Immunohistochemical Analysis of Paraoxonases and Chemokines in Arteries of Patients with Peripheral Artery Disease
Abstract
:1. Introduction
2. Results
| Parameter | Control (n = 8) | PAD (n = 66) | p-Value |
|---|---|---|---|
| Clinical characteristics | |||
| Age, years | 66 (30–76) | 70 (62–77) | 0.223 |
| Male, n (%) | 5 (62.5) | 55 (85.9) | 0.094 |
| Smokers, n (%) | 1 (14.3) | 16 (31.4) | 0.048 |
| Complete blood count | |||
| Red blood cells, ×1012/L | 4.32 (3.18–4.47) | 3.67 (3.14–4.24) | 0.449 |
| Hemoglobin, g/dL | 12.46 (9.99–13.28) | 10.85 (9.45–12.93) | 0.468 |
| Leukocytes, ×109/L | 9.22 (8.58–10.17) | 9.89 (7.44–12.20) | 0.668 |
| Platelets, ×109/L | 227.5 (163.7–246.2) | 312.5 (199.0–419.0) | 0.080 |
| Biochemical variables in serum or plasma | |||
| Glucose, mmol/L | 5.77 (5.11–6.77) | 6.38 (5.11–8.83) | 0.406 |
| Total cholesterol, mmol/L | 4.77 (3.87–6.39) | 3.39 (2.90–4.47) | 0.030 |
| HDL cholesterol, mmol/L | 1.24 (0.98–1.40) | 0.93 (0.83–1.20) | 0.074 |
| LDL cholesterol, mmol/L | 3.54 (3.11–4.42) | 1.95 (1.68–2.69) | 0.001 |
| Triglycerides, mmol/L | 1.47 (1.13–2.15) | 1.31 (1.00–1.87) | 0.449 |
| Fibrinogen, g/L | 5.51 (4.48–7.54) | 6.96 (5.34–8.11) | 0.237 |
| C-reactive protein, mg/L | 6.1 (0.6–7.2) | 8.1 (2.7–16.0) | 0.147 |
| Total proteins, g/L | 65 (55–68) | 60 (55–69) | 0.743 |
| CCL2, ng/L | 373.4 (255.2–431.8) | 622.8 (472.7–898.4) | <0.001 |
| PON1, mg/L | 75.4 (56.7–143.8) | 25.2 (18.4–35.8) | <0.001 |
| PON3, mg/L | 1.95 (1.51–2.50) | 1.73 (1.43–2.27) | 0.490 |
| 8-Isoprostanes, ng/L | 14.2 (2.0–37.2) | 100.8 (37.6–314.7) | <0.001 |
| PON1 lactonase activity, U/L | 5.69 (5.02–6.29) | 3.04 (2.11–3.73) | <0.001 |
| Parameter | Control (n = 8) | PAD (n = 66) | p-Value |
|---|---|---|---|
| IMT (mm) | 1.00 (0.70–1.30) | 1.29 (1.00–1.74) | 0.150 |
| I/M ratio | 0.16 (0.13–0.65) | 2.10 (1.33–3.22) | <0.001 |
| % PON1 staining | 1.70 (1.54–3.72) | 11.19 (7.25–20.81) | <0.001 |
| % PON3 staining | 0.55 (0.22–0.73) | 3.25 (2.01–4.37) | <0.001 |
| % CCL2 staining | 2.26 (0.36–3.65) | 30.75 (9.63–44.41) | <0.001 |
| % CCR2 staining | 18.29 (7.02–27.56) | 22.99 (13.21–42.71) | 0.263 |
| % CD68 staining | 1.10 (0.65–2.88) | 4.57 (2.40–9.24) | 0.007 |
| % D6 staining | 0.83 (0.22–12.9) | 41.21 (24.55–58.39) | <0.001 |
| % DARC staining | 3.29 (2.01–5.06) | 37.26 (18.06–51.85) | <0.001 |
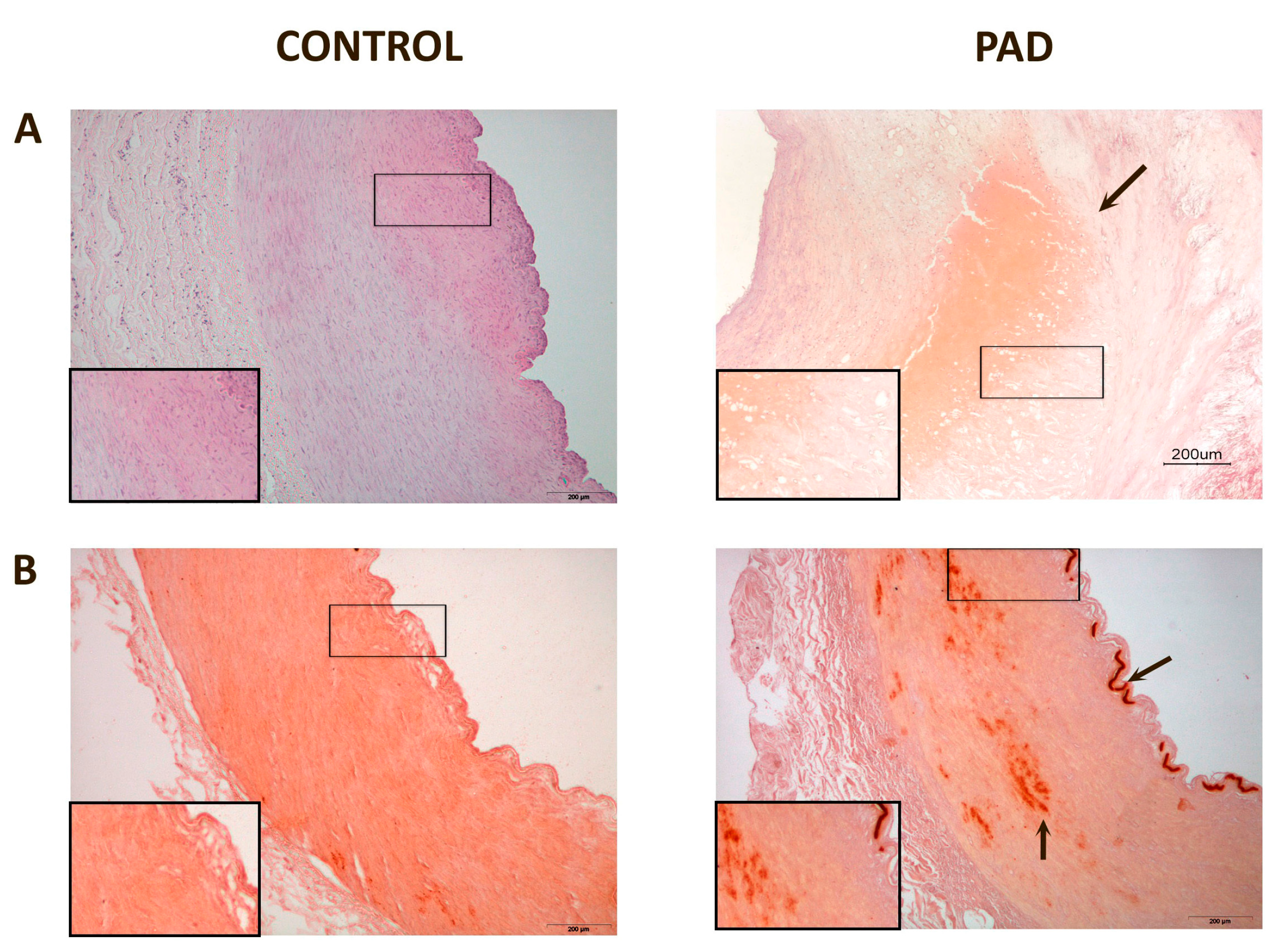

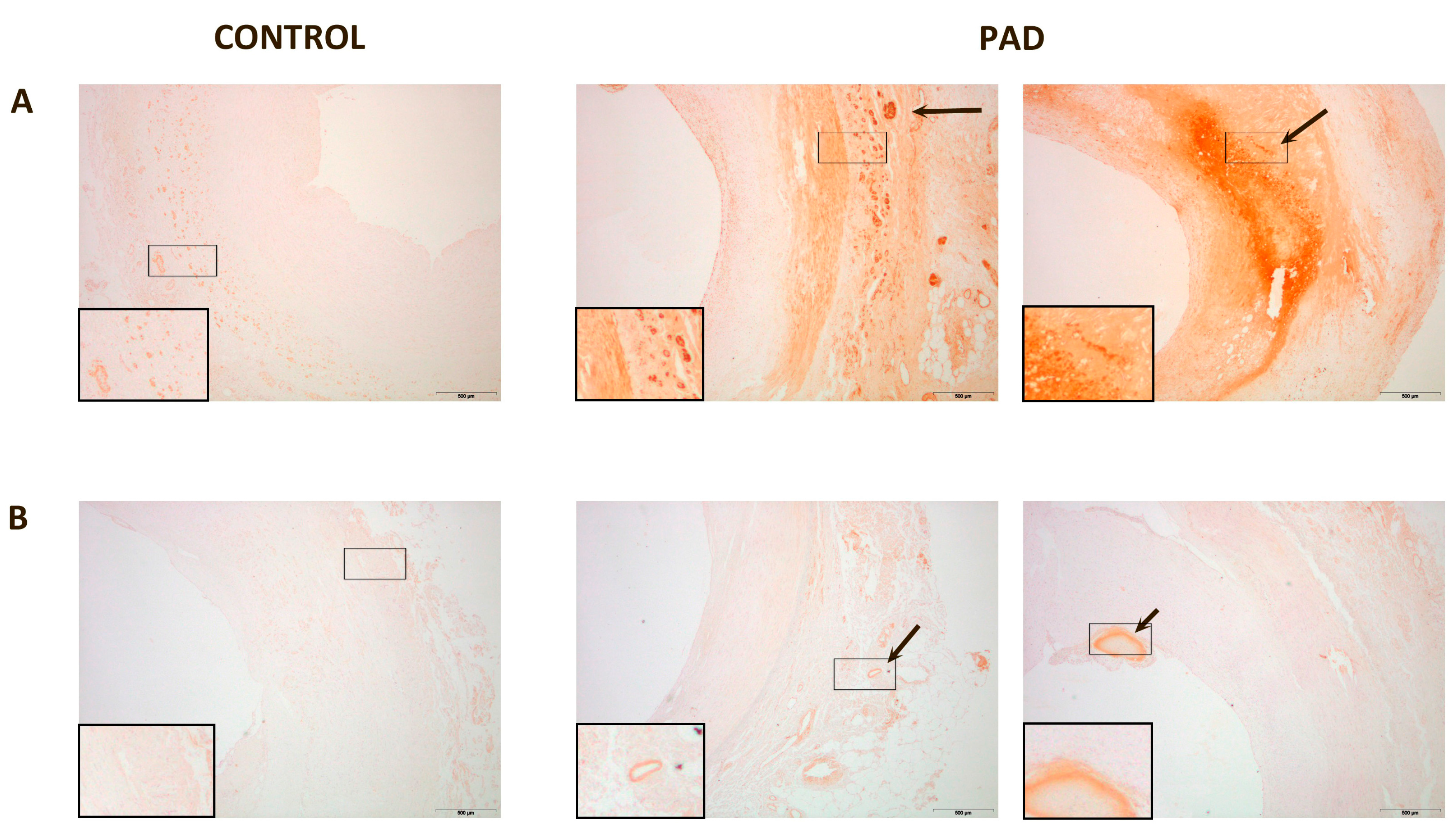
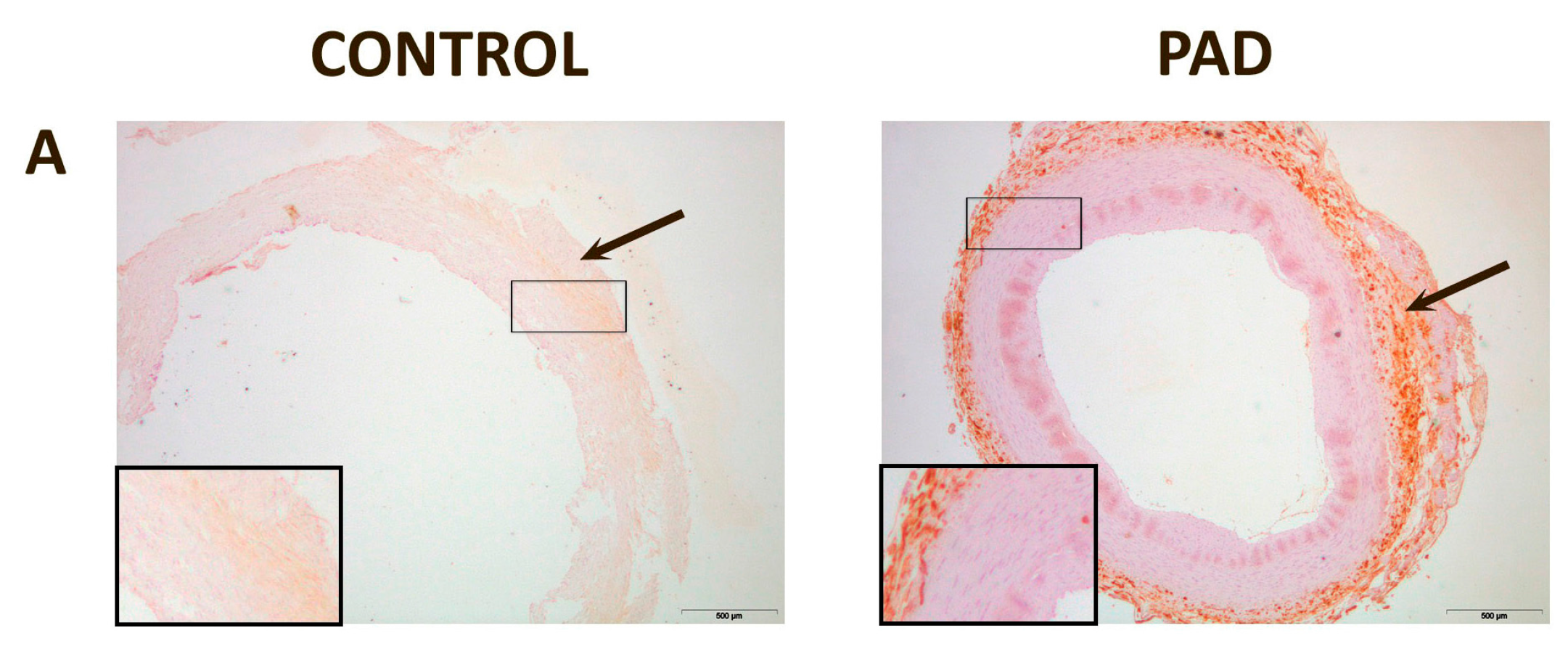
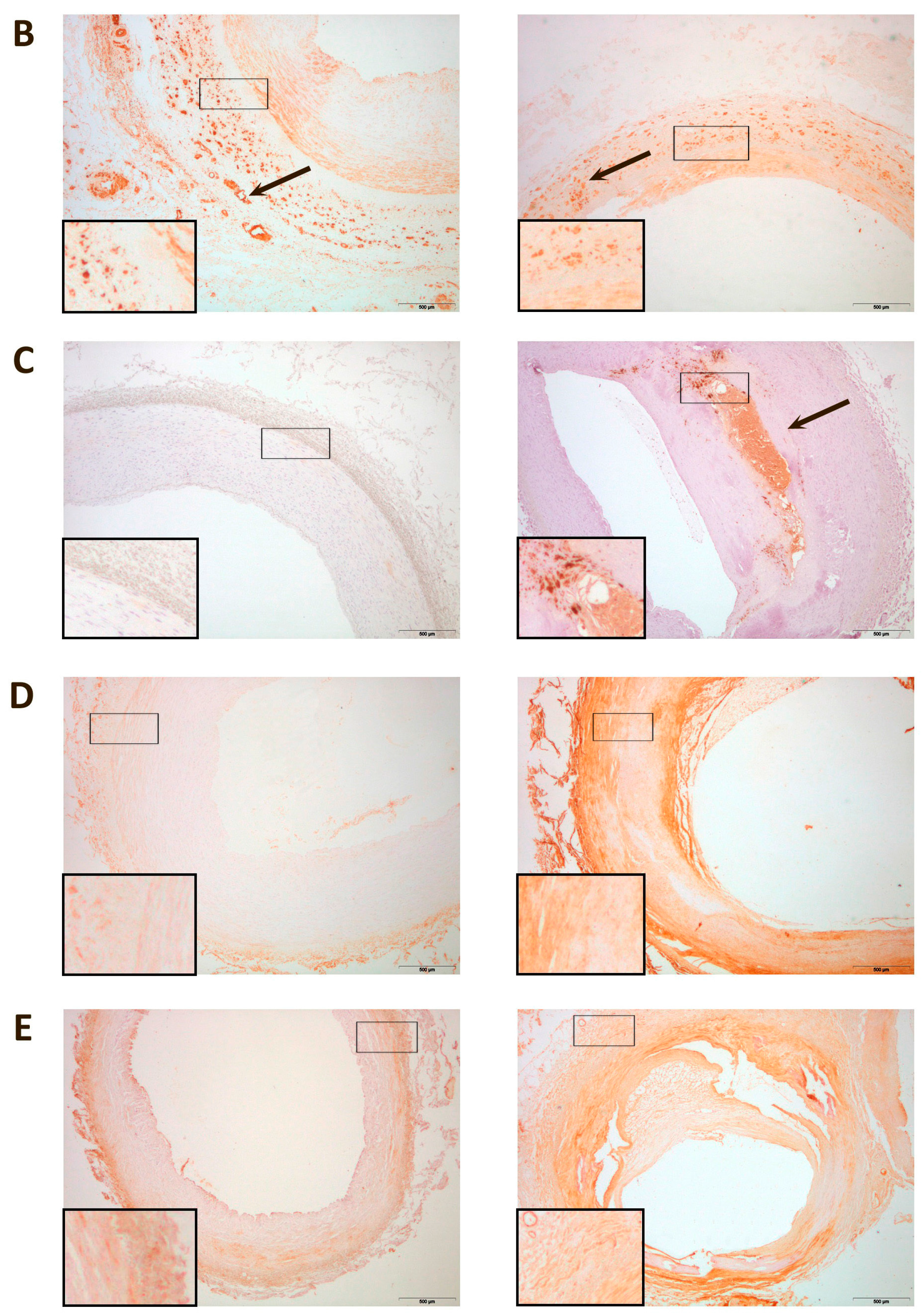
3. Discussion
4. Experimental Section
4.1. Study Population
4.2. Biochemical Analyses
4.3. Histological and Immunohistochemical Analyses
4.4. Statistical Analyses
5. Conclusions
Acknowledgments
Author Contributions
Supplementary Materials
Conflicts of Interest
References
- Golomb, B.A.; Dang, T.T.; Criqui, M.H. Peripheral arterial disease: Morbidity and mortality implications. Circulation 2006, 114, 688–699. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.B.; Shemanski, L.; Manolio, T.A.; Cushman, M.; Mittelmark, M.; Polak, J.F.; Powe, N.R.; Siscovick, D. Ankle-arm index as a predictor of cardiovascular disease and mortality in the cardiovascular health study. Arterioscler. Thromb. Vasc. Biol. 1999, 9, 538–545. [Google Scholar] [CrossRef]
- Criqui, M.H. Peripheral arterial disease—Epidemiological aspects. Vasc. Med. 2001, 6, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Mehlsen, J.; Wiinberg, N.; Joergensen, B.S.; Schultz-Larsen, P. High prevalence of peripheral arterial disease in patients with previous cerebrovascular or coronary event. Blood Press. 2010, 19, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Gornik, H.L.; Creager, M.A. Contemporary management of peripheral arterial disease: Cardiovascular risk-factor modification. Clevel. Clin. J. Med. 2006, 73, S30–S37. [Google Scholar] [CrossRef]
- Hirsch, A.T.; Criqui, M.H.; Treat-Jacobson, D.; Regensteiner, J.G.; Creager, M.A.; Olin, J.W.; Krook, H.; Hunninghake, D.B.; Comerota, A.J.; Walsh, M.E.; et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA 2001, 286, 1317–1324. [Google Scholar] [CrossRef] [PubMed]
- McDermott, M.M.; Liu, K.; Greenland, P.; Guralnik, J.M.; Criqui, M.H.; Chan, C.; Pearce, W.H.; Schneider, J.R.; Ferrucci, L.; Celic, L.; et al. Functional decline in peripheral arterial disease: Associations with the ankle brachial index and leg symptoms. JAMA 2004, 292, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Criqui, M.H.; Langer, R.D.; Fronek, A.; Feigelson, H.S.; Klauber, M.R.; McCann, T.J.; Browner, D. Mortality over a period of 10 years in patients with peripheral arterial disease. N. Engl. J. Med. 1992, 326, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Strzyżewski, K.W.; Pioruńska-Stolzmann, M.; Majewski, W.; Kasprzak, M.; Strzyżewski, W. Effect of surgical treatment on lipid peroxidation parameters and antioxidant status in the serum of patients with peripheral arterial disease. Dis. Markers 2013, 35, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Arslan, C.; Altan, H.; Beşirli, K.; Aydemir, B.; Kiziler, A.R.; Denli, S. The role of oxidative stress and antioxidant defenses in Buerger disease and atherosclerotic peripheral arterial occlusive disease. Ann. Vasc. Surg. 2010, 24, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Abelló, D.; Sancho, E.; Camps, J.; Joven, J. Exploring the role of paraoxonases in the pathogenesis of coronary artery disease: A systematic review. Int. J. Mol. Sci. 2014, 15, 20997–21010. [Google Scholar] [CrossRef] [PubMed]
- Primo-Parmo, S.L.; Sorenson, R.C.; Teiber, J.; la Du, B.N. The human serum paraoxonase/arylesterase gene (PON1) is one member of a multigene family. Genomics 1996, 33, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Sorenson, R.C.; Primo-Parmo, S.L.; Camper, S.A.; la Du, B.N. The genetic mapping and gene structure of mouse paraoxonase/arylesterase. Genomics 1995, 30, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Camps, J.; Marsillach, J.; Joven, J. The paraoxonases: Role in human diseases and methodological difficulties in measurement. Crit. Rev. Clin. Lab. Sci. 2009, 46, 83–106. [Google Scholar] [CrossRef] [PubMed]
- Hine, D.; Mackness, B.; Mackness, M. Coincubation of PON1, APO A1, and LCAT increases the time HDL is able to prevent LDL oxidation. IUBMB Life 2012, 64, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Mackness, M.I.; Durrington, P.N.; Mackness, B. The role of paraoxonase 1 activity in cardiovascular disease: Potential for therapeutic intervention. Am. J. Cardiovasc. Drugs 2004, 4, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Mackness, M.I.; Mackness, B.; Durrington, P.N. Paraoxonase and coronary artery disease. Atheroscler. Suppl. 2002, 3, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Mackness, M.I.; Arrol, S.; Durrington, P.N. Paraoxonase prevents accumulation of lipoperoxides in low-density lipoprotein. FEBS Lett. 1991, 286, 152–154. [Google Scholar] [CrossRef] [PubMed]
- Mackness, M.I.; Arrol, S.; Abbott, C.; Durrington, P.N. Protection of low-density lipoprotein against oxidative modification by high-density lipoprotein associated paraoxonase. Atherosclerosis 1993, 104, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Mackness, B.; Hine, D.; Liu, Y.; Mastorikou, M.; Mackness, M. Paraoxonase-1 inhibits oxidised LDL-induced MCP-1 production by endothelial cells. Biochem. Biophys. Res. Commun. 2004, 318, 680–683. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, M. Many cytokines are very useful therapeutic targets in disease. J. Clin. Investig. 2008, 118, 3533–3536. [Google Scholar] [CrossRef] [PubMed]
- Charo, I.F.; Taubman, M.B. Chemokines in the pathogenesis of vascular disease. Circ. Res. 2004, 95, 858–866. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Sanabria, F.; Rull, A.; Beltrán-Debón, R.; Aragonès, G.; Camps, J.; Mackness, B.; Mackness, M.; Joven, J. Tissue distribution and expression of paraoxonases and chemokines in mouse: The ubiquitous and joint localisation suggest a systemic and coordinated role. J. Mol. Histol. 2010, 41, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Rull, A.; Camps, J.; Alonso-Villaverde, C.; Joven, J. Insulin resistance, inflammation, and obesity: Role of monocyte chemoattractant protein-1 (or CCL2) in the regulation of metabolism. Mediat. Inflamm. 2010, 2010, 326580. [Google Scholar] [CrossRef]
- Petrkova, J.; Szotkowska, J.; Hermanova, Z.; Lukl, J.; Petrek, M. Monocyte chemoattractant protein-1 in patients with peripheral arterial disease. Mediat. Inflamm. 2004, 13, 39–43. [Google Scholar] [CrossRef]
- Van Wijk, D.F.; van Leuven, S.I.; Sandhu, M.S.; Tanck, M.W.; Hutten, B.A.; Wareham, N.J.; Kastelein, J.J.; Stroes, E.S.; Khaw, K.T.; Boekholdt, S.M. Chemokine ligand 2 genetic variants, serum monocyte chemoattractant protein-1 levels, and the risk of coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1460–1466. [Google Scholar] [CrossRef] [PubMed]
- Marsillach, J.; Aragonès, G.; Beltrán, R.; Caballeria, J.; Pedro-Botet, J.; Morcillo-Suárez, C.; Navarro, A.; Joven, J.; Camps, J. The measurement of the lactonase activity of paraoxonase-1 in the clinical evaluation of patients with chronic liver impairment. Clin. Biochem. 2009, 42, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Ferré, N.; Marsillach, J.; Camps, J.; Mackness, B.; Mackness, M.; Riu, F.; Coll, B.; Tous, M.; Joven, J. Paraoxonase-1 is associated with oxidative stress, fibrosis and FAS expression in chronic liver diseases. J. Hepatol. 2006, 45, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Pasqualini, L.; Cortese, C.; Marchesi, S.; Siepi, D.; Pirro, M.; Vaudo, G.; Liberatoscioli, L.; Gnasso, A.; Schillaci, G.; Mannarino, E. Paraoxonase-1 activity modulates endothelial function in patients with peripheral arterial disease. Atherosclerosis 2005, 183, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Rull, A.; García, R.; Fernández-Sender, L.; Beltrán-Debón, R.; Aragonès, G.; Alegret, J.M.; Alonso-Villaverde, C.; Mackness, B.; Mackness, M.; Camps, J.; et al. The role of combined assessment of defense against oxidative stress and inflammation in the evaluation of peripheral arterial disease. Curr. Mol. Med. 2011, 11, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Rull, A.; García, R.; Fernández-Sender, L.; García-Heredia, A.; Aragonès, G.; Beltrán-Debón, R.; Marsillach, J.; Alegret, J.M.; Martín-Paredero, V.; Mackness, B.; et al. Serum paraoxonase-3 concentration is associated with insulin sensitivity in peripheral artery disease and with inflammation in coronary artery disease. Atherosclerosis 2012, 220, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, G.Z.; Rabinovitch, P.S.; Tabas, I. Macrophage mitochondrial oxidative stress promotes atherosclerosis and nuclear factor-κB-mediated inflammation in macrophages. Circ. Res. 2014, 114, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.T.; Devarajan, A.; Bourquard, N.; Shih, D.; Fogelman, A.M. Is it just paraoxonase 1 or are other members of the paraoxonase gene family implicated in atherosclerosis? Curr. Opin. Lipidol. 2008, 19, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Mastorikou, M.; Mackness, B.; Liu, Y.; Mackness, M. Glycation of paraoxonase-1 inhibits its activity and impairs the ability of high-density lipoprotein to metabolise membrane lipid hydroperoxides. Diabet. Med. 2008, 25, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- Mackness, B.; Beltran-Debon, R.; Aragones, G.; Joven, J.; Camps, J.; Mackness, M. Human tissue distribution of paraoxonases 1 and 2 mRNA. IUBMB Life 2010, 62, 480–482. [Google Scholar] [PubMed]
- Draganov, D.I.; Stetson, P.L.; Watson, D.E.; Billecke, S.S.; la Du, B.N. Rabbit serum paraoxonase 3 (PON3) is a high density lipoprotein associated lactonase and protects low density lipoprotein against oxidation. J. Biol. Chem. 2000, 275, 33435–33442. [Google Scholar] [CrossRef] [PubMed]
- Teiber, J.F.; Draganov, D.I.; la Du, B.N. Lactonase and lactonising activities of human serum paraoxonase (PON1) and rabbit serum PON3. Biochem. Pharmacol. 2003, 66, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Mackness, B.; Mackness, M. Comparison of the ability of paraoxonases 1 and 3 to attenuate the in vitro oxidation of low-density lipoprotein and reduce macrophage oxidative stress. Free Radic. Biol. Med. 2008, 45, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Shih, D.M.; Xia, Y.R.; Wang, X.P.; Bourquard, N.; Fogelman, A.M.; Lusis, A.J.; Reddy, S.T. Decreased obesity and atherosclerosis in human paraoxonase 3 transgenic mice. Circ. Res. 2007, 100, 1200–1207. [Google Scholar] [CrossRef] [PubMed]
- Marsillach, J.; Camps, J.; Beltran-Debón, R.; Rull, A.; Aragones, G.; Maestre-Martínez, C.; Sabench, F.; Hernández, M.; Castillo, D.D.; Joven, J.; et al. Immunohistochemical analysis of paraoxonases-1 and 3 in human atheromatous plaques. Eur. J. Clin. Investig. 2011, 41, 308–314. [Google Scholar] [CrossRef]
- Satiroglu, O.; Uydu, H.A.; Demir, A.; Bostan, M.; Atak, M.; Bozkurt, E. Association between plasma monocyte chemoattractant protein-1 levels and the extent of atherosclerotic peripheral artery disease. Tohoku J. Exp. Med. 2011, 224, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Coll, B.; Alonso-Villaverde, C.; Joven, J. Monocyte chemoattractant protein-1 and atherosclerosis: Is there room for an additional biomarker? Clin. Chim. Acta 2007, 383, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, R.B.; Baumert, J.; Barbalic, M.; Dupuis, J.; Ellinor, P.T.; Durda, P.; Dehghan, A.; Bis, J.C.; Illig, T.; Morrison, A.C.; et al. Duffy antigen receptor for chemokines (Darc) polymorphism regulates circulating concentrations of monocyte chemoattractant protein-1 and other inflammatory mediators. Blood 2010, 115, 5289–5299. [Google Scholar] [CrossRef] [PubMed]
- Aragones, G.; Ercilla, A.; Barreda, M.; Rull, A.; Beltrán-Debón, R.; Rodríguez-Gallego, E.; Alonso-Villaverde, C.; Camps, J.; Joven, J. Human Duffy blood group alloantigen system influences the measurement of monocyte chemoattractant protein-1 (MCP-1) in serum but not in plasma. Clin. Lab. 2012, 58, 185–188. [Google Scholar] [PubMed]
- Reckless, J.; Rubin, E.M.; Verstuyft, J.B.; Metcalfe, J.C.; Grainger, D.J. Monocyte chemoattractant protein-1 but not tumor necrosis factor-α is correlated with monocyte infiltration in mouse lipid lesions. Circulation 1999, 99, 2310–2316. [Google Scholar] [CrossRef] [PubMed]
- Graham, G.J.; Locati, M.; Mantovani, A.; Rot, A.; Thelen, M. The biochemistry and biology of the atypical chemokine receptors. Immunol. Lett. 2012, 145, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Manea, A.; Manea, S.A.; Todirita, A.; Albulescu, I.C.; Raicu, M.; Sasson, S.; Simionescu, M. High-glucose-increased expression and activation of NADPH oxidase in human vascular smooth muscle cells is mediated by 4-hydroxynonenal-activated PPARα and PPARβ/δ. Cell Tissue Res. 2015. [Google Scholar] [CrossRef]
- Fuhrman, B. Regulation of hepatic paraoxonase-1 expression. J. Lipids 2012, 2012, 684010. [Google Scholar] [CrossRef] [PubMed]
- Holvoet, P.; Rull, A.; García-Heredia, A.; López-Sanromà, S.; Geeraert, B.; Joven, J.; Camps, J. Stevia-derived compounds attenuate the toxic effects of ectopic lipid accumulation in the liver of obese mice: A transcriptomic and metabolomic study. Food Chem. Toxicol. 2015, 77, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Marsillach, J.; Camps, J.; Ferré, N.; Beltrán, R.; Rull, A.; Mackness, B.; Mackness, M.; Joven, J. Paraoxonase-1 is related to inflammation, fibrosis and PPARδ in experimental liver disease. BMC Gastroenterol. 2009, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, R.; Kim, M.; Kieny, R. Surgical treatment of peripheral circulation disorders. Helv. Chir. Acta 1954, 21, 499–533. [Google Scholar] [PubMed]
- Ng, C.J.; Wadleigh, D.J.; Gangopadhyay, A.; Hama, S.; Grijalva, V.R.; Navab, M.; Fogelman, A.M.; Reddy, S.T. Paraoxonase-2 is a ubiquitously expressed protein with antioxidant properties and is capable of preventing cell-mediated oxidative modification of low density lipoprotein. J. Biol. Chem. 2001, 276, 4444–4449. [Google Scholar]
- Reddy, S.T.; Wadleigh, D.J.; Grijalva, V.; Ng, C.; Hama, S.; Gangopadhyay, A.; Shih, D.M.; Lusis, A.J.; Navab, M.; Fogelman, A.M. Human paraoxonase-3 is an HDL-associated enzyme with biological activity similar to paraoxonase-1 protein but is not regulated by oxidised lipids. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Marsillach, J.; Mackness, B.; Mackness, M.; Riu, F.; Beltran, R.; Joven, J.; Camps, J. Immunohistochemical analysis of paraoxonase-1, 2 and 3 expression in normal mouse tissues. Free Radic. Biol. Med. 2008, 45, 146–157. [Google Scholar] [CrossRef] [PubMed]
- AnalySIS® 3.1. Step by Step Analysis. Available online: ftp://ftp.ccmr.cornell.edu/utility/FEI%20temp/AnalySIS%20docs/Getting%20Started.pdf (accessed on 27 April 2015).
- Suzme, R.; Yalcin, O.; Gurdol, F.; Gungor, F.; Bilir, A. Connective tissue alterations in women with pelvic organ prolapse and urinary incontinence. Acta Obstet. Gynecol. Scand. 2007, 86, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Palmowski, M.; Huppert, J.; Ladewig, G.; Hauff, P.; Reinhardt, M.; Mueller, M.M.; Woenne, E.C.; Jenne, J.W.; Maurer, M.; Kauffmann, G.W.; et al. Molecular profiling of angiogenesis with targeted ultrasound imaging: Early assessment of antiangiogenic therapy effects. Mol. Cancer Ther. 2008, 7, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Białas, M.; Okon, K.; Czopek, J. Assessing microvessel density in gastric carcinoma: A comparison of three markers. Pol. J. Pathol. 2003, 4, 249–252. [Google Scholar]
- Sander, C.S.; Hamm, F.; Elsner, P.; Thiele, J.J. Oxidative stress in malignant melanoma and non-melanoma skin cancer. Br. J. Dermatol. 2003, 148, 913–922. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Aguilera, A.; Sepúlveda, J.; Rodríguez-Gallego, E.; Guirro, M.; García-Heredia, A.; Cabré, N.; Luciano-Mateo, F.; Fort-Gallifa, I.; Martín-Paredero, V.; Joven, J.; et al. Immunohistochemical Analysis of Paraoxonases and Chemokines in Arteries of Patients with Peripheral Artery Disease. Int. J. Mol. Sci. 2015, 16, 11323-11338. https://doi.org/10.3390/ijms160511323
Hernández-Aguilera A, Sepúlveda J, Rodríguez-Gallego E, Guirro M, García-Heredia A, Cabré N, Luciano-Mateo F, Fort-Gallifa I, Martín-Paredero V, Joven J, et al. Immunohistochemical Analysis of Paraoxonases and Chemokines in Arteries of Patients with Peripheral Artery Disease. International Journal of Molecular Sciences. 2015; 16(5):11323-11338. https://doi.org/10.3390/ijms160511323
Chicago/Turabian StyleHernández-Aguilera, Anna, Julio Sepúlveda, Esther Rodríguez-Gallego, Maria Guirro, Anabel García-Heredia, Noemí Cabré, Fedra Luciano-Mateo, Isabel Fort-Gallifa, Vicente Martín-Paredero, Jorge Joven, and et al. 2015. "Immunohistochemical Analysis of Paraoxonases and Chemokines in Arteries of Patients with Peripheral Artery Disease" International Journal of Molecular Sciences 16, no. 5: 11323-11338. https://doi.org/10.3390/ijms160511323






