A Moonlighting Human Protein Is Involved in Mitochondrial Import of tRNA
Abstract
:1. Introduction
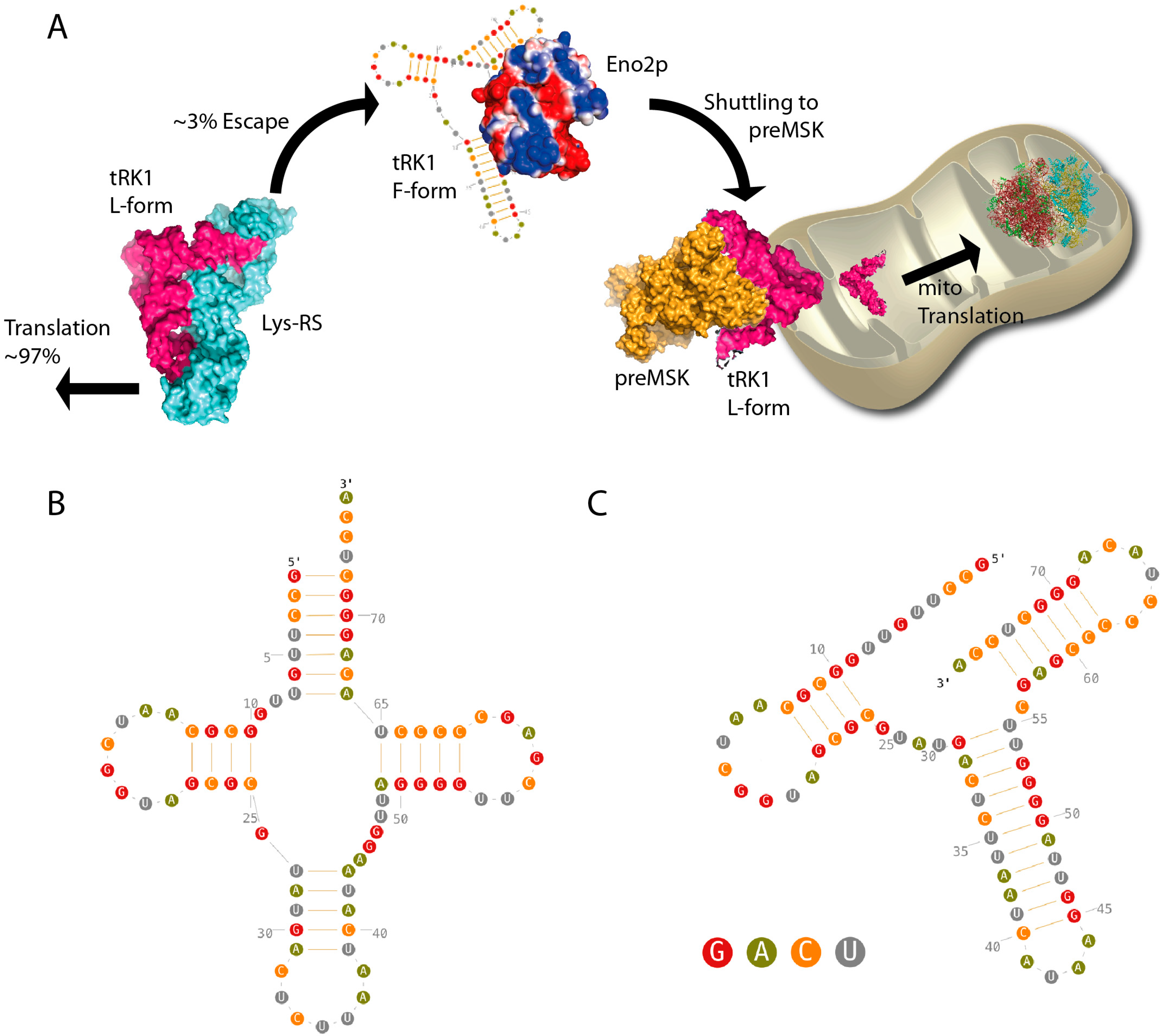
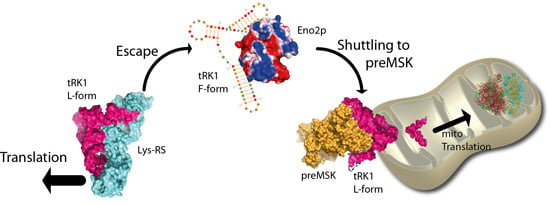
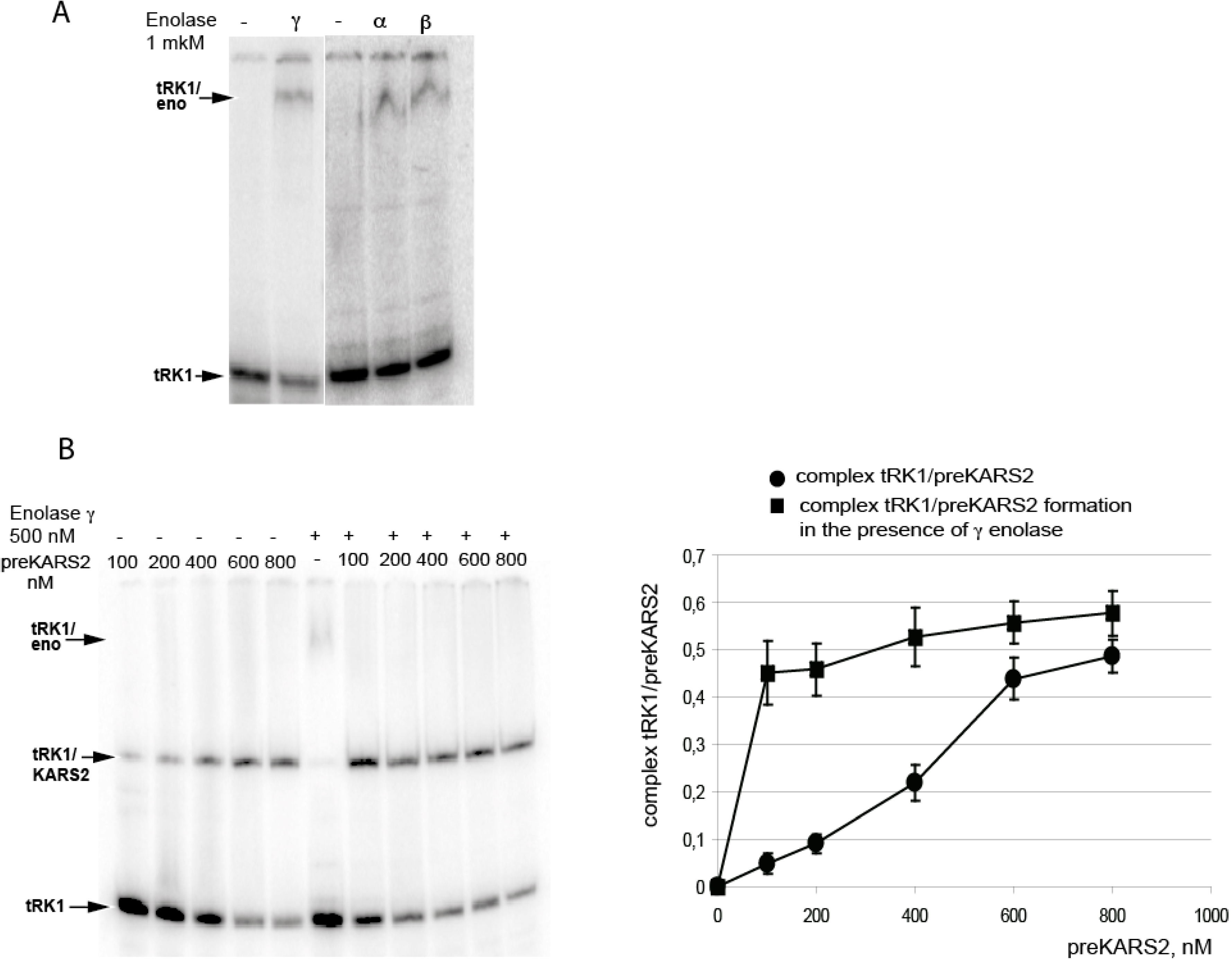
2. Results
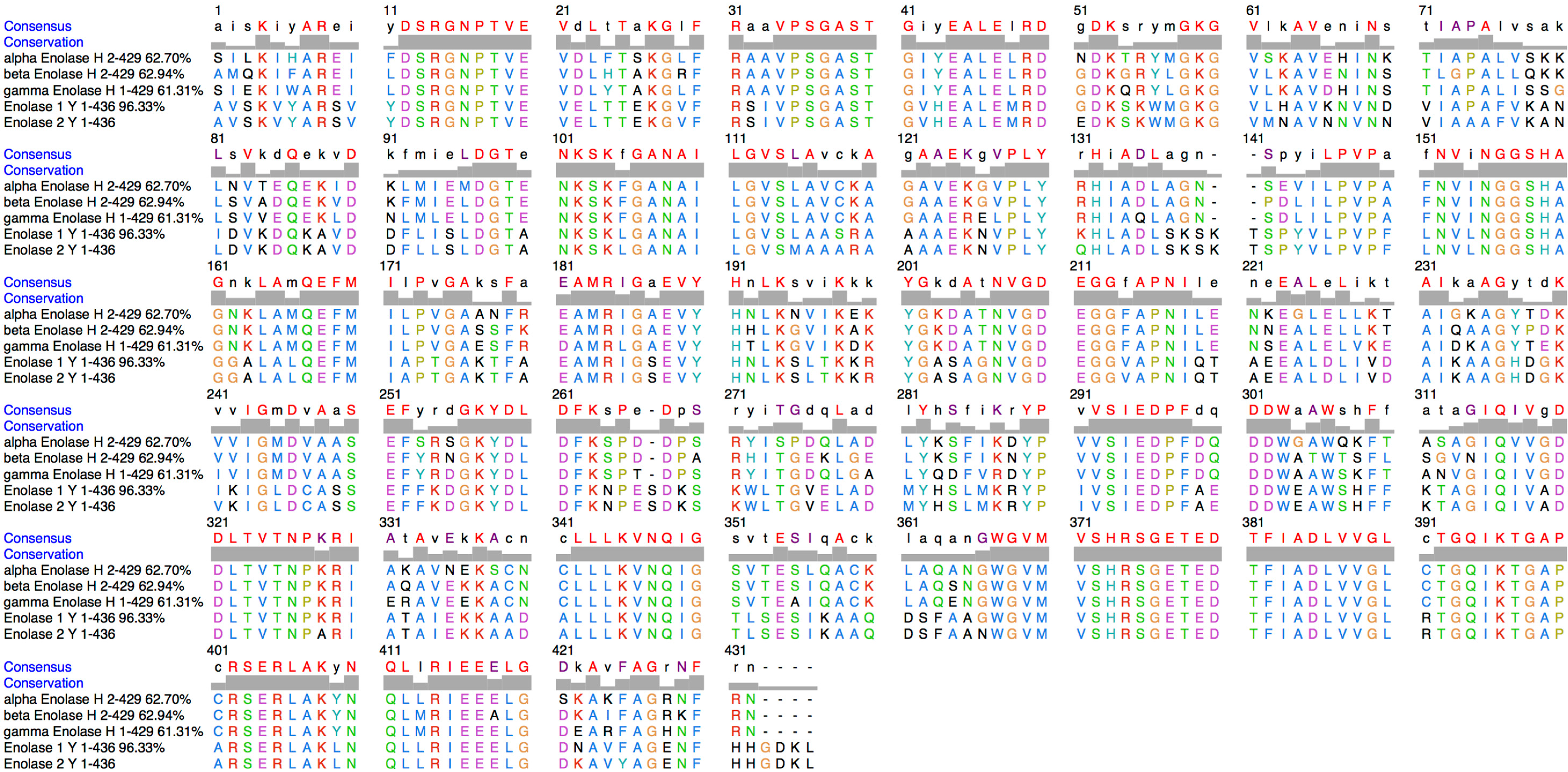

3. Discussion

4. Experimental Section
4.1. Expression and Purification of Recombinant Proteins
4.2. Electrophoretic Mobility Shift Assay (EMSA)
4.3. In Vitro Import Assay
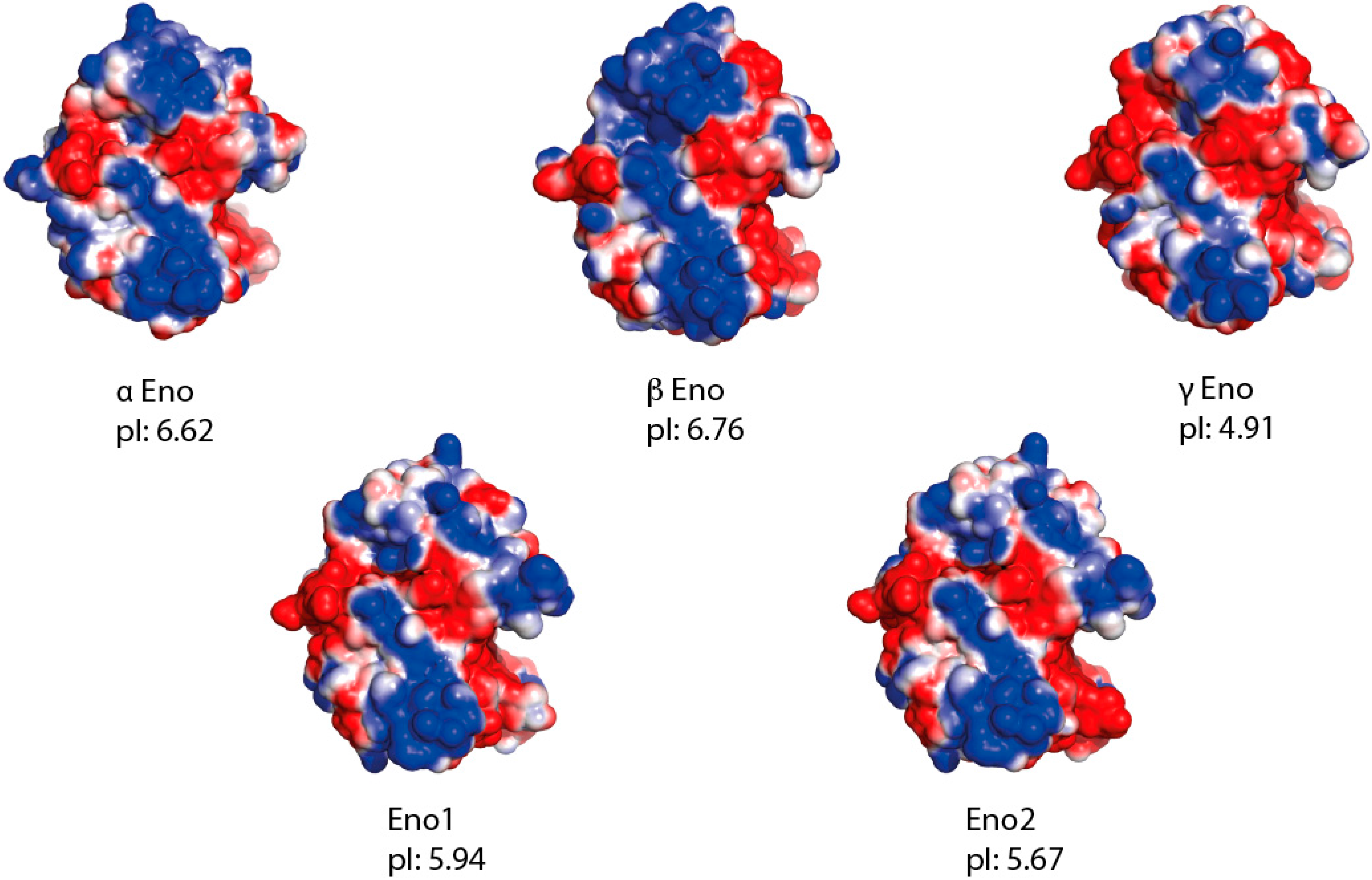
4.4. Protein Alignment, Molecular Modelling and Electrostatic Surface Calculation
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Frechin, M.; Enkler, L.; Tetaud, E.; Laporte, D.; Senger, B.; Blancard, C.; Hammann, P.; Bader, G.; Clauder-Münster, S.; Steinmetz, L.M.; et al. Expression of nuclear and mitochondrial genes encoding atp synthase is synchronized by disassembly of a multisynthetase complex. Mol. Cell 2014, 56, 763–776. [Google Scholar] [CrossRef]
- Entelis, N.S.; Kolesnikova, O.A.; Martin, R.P.; Tarassov, I.A. RNA delivery into mitochondria. Adv. Drug Deliv. Rev. 2001, 49, 199–215. [Google Scholar] [CrossRef] [PubMed]
- Sieber, F.; Duchêne, A.-M.; Maréchal-Drouard, L. Chapter four—Mitochondrial RNA import: From diversity of natural mechanisms to potential applications. In International Review of Cell and Molecular Biology; Kwang, W.J., Ed.; Academic Press: New York, NY, USA, 2011; Volume 287, pp. 145–190. [Google Scholar]
- Emblem, A.; Karlsen, B.O.; Evertsen, J.; Johansen, S.D. Mitogenome rearrangement in the cold-water scleractinian coral lophelia pertusa (cnidaria, anthozoa) involves a long-term evolving group I intron. Mol. Phylogenet. Evol. 2011, 61, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Jackson, K.E.; Habib, S.; Frugier, M.; Hoen, R.; Khan, S.; Pham, J.S.; Pouplana, L.R.D.; Royo, M.; Santos, M.A.S.; Sharma, A.; et al. Protein translation in plasmodium parasites. Trends Parasitol. 2011, 27, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.P.; Schneller, J.M.; Stahl, A.J.; Dirheimer, G. Import of nuclear deoxyribonucleic acid coded lysine-accepting transfer ribonucleic acid (anticodon CUU) into yeast mitochondria. Biochemistry 1979, 18, 4600–4605. [Google Scholar] [CrossRef] [PubMed]
- Kamenski, P.; Kolesnikova, O.; Jubenot, V.; Entelis, N.; Krasheninnikov, I.A.; Martin, R.P.; Tarassov, I. Evidence for an adaptation mechanism of mitochondrial translation via tRNA import from the cytosol. Mol. Cell 2007, 26, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Entelis, N.S.; Kolesnikova, O.A.; Dogan, S.; Martin, R.P.; Tarassov, I.A. 5 S rRNA and tRNA import into human mitochondria. Comparison of in vitro requirements. J. Biol. Chem. 2001, 276, 45642–45653. [Google Scholar] [CrossRef] [PubMed]
- Kolesnikova, O.A.; Entelis, N.S.; Jacquin-Becker, C.; Goltzene, F.; Chrzanowska-Lightowlers, Z.M.; Lightowlers, R.N.; Martin, R.P.; Tarassov, I. Nuclear DNA-encoded tRNAs targeted into mitochondria can rescue a mitochondrial DNA mutation associated with the MERRF syndrome in cultured human cells. Hum. Mol. Genet. 2004, 13, 2519–2534. [Google Scholar] [CrossRef] [PubMed]
- Comte, C.; Tonin, Y.; Heckel-Mager, A.M.; Boucheham, A.; Smirnov, A.; Aure, K.; Lombes, A.; Martin, R.P.; Entelis, N.; Tarassov, I. Mitochondrial targeting of recombinant RNAs modulates the level of a heteroplasmic mutation in human mitochondrial DNA associated with kearns sayre syndrome. Nucleic Acids Res. 2013, 41, 418–433. [Google Scholar] [CrossRef] [PubMed]
- Tonin, Y.; Heckel, A.M.; Dovydenko, I.; Meschaninova, M.; Comte, C.; Venyaminova, A.; Pyshnyi, D.; Tarassov, I.; Entelis, N. Characterization of chemically modified oligonucleotides targeting a pathogenic mutation in human mitochondrial DNA. Biochimie 2014, 100, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Tonin, Y.; Heckel, A.M.; Vysokikh, M.; Dovydenko, I.; Meschaninova, M.; Rotig, A.; Munnich, A.; Venyaminova, A.; Tarassov, I.; Entelis, N. Modeling of antigenomic therapy of mitochondrial diseases by mitochondrially addressed RNA targeting a pathogenic point mutation in mitochondrial DNA. J. Biol. Chem. 2014, 289, 13323–13334. [Google Scholar] [CrossRef] [PubMed]
- Gowher, A.; Smirnov, A.; Tarassov, I.; Entelis, N. Induced tRNA import into human mitochondria: Implication of a host aminoacyl-tRNA-synthetase. PLoS ONE 2013, 8, e66228. [Google Scholar] [CrossRef] [PubMed]
- Brandina, I.; Graham, J.; Lemaitre-Guillier, C.; Entelis, N.; Krasheninnikov, I.; Sweetlove, L.; Tarassov, I.; Martin, R.P. Enolase takes part in a macromolecular complex associated to mitochondria in yeast. Biochim. Biophys. Acta 2006, 1757, 1217–1228. [Google Scholar] [CrossRef] [PubMed]
- Entelis, N.; Brandina, I.; Kamenski, P.; Krasheninnikov, I.A.; Martin, R.P.; Tarassov, I. A glycolytic enzyme, enolase, is recruited as a cofactor of tRNA targeting toward mitochondria in Saccharomyces cerevisiae. Genes Dev. 2006, 20, 1609–1620. [Google Scholar] [CrossRef] [PubMed]
- Kamenski, P.; Smirnova, E.; Kolesnikova, O.; Krasheninnikov, I.A.; Martin, R.P.; Entelis, N.; Tarassov, I. tRNA mitochondrial import in yeast: Mapping of the import determinants in the carrier protein, the precursor of mitochondrial lysyl-tRNA synthetase. Mitochondrion 2010, 10, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Entelis, N.S.; Kieffer, S.; Kolesnikova, O.A.; Martin, R.P.; Tarassov, I.A. Structural requirements of tRNALys for its import into yeast mitochondria. Proc. Natl. Acad. Sci. USA 1998, 95, 2838–2843. [Google Scholar] [CrossRef] [PubMed]
- Kolesnikova, O.; Kazakova, H.; Comte, C.; Steinberg, S.; Kamenski, P.; Martin, R.P.; Tarassov, I.; Entelis, N. Selection of RNA aptamers imported into yeast and human mitochondria. RNA 2010, 16, 926–941. [Google Scholar] [CrossRef] [PubMed]
- Pancholi, V. Multifunctional α-enolase: Its role in diseases. Cell. Mol. Life Sci. 2001, 58, 902–920. [Google Scholar] [CrossRef] [PubMed]
- Kolesnikova, O.; Entelis, N.; Kazakova, H.; Brandina, I.; Martin, R.P.; Tarassov, I. Targeting of tRNA into yeast and human mitochondria: The role of anticodon nucleotides. Mitochondrion 2002, 2, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Chai, G.; Brewer, J.M.; Lovelace, L.L.; Lebioda, L. Structures of asymmetric complexes of human neuron specific enolase with resolved substrate and product and an analogous complex with two inhibitors indicate subunit interaction and inhibitor cooperativity. J. Inorg. Biochem. 2012, 111, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Ramos, A.; Roig-Borrellas, A.; Garcia-Melero, A.; Lopez-Alemany, R. Enolase, a multifunctional protein: Its role on pathophysiological situations. J. Biomed. Biotechnol. 2012, 2012, 12. [Google Scholar] [CrossRef]
- Sedoris, K.C.; Thomas, S.D.; Miller, D.M. c-Myc promoter binding protein regulates the cellular response to an altered glucose concentration. Biochemistry 2007, 46, 8659–8668. [Google Scholar] [CrossRef] [PubMed]
- Wistow, G.J.; Lietman, T.; Williams, L.A.; Stapel, S.O.; de Jong, W.W.; Horwitz, J.; Piatigorsky, J. Tau-crystallin/α-enolase: One gene encodes both an enzyme and a lens structural protein. J. Cell Biol. 1988, 107, 2729–2736. [Google Scholar] [CrossRef] [PubMed]
- Kaminska, M.; Deniziak, M.; Kerjan, P.; Barciszewski, J.; Mirande, M. A recurrent general RNA binding domain appended to plant methionyl-tRNA synthetase acts as a cis-acting cofactor for aminoacylation. EMBO J. 2000, 19, 6908–6917. [Google Scholar] [CrossRef] [PubMed]
- Smirnov, A.; Entelis, N.; Martin, R.P.; Tarassov, I. Biological significance of 5 S rRNA import into human mitochondria: Role of ribosomal protein MRP-L18. Genes Dev. 2011, 25, 1289–1305. [Google Scholar] [CrossRef] [PubMed]
- Henis, Y.I.; Levitzki, A. An analysis on the slope of scatchard plots. Eur. J. Biochem. 1976, 71, 529–532. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped blast and psi-blast: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Biasini, M.; Bienert, S.; Waterhouse, A.; Arnold, K.; Studer, G.; Schmidt, T.; Kiefer, F.; Cassarino, T.G.; Bertoni, M.; Bordoli, L.; et al. Swiss-model: Modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 2014, 42, W252–W258. [Google Scholar] [CrossRef] [PubMed]
- Sims, P.A.; Menefee, A.L.; Larsen, T.M.; Mansoorabadi, S.O.; Reed, G.H. Structure and catalytic properties of an engineered heterodimer of enolase composed of one active and one inactive subunit. J. Mol. Biol. 2006, 355, 422–431. [Google Scholar] [CrossRef] [PubMed]
- The Pymol Molecular Graphics System, version 1.3r1; Schrodinger, LLC: New York, NY, USA, 2010.
- Dolinsky, T.J.; Czodrowski, P.; Li, H.; Nielsen, J.E.; Jensen, J.H.; Klebe, G.; Baker, N.A. PDB2PQR: Expanding and upgrading automated preparation of biomolecular structures for molecular simulations. Nucleic Acids Res. 2007, 35, W522–W525. [Google Scholar] [CrossRef]
- Baker, N.A.; Sept, D.; Joseph, S.; Holst, M.J.; McCammon, J.A. Electrostatics of nanosystems: Application to microtubules and the ribosome. Proc. Natl. Acad. Sci. USA 2001, 98, 10037–10041. [Google Scholar] [CrossRef] [PubMed]
- Gasteiger, E.; Gattiker, A.; Hoogland, C.; Ivanyi, I.; Appel, R.D.; Bairoch, A. Expasy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003, 31, 3784–3788. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baleva, M.; Gowher, A.; Kamenski, P.; Tarassov, I.; Entelis, N.; Masquida, B. A Moonlighting Human Protein Is Involved in Mitochondrial Import of tRNA. Int. J. Mol. Sci. 2015, 16, 9354-9367. https://doi.org/10.3390/ijms16059354
Baleva M, Gowher A, Kamenski P, Tarassov I, Entelis N, Masquida B. A Moonlighting Human Protein Is Involved in Mitochondrial Import of tRNA. International Journal of Molecular Sciences. 2015; 16(5):9354-9367. https://doi.org/10.3390/ijms16059354
Chicago/Turabian StyleBaleva, Maria, Ali Gowher, Piotr Kamenski, Ivan Tarassov, Nina Entelis, and Benoît Masquida. 2015. "A Moonlighting Human Protein Is Involved in Mitochondrial Import of tRNA" International Journal of Molecular Sciences 16, no. 5: 9354-9367. https://doi.org/10.3390/ijms16059354