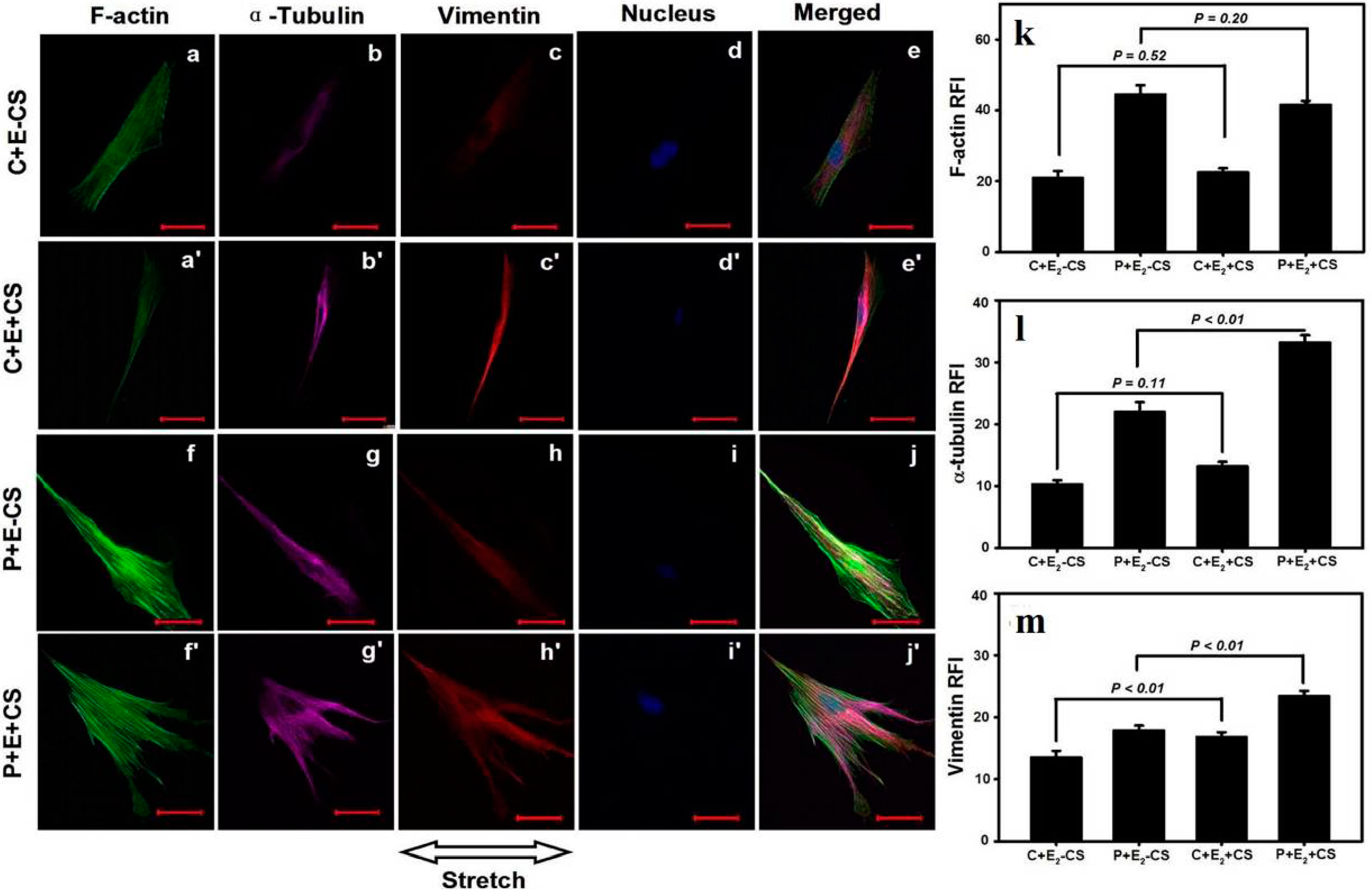
3. Discussion
4. Experimental Section
4.1. Patient Selection and Tissue Collection
4.2. Primary Culture of Human Vaginal Fibroblasts
4.3. Phenotype Identification of the Vaginal Fibroblasts
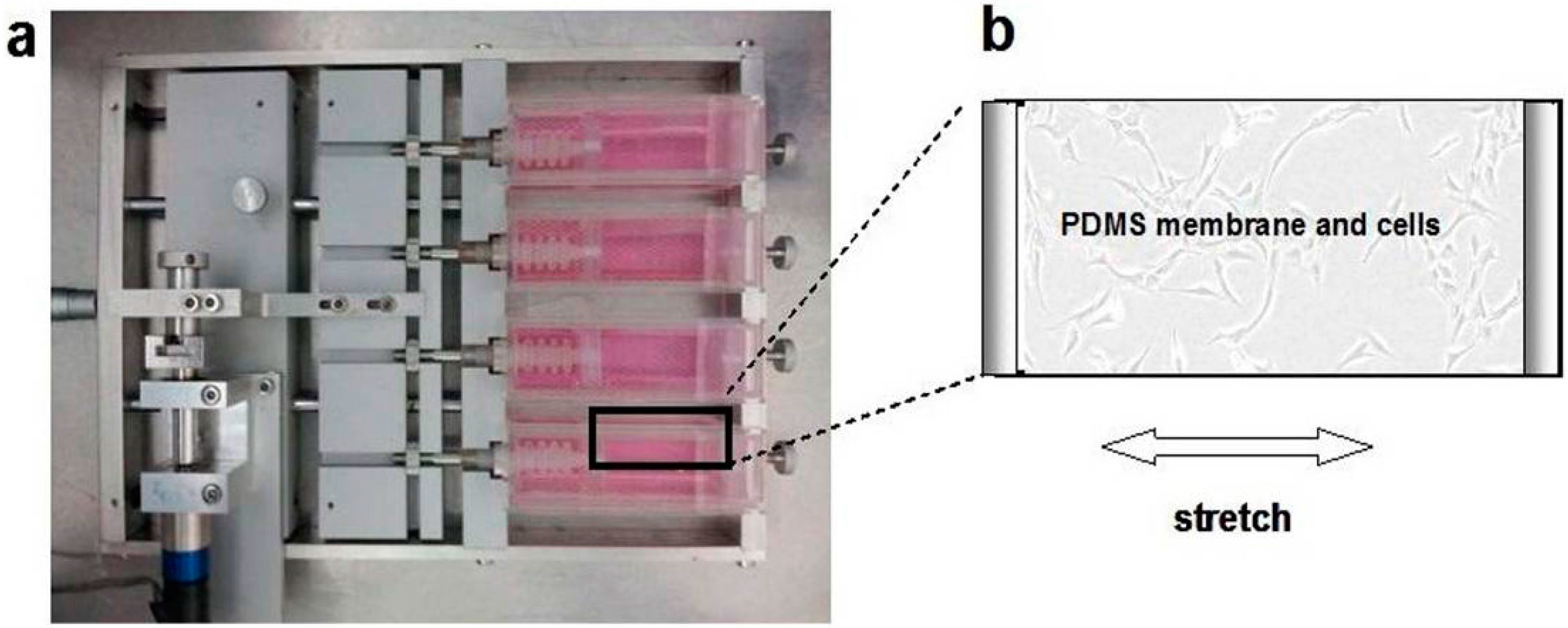
4.4. Loading of Cyclic Mechanical Stretch and the Administration of E2
4.5. Fibroblast Counting
4.6. Immunological Staining and Imaging with Confocal Microscopy
4.7. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mokrzycki, M.L.; Mittal, K.; Smilen, S.W.; Blechman, A.N.; Porges, R.F.; Demopolous, R.I. Estrogen and progesterone receptors in the uterosacral ligament. Obstet. Gynecol. 1997, 90, 402–404. [Google Scholar] [CrossRef] [PubMed]
- Hendrix, S.L.; Clark, A.; Nygaard, I.; Aragaki, A.; Barnabei, V.; McTiernan, A. Pelvic organ prolapse in the Women’s Health Initiative: Gravity and gravidity. Am. J. Obstet. Gynecol. 2002, 186, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Olsen, A.L.; Smith, V.J.; Bergstrom, J.O.; Colling, J.C.; Clark, A.L. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet. Gynecol. 1997, 89, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Jelovsek, J.E.; Maher, C.; Barber, M.D. Pelvic organ prolapse. Lancet 2007, 369, 1027–1038. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.M.; Jeon, M.J.; Chung, D.J.; Kim, S.K.; Kim, J.W.; Bai, S.W. Risk factors for pelvic organ prolapse. Int. J. Gynaecol. Obstet. 2007, 98, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Word, R.A.; Pathi, S.; Schaffer, J.I. Pathophysiology of pelvic organ prolapse. Obstet. Gynecol. Clin. N. Am. 2009, 36, 521–539. [Google Scholar] [CrossRef]
- DeLancey, J.O. Anatomic aspects of vaginal eversion after hysterectomy. Am. J. Obstet. Gynecol. 1992, 166, 1717–1728. [Google Scholar] [CrossRef] [PubMed]
- Langevin, H.M.; Bouffard, N.A.; Fox, J.R.; Palmer, B.M.; Wu, J.; Iatridis, J.C.; Barnes, W.D.; Badger, G.J.; Howe, A.K. Fibroblast cytoskeletal remodeling contributes to connective tissue tension. J. Cell. Physiol. 2011, 226, 1166–1175. [Google Scholar] [CrossRef] [PubMed]
- Provenzano, P.; Lakes, R.; Keenan, T.; Vanderby, R., Jr. Nonlinear ligament viscoelasticity. Ann. Biomed. Eng. 2001, 29, 908–914. [Google Scholar] [CrossRef] [PubMed]
- Buck, R.C. Reorientation response of cells to repeated stretch and recoil of the substratum. Exp. Cell Res. 1980, 127, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Ji, B.; Dai, L. Stability of adhesion clusters and cell reorientation under lateral cyclic tension. Biophys. J. 2008, 95, 4034–4044. [Google Scholar] [CrossRef] [PubMed]
- Chiquet, M.; Gelman, L.; Lutz, R.; Maier, S. From mechanotransduction to extracellular matrix gene expression in fibroblasts. Biochim. Biophys. Acta 2009, 1793, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Thampatty, B.P.; Lin, J.S.; Im, H.J. Mechanoregulation of gene expression in fibroblasts. Gene 2007, 391, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Zapata, A.M.; Kerkhof, M.H.; Zandieh-Doulabi, B.; Brölmann, H.A.; Smit, T.H.; Helder, M.N. Fibroblasts from women with pelvic organ prolapse show differential mechanoresponses depending on surface substrates. Int. Urogynecol. J. 2013, 24, 1567–1575. [Google Scholar] [CrossRef] [PubMed]
- Lelièvre, S.A. Contributions of extracellular matrix signaling and tissue architecture to nuclear mechanisms and spatial organization of gene expression control. Biochim. Biophys. Acta 2009, 1790, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Tomasek, J.J.; Gabbiani, G.; Hinz, B.; Chaponnier, C.; Brown, R.A. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Biol. 2002, 3, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Goh, J.T. Biomechanical and biochemical assessments for pelvic organ prolapse. Curr. Opin. Obstet. Gynecol. 2003, 15, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Rubod, C.; Brieu, M.; Cosson, M.; Rivaux, G.; Clay, J.C.; de Landsheere, L.; Gabriel, B. Biomechanical properties of human pelvic organs. Urology 2012, 79, e17–e22. [Google Scholar] [CrossRef] [PubMed]
- Ingber, D.E.; Tensegrity, I. Cell structure and hierarchical systems biology. J. Cell Sci. 2003, 116, 1157–1173. [Google Scholar]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002; pp. 907–982. [Google Scholar]
- Ewies, A.A.; Elshafie, M.; Li, J.; Stanley, A.; Thompson, J.; Styles, J.; White, I.; Al-Azzawi, F. Changes in transcription profile and cytoskeleton morphology in pelvic ligament fibroblasts in response to stretch: The effects of estradiol and levormeloxifene. Mol. Hum. Reprod. 2008, 14, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Zong, W.; Jallah, Z.C.; Stein, S.E.; Abramowitch, S.D.; Moalli, P.A. Repetitive mechanical stretch increases extracellular collagenase activity in vaginal fibroblasts. Female Pelvic Med. Reconstr. Surg. 2010, 16, 257–262. [Google Scholar] [CrossRef]
- Martins, P.; Lopes Silva-Filho, A.; Maciel da Fonseca, R.; Santos, A.; Santos, L.; Mascarenhas, T.; Natal Jorge, R.M.; Ferreira, A.J. Biomechanical properties of vaginal tissue in women with pelvic organ prolapse. Gynecol. Obstet. Investig. 2013, 75, 85–92. [Google Scholar] [CrossRef]
- DeLancey, J.O. Anatomy and biomechanics of genital prolapse. Clin. Obstet. Gynecol. 1993, 36, 897–909. [Google Scholar] [CrossRef] [PubMed]
- Al-Taher, H.; Sutherst, J.R.; Richmond, D.H.; Brown, M.C. Vaginal pressure as an index of intra-abdominal pressure during urodynamic evaluation. Br. J. Urol. 1987, 59, 529–532. [Google Scholar] [CrossRef] [PubMed]
- Mouritsen, L.; Hulbaek, M.; Brostrøm, S.; Bogstad, J. Vaginal pressure during daily activities before and after vaginal repair. Int. Urogynecol. J. 2007, 18, 943–948. [Google Scholar] [CrossRef]
- O’Dell, K.K.; Morse, A.N.; Crawford, S.L.; Howard, A. Vaginal pressure during lifting, floor exercises, jogging, and use of hydraulic exercise machines. Int. Urogynecol. J. 2007, 18, 1481–1489. [Google Scholar] [CrossRef]
- Liu, Y.M.; Choy, K.W.; Lui, W.T.; Pang, M.W.; Wong, Y.F.; Yip, S.K. 17 β-estradiol suppresses proliferation of fibroblasts derived from cardinal ligaments in patients with or without pelvic organ prolapse. Hum. Reprod. 2006, 21, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.D.; Oliver, R.H.; Leung, B.S.; Lin, L.Y.; Yeh, J. Estrogen receptor α and β expression in the vaginal walls and uterosacral ligaments of premenopausal and postmenopausal women. Fertil. Steril. 1999, 71, 1099–1102. [Google Scholar] [CrossRef] [PubMed]
- Ewies, A.A.; Thompson, J.; Al-Azzawi, F. Changes in gonadal steroid receptors in the cardinal ligaments of prolapsed uteri: immunohistomorphometric data. Hum. Reprod. 2004, 19, 1622–1628. [Google Scholar] [CrossRef] [PubMed]
- Bump, R.C.; Mattiasson, A.; Bø, K.; Brubaker, L.P.; DeLancey, J.O.; Klarskov, P.; Shull, B.L.; Smith, A.R. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am. J. Obstet. Gynecol. 1996, 175, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.Z.; Yang, W.T. Judging standard of immunohistochemical results. Chin. Oncol. 1996, 6, 229–231. [Google Scholar]
- Zhang, R.G.; Wang, C.S.; Gao, C.F. Prevalence and pathogenesis of Barrett’s esophagus in Luoyang, China. Asian Pac. J. Cancer Prev. 2012, 13, 2185–2191. [Google Scholar] [CrossRef] [PubMed]
- Lü, D.; Liu, X.; Gao, Y.; Huo, B.; Kang, Y.; Chen, J.; Sun, S.; Chen, L.; Luo, X.; Long, M. Asymmetric migration of human keratinocytes under mechanical stretch and cocultured fibroblasts in a wound repair model. PLoS ONE 2013, 8, e74563. [Google Scholar] [CrossRef] [PubMed]
- Sbardella, D.; Fasciglione, G.F.; Gioia, M.; Ciaccio, C.; Tundo, G.R.; Marini, S.; Coletta, M. Human matrix metalloproteinases: An ubiquitarian class of enzymes involved in several pathological processes. Mol. Asp. Med. 2012, 33, 119–208. [Google Scholar] [CrossRef] [Green Version]
- Lü, D.; Luo, C.; Zhang, C.; Li, Z.; Long, M. Differential regulation of morphology and stemness of mouse embryonic stem cells by substrate stiffness and topography. Biomaterials 2014, 35, 3945–3955. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Zhang, Z.; Lü, D.; Xu, Q. Effects of Mechanical Stretching on the Morphology and Cytoskeleton of Vaginal Fibroblasts from Women with Pelvic Organ Prolapse. Int. J. Mol. Sci. 2015, 16, 9406-9419. https://doi.org/10.3390/ijms16059406
Wang S, Zhang Z, Lü D, Xu Q. Effects of Mechanical Stretching on the Morphology and Cytoskeleton of Vaginal Fibroblasts from Women with Pelvic Organ Prolapse. International Journal of Molecular Sciences. 2015; 16(5):9406-9419. https://doi.org/10.3390/ijms16059406
Chicago/Turabian StyleWang, Sumei, Zhenyu Zhang, Dongyuan Lü, and Qiuxiang Xu. 2015. "Effects of Mechanical Stretching on the Morphology and Cytoskeleton of Vaginal Fibroblasts from Women with Pelvic Organ Prolapse" International Journal of Molecular Sciences 16, no. 5: 9406-9419. https://doi.org/10.3390/ijms16059406





