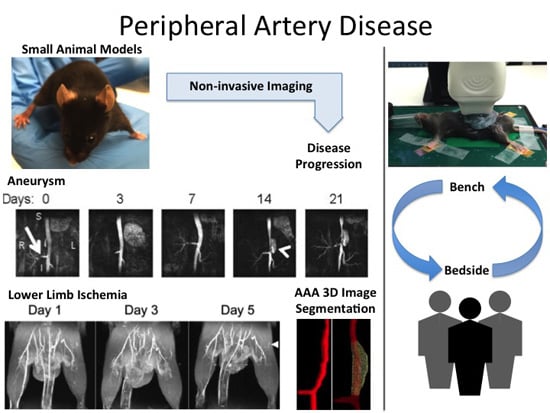
Imaging of Small Animal Peripheral Artery Disease Models: Recent Advancements and Translational Potential
Abstract
:1. Introduction
| Acronym | Definition | Acronym | Definition |
|---|---|---|---|
| 18F-FDG | 18F-Fluorodeoxyglucose | MCA | Middle Cerebral Artery |
| AAA | Abdominal Aortic Aneurysm | MMP | Matrix Metalloproteinase |
| ADC | Apparent Diffusion Coefficient | MRA | Magnetic Resonance Angiography |
| Alk1 | Activin receptor-like kinase 1 | MRI | Magnetic Resonance Imaging |
| AngII | Angiotensin II | MSC | Mesenchymal Stem Cell |
| apoE−/− | Apolipoprotein E-deficient | NIR | Near Infrared |
| AVM | Arteriovenous Malformation | NIRF | Near Infrared Fluorescence |
| B-mode | Brightness Mode | NO | Nitric Oxide |
| BM-MNC | Bone-Marrow-derived Mononuclear Cell | PAD | Peripheral Artery Disease |
| BOLD | Blood-Oxygen-Level Dependent | PET | Positron Emission Tomography |
| CAD | Coronary Artery Disease | PW Doppler | Pulsed Wave Doppler |
| CEUS | Contrast-enhanced Ultrasound | PWI | Perfusion Weighted Imaging |
| CLI | Critical Limb Ischemia | rmVEGF | Recombinant murine Vascular Endothelial Growth Factor |
| CT | Computed Tomography | rt-PA | Recombinant Tissue Plasminogen Activator |
| CTA | Computed Tomography Angiography | SAH | Subarachnoid Hemorrhage |
| DE | Delayed-enhancement | smLRP1−/− | Smooth Muscle Specific Low-density Lipoprotein Receptor-related Protein 1-deficient |
| ECG | Electrocardiogram | SPECT | Single Photon Emission Computed Tomography |
| eNOS | Endothelial Nitric Oxide Synthase | TAA | Thoracic Aortic Aneurysm |
| HHT | Hereditary Hemorrhagic Telangiectasia | TOF-MRA | Time-of-flight Magnetic Resonance Angiography |
| LDLR−/− | Low-density Lipoprotein Receptor-deficient | US | Ultrasound |
| LDPI | Laser Doppler Perfusion Imaging | VCAM-1 | Vascular Cell Adhesion Molecule 1 |
| M-mode | Motion Mode | VEGF | Vascular Endothelial Growth Factor |
1.1. Ethics and Regulations of Animal Models
1.2. Pathophysiology of Atherosclerosis
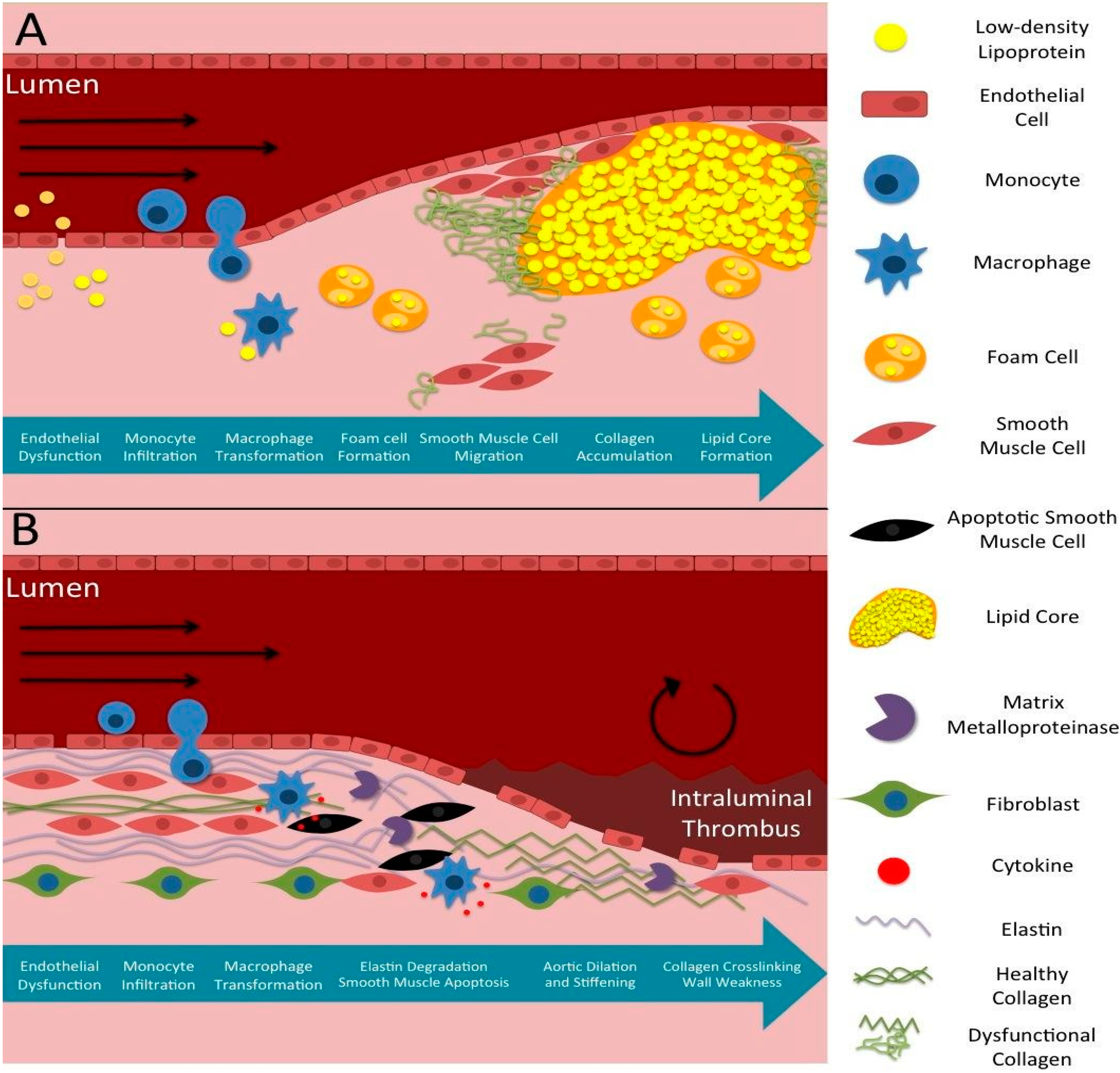
1.3. Pathophysiology of Aneurysms
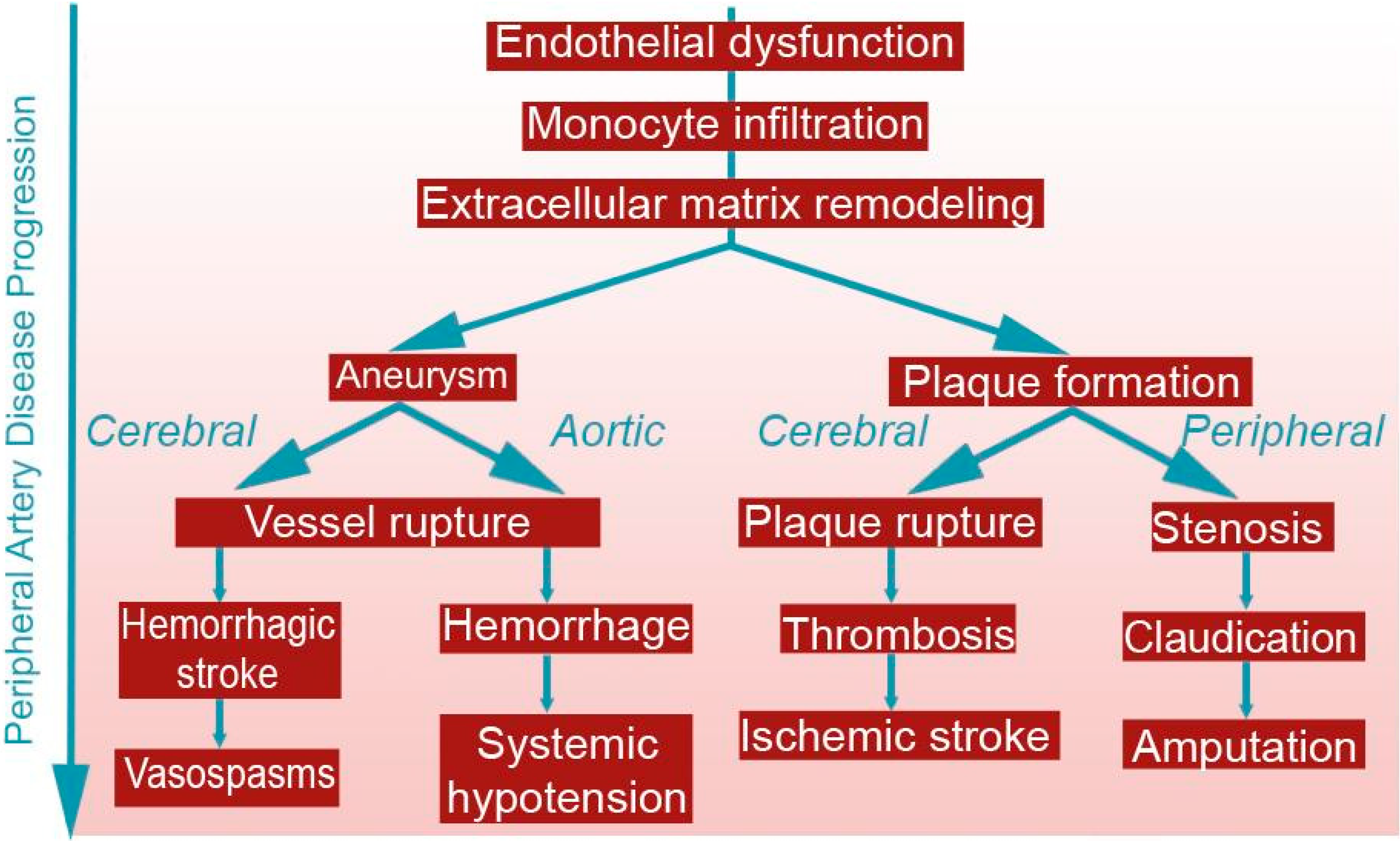
1.4. The Clinical Need for Early Diagnosis of PAD
1.5. Non-Invasive Imaging Strategies in Small Animals
| Imaging Modality | Advantages | Disadvantages | Cardiovascular Imaging Contrast Agents |
|---|---|---|---|
| Ultrasound | Fast acquisition time | Acoustic artifacts Often difficult to interpret | Microbubbles |
| Portable | |||
| High spatiotemporal resolution | |||
| No harmful radiation | |||
| Magnetic Resonance Imaging | Non-ionizing | Lower temporal resolution compared to ultrasound Difficult to time bolus injections of contrast agents High cost | Gadolinium-based contrast agents Iron oxide and other paramagnetic particles |
| Superior tissue differentiation | |||
| Provides anatomical, functional, and molecular information | |||
| Whole body imaging capability | |||
| Positron Emission Tomography | Provides quantitative pharmacokinetic information on radiotracer distribution throughout the body | Requires radioactive contrast agents with short half-lives | 11C, 18F, 13N, 15O, 82Rb |
| Limited spatial resolution (1–2 mm) | |||
| Single-Photon Emission Computed Tomography | Provides molecular and functional parameters | Dependent on the pharmacodynamics and kinetics of the tracer | 99mTc, 111In chelates |
| No depth limitation | |||
| Computed Tomography | Fast acquisition time | Ionizing radiation Lacks soft tissue differentiation May require contrast agent | Iodine Barium |
| High spatiotemporal resolution | |||
| Provides information about the spatial geometry, luminal patency, and vascular networks | |||
| Diffuse Optical Imaging | High sensitivity | Limited depth of penetration (1–2 mm) Susceptibility to photobleaching | Fluorophores Luciferin/luciferase |
| No ionizing radiation | |||
| Availability |
1.5.1. Magnetic Resonance Imaging
1.5.2. Computed Tomography
1.5.3. Ultrasound
1.5.4. Optical Imaging
1.5.5. Positron Emission Tomography and Single Photon Emission Computed Tomography
1.6. Advances in PAD Imaging
2. Aortic Disease
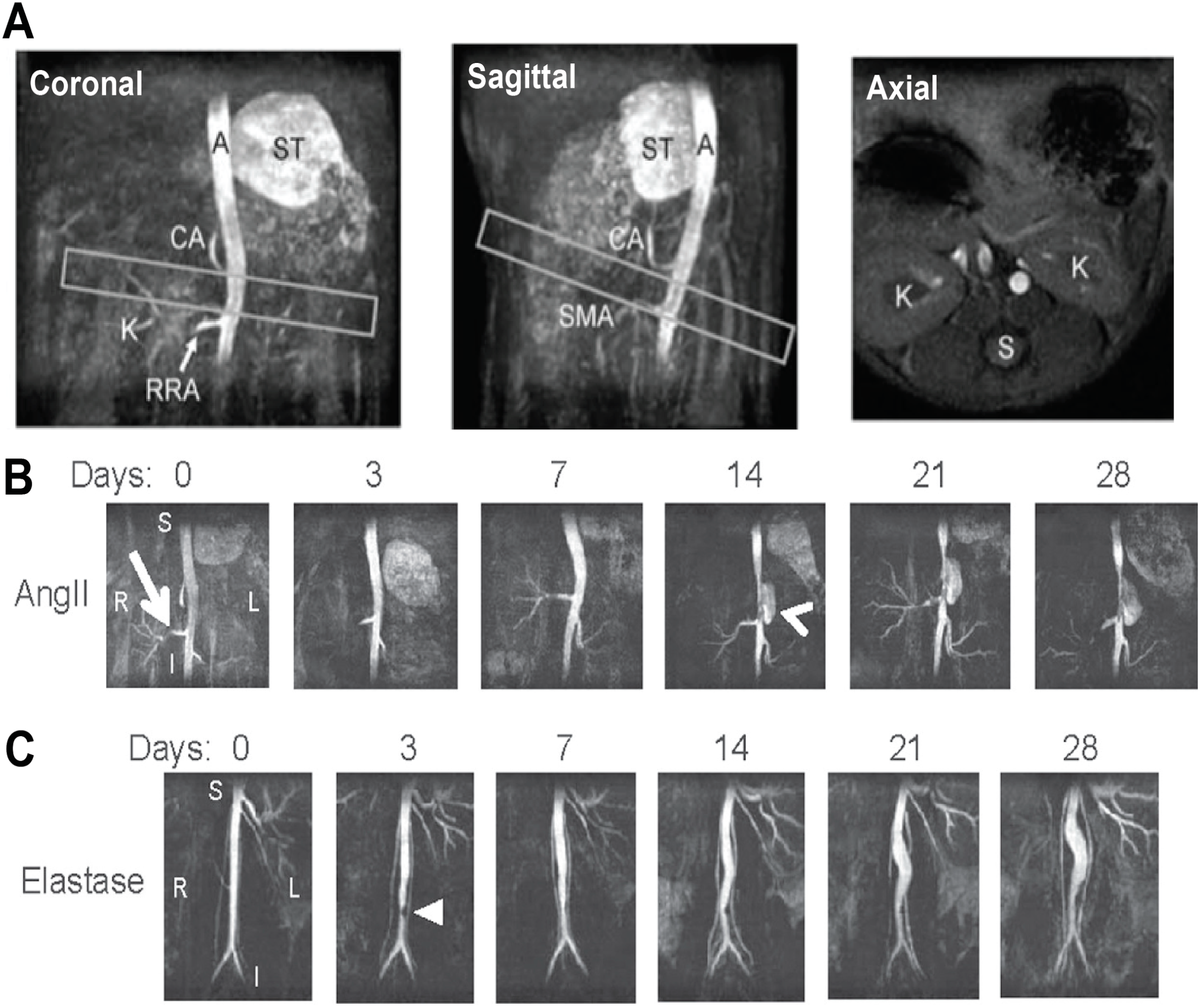
2.1. Small Animal Models of Aortic Disease
2.2. Anatomic and Biomechanical Measurements
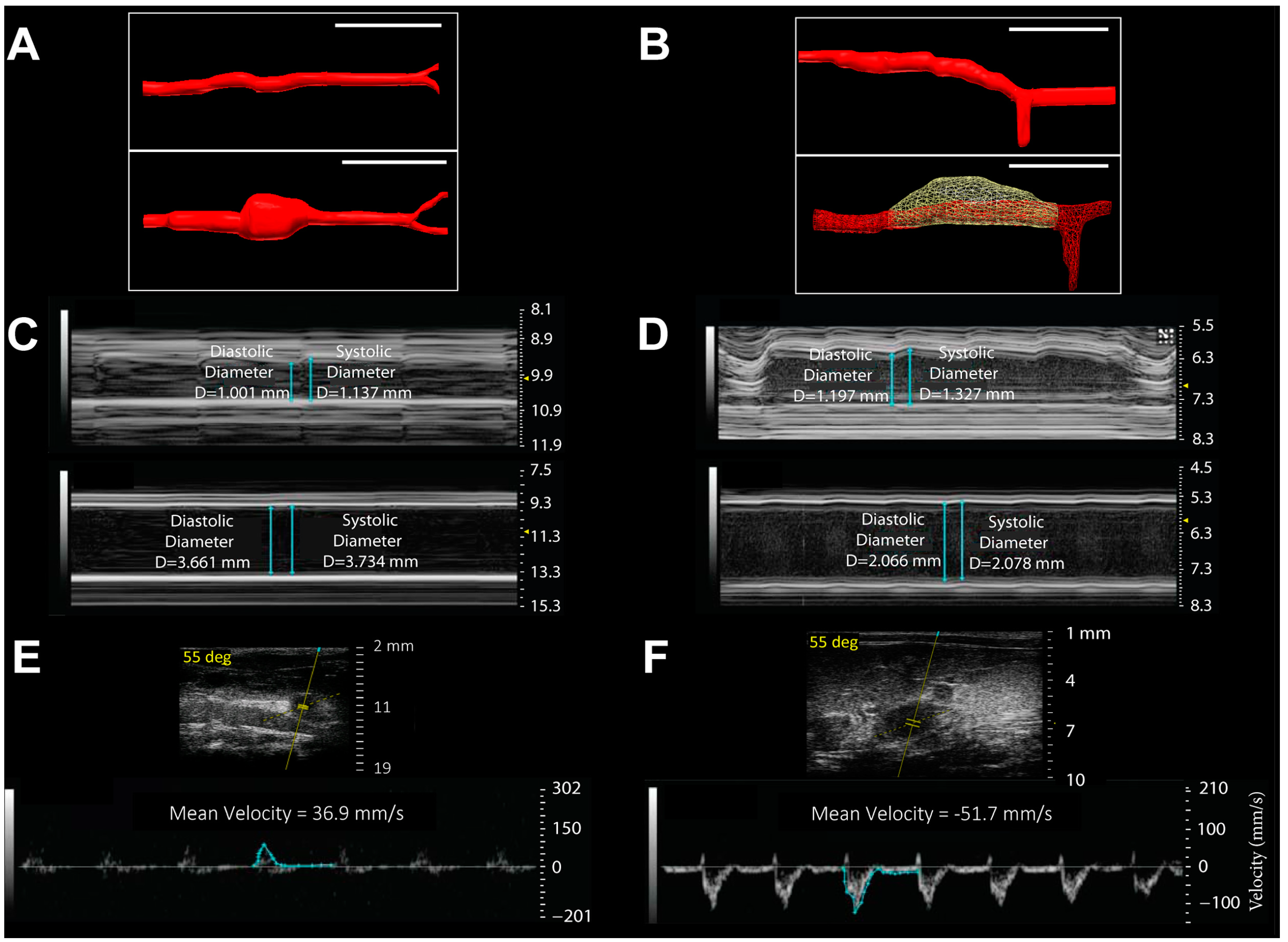
2.3. Novel Molecular Imaging of Aortic Disease

3. Carotid and Cerebrovascular Disease
3.1. Technologies for Carotid and Cerebrovascular Imaging
3.2. Carotid Atherosclerosis and Cerebral Ischemia
3.2.1. Small Animal Models of Carotid Stenosis and Cerebral Ischemia
3.2.2. Structural and Hemodynamic Imaging of Carotid Stenosis and Ischemic Stroke
3.2.3. Molecular Imaging of Carotid Plaques

3.3. Cerebrovascular Aneurysms
3.3.1. Small Animal Models of Intracranial Aneurysms
3.3.2. SAH Imaging
3.4. Cerebral AVMs
3.4.1. Small Animal Models of AVM
3.4.2. Structural and Hemodynamic Imaging of AVM
3.4.3. Optical Imaging and the “Response-to-Injury” Paradigm of AVM Pathogenesis
4. Lower Limb Athero-Thrombosis
4.1. Small Animal Models for Lower Limb PAD
4.2. Factors that Impair Perfusion Recovery
4.3. Imaging of Angiogenic Factors in Ischemic Animal Models
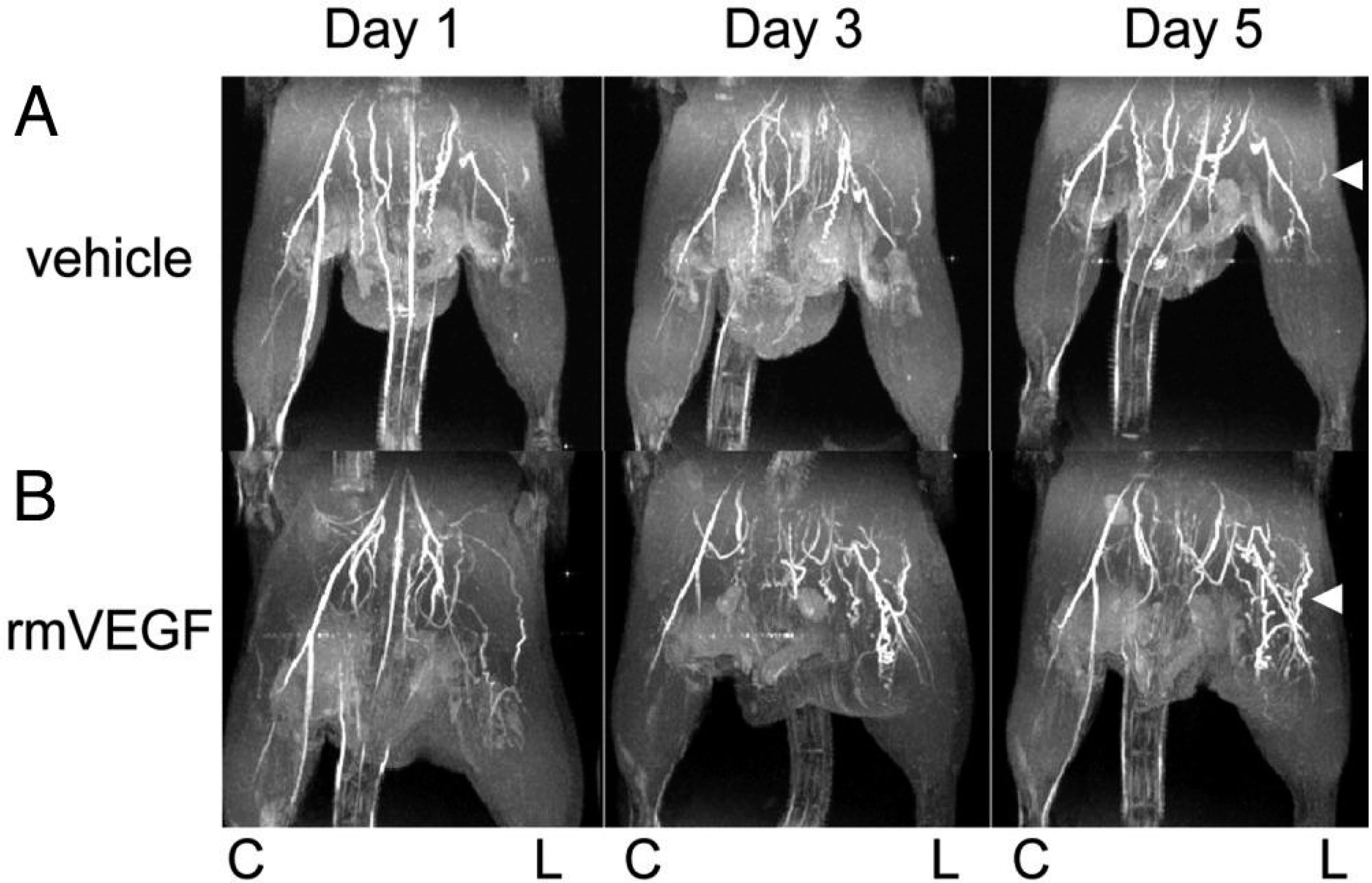
4.4. Quantification of Lower Limb PAD Induced Biomarkers
5. Emerging Technologies and Future Directions
5.1. Theranostic Micro- and Nanoparticles
5.2. Imaging Advancements in Depth Penetration and Resolution
5.3. Stem Cells as a Treatment of PAD
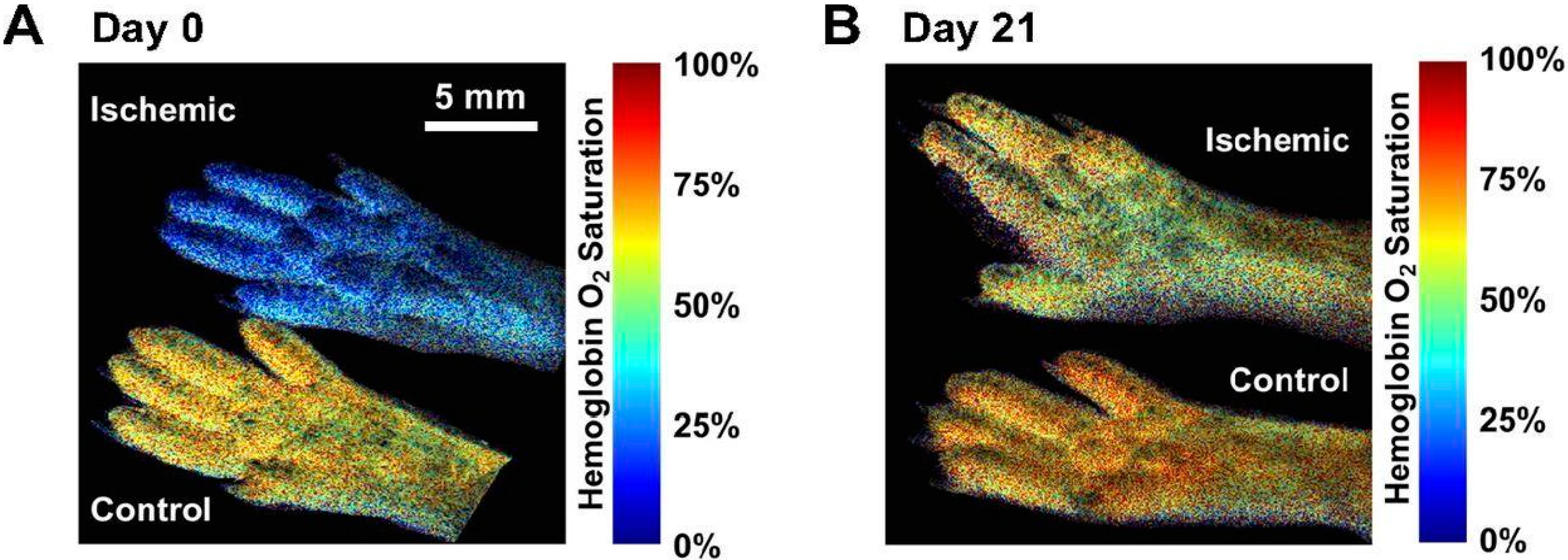
6. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hiatt, W.R. Medical treatment of peripheral arterial disease and claudication. N. Engl. J. Med. 2001, 344, 1608–1621. [Google Scholar] [CrossRef] [PubMed]
- Valentine, R.J.; Decaprio, J.D.; Castillo, J.M.; Modrall, J.G.; Jackson, M.R.; Clagett, G.P. Watchful waiting in cases of small abdominal aortic aneurysms- appropriate for all patients? J. Vasc. Surg. 2000, 32, 441–448, discussion 448–450. [Google Scholar] [CrossRef] [PubMed]
- Ferdowsian, H.R.; Beck, N. Ethical and scientific considerations regarding animal testing and research. PLoS ONE 2011, 6, e24059. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Tang, D. Computer simulations of atherosclerotic plaque growth in coronary arteries. Mol. Cell. Biomech. 2010, 7, 193–202. [Google Scholar] [PubMed]
- Botnar, R.; Rappitsch, G.; Scheidegger, M.B.; Liepsch, D.; Perktold, K.; Boesiger, P. Hemodynamics in the carotid artery bifurcation: A comparison between numerical simulations and in vitro mri measurements. J. Biomech. 2000, 33, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Munir, J.A. Atherosclerosis: Clinical Perspectives through Imaging; Taylor, A.J., Villines, T.C., Eds.; Springer: New York, NY, USA, 2013; p. 233. [Google Scholar]
- Falk, E. Pathogenesis of atherosclerosis. J. Am. Coll. Cardiol. 2006, 47, C7–C12. [Google Scholar] [CrossRef] [PubMed]
- Naghavi, M.; Libby, P.; Falk, E.; Casscells, S.W.; Litovsky, S.; Rumberger, J.; Badimon, J.J.; Stefanadis, C.; Moreno, P.; Pasterkamp, G.; et al. From vulnerable plaque to vulnerable patient: A call for new definitions and risk assessment strategies: Part I. Circulation 2003, 108, 1664–1672. [Google Scholar] [CrossRef] [PubMed]
- Glagov, S.; Zarins, C.; Giddens, D.P.; Ku, D.N. Hemodynamics and atherosclerosis. Insights and perspectives gained from studies of human arteries. Arch. Pathol. Lab. Med. 1988, 112, 1018–1031. [Google Scholar] [PubMed]
- Ku, D.N. Blood flow in arteries. Annu. Rev. Fluid Mech. 1997, 29, 399–434. [Google Scholar] [CrossRef]
- Go, A.S.; Mozaffarian, D.; Roger, V.L.; Benjamin, E.J.; Berry, J.D.; Blaha, M.J.; Dai, S.; Ford, E.S.; Fox, C.S.; Franco, S.; et al. Executive summary: Heart disease and stroke statistics—2014 update: A report from the american heart association. Circulation 2014, 129, 399–410. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, D.H.; Polak, J.F.; Kronmal, R.A.; Manolio, T.A.; Burke, G.L.; Wolfson, S.K., Jr. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular health study collaborative research group. N. Engl. J. Med. 1999, 340, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Hertzer, N.R.; Beven, E.G.; Young, J.R.; O’Hara, P.J.; Ruschhaupt, W.F., 3rd; Graor, R.A.; Dewolfe, V.G.; Maljovec, L.C. Coronary artery disease in peripheral vascular patients. A classification of 1000 coronary angiograms and results of surgical management. Ann. Surg. 1984, 199, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Hertzer, N.R.; Young, J.R.; Beven, E.G.; Graor, R.A.; O’Hara, P.J.; Ruschhaupt, W.F., 3rd; deWolfe, V.G.; Maljovec, L.C. Coronary angiography in 506 patients with extracranial cerebrovascular disease. Arch. Intern. Med. 1985, 145, 849–852. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.S.; Olson, C.M.; Johnson, E.S.; Senger, C.A.; Soh, C.B.; Whitlock, E.P. The Ankle Brachial Index for Peripheral Artery Disease Screening and Cardiovascular Disease Prediction in Asymptomatic Adults: A Systematic Evidence Review for the U.S. Preventive Services Task Force; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2013. [Google Scholar]
- Madamanchi, N.R.; Vendrov, A.; Runge, M.S. Oxidative stress and vascular disease. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Kuhlencordt, P.J.; Gyurko, R.; Han, F.; Scherrer-Crosbie, M.; Aretz, T.H.; Hajjar, R.; Picard, M.H.; Huang, P.L. Accelerated atherosclerosis, aortic aneurysm formation, and ischemic heart disease in apolipoprotein E/endothelial nitric oxide synthase double-knockout mice. Circulation 2001, 104, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Naderi, S. Etiopathogenic differences in coronary artery disease and peripheral artery disease: Results from the national health and nutrition examination survey. Angiology 2014, 65, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Ripa, R.S.; Kjaer, A.; Hesse, B. Non-invasive imaging for subclinical coronary atherosclerosis in patients with peripheral artery disease. Curr. Atheroscler. Rep. 2014, 16, 415. [Google Scholar] [CrossRef] [PubMed]
- Hussein, A.A.; Uno, K.; Wolski, K.; Kapadia, S.; Schoenhagen, P.; Tuzcu, E.M.; Nissen, S.E.; Nicholls, S.J. Peripheral arterial disease and progression of coronary atherosclerosis. J. Am. Coll. Cardiol. 2011, 57, 1220–1225. [Google Scholar] [CrossRef] [PubMed]
- Eraso, L.H.; Fukaya, E.; Mohler, E.R.; Xie, D.; Sha, D.; Berger, J.S. Peripheral arterial disease, prevalence and cumulative risk factor profile analysis. Eur. J. Prev. Cardiol. 2012, 21, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Murabito, J.M.; D’Agostino, R.B.; Silbershatz, H.; Wilson, W.F. Intermittent claudication. A risk profile from the framingham heart study. Circulation 1997, 96, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Fowkes, F.G.R.; Rudan, D.; Rudan, I.; Aboyans, V.; Denenberg, J.O.; McDermott, M.M.; Norman, P.E.; Sampson, U.K.A.; Williams, L.J.; Mensah, G.A.; et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: A systematic review and analysis. Lancet 2013, 382, 1329–1340. [Google Scholar] [CrossRef] [PubMed]
- Diehm, C.; Schuster, A.; Allenberg, J.R.; Darius, H.; Haberl, R.; Lange, S.; Pittrow, D.; von Stritzky, B.; Tepohl, G.; Trampisch, H.-J. High prevalence of peripheral arterial disease and co-morbidity in 6880 primary care patients: Cross-sectional study. Atherosclerosis 2004, 172, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Formosa, C.; Cassar, K.; Gatt, A.; Mizzi, A.; Mizzi, S.; Camileri, K.P.; Azzopardi, C.; DeRaffaele, C.; Falzon, O.; Cristina, S.; et al. Hidden dangers revealed by misdiagnosed peripheral arterial disease using ABPI measurement. Diabetes Res. Clin. Pract. 2013, 102, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Sampson, U.K.; Norman, P.E.; Fowkes, F.G.; Aboyans, V.; Song, Y.; Harrell, F.E., Jr.; Forouzanfar, M.H.; Naghavi, M.; Denenberg, J.O.; McDermott, M.M.; et al. Estimation of global and regional incidence and prevalence of abdominal aortic aneurysms 1990 to 2010. Glob. Heart 2014, 9, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Brisman, J.L.; Song, J.K.; Newell, D.W. Cerebral aneurysms. N. Engl. J. Med. 2006, 355, 928–939. [Google Scholar] [CrossRef] [PubMed]
- Redekop, G.; TerBrugge, K.; Montanera, W.; Willinsky, R. Arterial aneurysms associated with cerebral arteriovenous malformations: Classification, incidence, and risk of hemorrhage. J. Neurosurg. 1998, 89, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, L.; Wallace, M.C.; Ter Brugge, K.G.; O’Kelly, C.; Willinsky, R.A.; Tymianski, M. The natural history and predictive features of hemorrhage from brain arteriovenous malformations. Stroke J. Cereb. Circ. 2009, 40, 100–105. [Google Scholar] [CrossRef]
- Hai, J.; Ding, M.; Guo, Z.; Wang, B. A new rat model of chronic cerebral hypoperfusion associated with arteriovenous malformations. J. Neurosurg. 2002, 97, 1198–1202. [Google Scholar] [CrossRef] [PubMed]
- Hai, J.; Wu, Y.; Lin, Q.; Huang, X.; Zhang, G. Cerebral blood flow and metabolic changes in hippocampal regions of a modified rat model with chronic cerebral hypoperfusion. Acta Neurol. Belg. 2013, 113, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Sadasivan, C.; Fiorella, D.J.; Woo, H.H.; Lieber, B.B. Physical factors effecting cerebral aneurysm pathophysiology. Ann. Biomed. Eng. 2013, 41, 1347–1365. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.W.; Curci, J.A.; Ennis, T.L.; Mao, D.; Pagano, M.B.; Pham, C.T. Pathophysiology of abdominal aortic aneurysms: Insights from the elastase-induced model in mice with different genetic backgrounds. Ann. N. Y. Acad. Sci. 2006, 1085, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Fowkes, F.G.R.; Murray, G.D.; Butcher, I.; Heald, C.L.; Lee, R.J.; Chambless, L.E.; Folsom, A.R.; Hirsch, A.T.; Dramaix, M.; deBacker, G.; et al. Ankle brachial index combined with framingham risk score to predict cardiovascular events and mortality: A meta-analysis. JAMA 2008, 300, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Lawes, C.M.; Bennett, D.A.; Anderson, C.S. Stroke epidemiology: A review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol. 2003, 2, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Hackett, M.L.; Anderson, C.S. Health outcomes 1 year after subarachnoid hemorrhage: An international population-based study. The australian cooperative research on subarachnoid hemorrhage study group. Neurology 2000, 55, 658–662. [Google Scholar] [CrossRef] [PubMed]
- Hiratzka, L.F.; Bakris, G.L.; Beckman, J.A.; Bersin, R.M.; Carr, V.F.; Casey, D.E., Jr.; Eagle, K.A.; Hermann, L.K.; Isselbacher, E.M.; et al. Guidelines for the diagnosis and management of patients with thoracic aortic disease. A report of the american college of cardiology foundation/american heart association task force on practice guidelines, american association for thoracic surgery, american college of radiology, american stroke association, society of cardiovascular anesthesiologists, society for cardiovascular angiography and interventions, society of interventional radiology, society of thoracic surgeons, and society for vascular medicine. J. Am. Coll. Cardiol. 2010, 55, e27–e129. [Google Scholar] [CrossRef] [PubMed]
- Al-Shahi, R.; Warlow, C. A systematic review of the frequency and prognosis of arteriovenous malformations of the brain in adults. Brain: J. Neurol. 2001, 124, 1900–1926. [Google Scholar] [CrossRef]
- Howard, G.; Goff, D.C. Population shifts and the future of stroke: Forecasts of the future burden of stroke. Ann. N. Y. Acad. Sci. 2012, 1268, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Balaban, R.S.; Hampshire, V.A. Challenges in small animal noninvasive imaging. ILAR J/Natl. Res. Counc. Inst. Lab. Anim. Resour. 2001, 42, 248–262. [Google Scholar] [CrossRef]
- Webb, A.; Kagadis, G.C. Introduction to Biomedical Imaging; Wiley Interscience-IEEE: Hoboken, NJ, USA, 2003. [Google Scholar]
- Lee, G.H.; Chang, Y.; Kim, T.J. Blood-pool and targeting MRI contrast agents: From Gd-chelates to Gd-nanoparticles. Eur. J. Inorg. Chem. 2012, 2012, 1924–1933. [Google Scholar] [CrossRef]
- Bui, T.; Stevenson, J.; Hoekman, J.; Zhang, S.; Maravilla, K.; Ho, R.J. Novel gd nanoparticles enhance vascular contrast for high-resolution magnetic resonance imaging. PLoS ONE 2010, 5, e13082. [Google Scholar] [CrossRef] [PubMed]
- Millon, A.; Dickson, S.D.; Klink, A.; Izquierdo-Garcia, D.; Bini, J.; Lancelot, E.; Ballet, S.; Robert, P.; Mateo de Castro, J.; Corot, C.; et al. Monitoring plaque inflammation in atherosclerotic rabbits with an iron oxide (P904) and 18F-FDG using a combined PET/MR scanner. Atherosclerosis 2013, 228, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Uppal, R.; Ay, I.; Dai, G.; Kim, Y.R.; Sorensen, A.G.; Caravan, P. Molecular mri of intracranial thrombus in a rat ischemic stroke model. Stroke J. Cereb. Circ. 2010, 41, 1271–1277. [Google Scholar] [CrossRef]
- Klink, A.; Heynens, J.; Herranz, B.; Lobatto, M.E.; Arias, T.; Sanders, H.M.; Strijkers, G.J.; Merkx, M.; Nicolay, K.; Fuster, V.; et al. In vivo characterization of a new abdominal aortic aneurysm mouse model with conventional and molecular magnetic resonance imaging. J. Am. Coll. Cardiol. 2011, 58, 2522–2530. [Google Scholar] [CrossRef] [PubMed]
- Botnar, R.M.; Wiethoff, A.J.; Ebersberger, U.; Lacerda, S.; Blume, U.; Warley, A.; Jansen, C.H.; Onthank, D.C.; Cesati, R.R.; Razavi, R.; et al. In vivo assessment of aortic aneurysm wall integrity using elastin-specific molecular magnetic resonance imaging. Circ. Cardiovasc. Imag. 2014, 7, 679–689. [Google Scholar] [CrossRef]
- Joshi, R.; Yanasak, N. Magnetic resonance angiography study of a normal mouse brain for creating a three-dimensional cerebral vasculature atlas and software for labeling vessels. In Proceedings of the 2011 IEEE International Conference on Bioinformatics and Biomedicine Workshops (BIBMW), Atlanta, GA, USA, 12–15 November; pp. 966–968.
- Klohs, J.; Baltes, C.; Princz-Kranz, F.; Ratering, D.; Nitsch, R.M.; Knuesel, I.; Rudin, M. Contrast-enhanced magnetic resonance microangiography reveals remodeling of the cerebral microvasculature in transgenic arcabeta mice. J. Neurosci. Off. J. Soc. Neurosci. 2012, 32, 1705–1713. [Google Scholar] [CrossRef]
- Goergen, C.J.; Azuma, J.; Barr, K.N.; Magdefessel, L.; Kallop, D.Y.; Gogineni, A.; Grewall, A.; Weimer, R.M.; Connolly, A.J.; Dalman, R.L.; et al. Influences of aortic motion and curvature on vessel expansion in murine experimental aneurysms. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Greve, J.M.; Les, A.S.; Tang, B.T.; Draney Blomme, M.T.; Wilson, N.M.; Dalman, R.L.; Pelc, N.J.; Taylor, C.A. Allometric scaling of wall shear stress from mice to humans: Quantification using cine phase-contrast mri and computational fluid dynamics. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H1700–H1708. [Google Scholar] [CrossRef] [PubMed]
- Pieles, G.E.; Szantho, G.; Rodrigues, J.C.; Lawton, C.B.; Stuart, A.G.; Bucciarelli-Ducci, C.; Turner, M.S.; Williams, C.A.; Tulloh, R.M.; Hamilton, M.C. Adaptations of aortic and pulmonary artery flow parameters measured by phase-contrast magnetic resonance angiography during supine aerobic exercise. Eur. J. Appl. Physiol. 2014, 114, 1013–1023. [Google Scholar] [CrossRef] [PubMed]
- Muir, E.R.; Watts, L.T.; Tiwari, Y.V.; Bresnen, A.; Shen, Q.; Duong, T.Q. Quantitative cerebral blood flow measurements using mri. Methods Mol. Biol. 2014, 1135, 205–211. [Google Scholar] [PubMed]
- Klink, A.; Hyafil, F.; Rudd, J.; Faries, P.; Fuster, V.; Mallat, Z.; Meilhac, O.; Mulder, W.J.; Michel, J.B.; Ramirez, F.; et al. Diagnostic and therapeutic strategies for small abdominal aortic aneurysms. Nat. Rev. Cardiol. 2011, 8, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.; Lee, T.-M.; Kay, A.R.; Tank, D.W. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc. Natl. Acad. Sci. USA 1990, 87, 9868–9872. [Google Scholar] [CrossRef] [PubMed]
- Greve, J.M.; Williams, S.P.; Bernstein, L.J.; Goldman, H.; Peale, F.V.; Bunting, S.; van Bruggen, N. Reactive hyperemia and BOLD MRI demonstrate that VEGF inhibition, age, and atherosclerosis adversely affect functional recovery in a murine model of peripheral artery disease. J. Magn. Reson. Imaging 2008, 28, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Kwong, R.Y.; Yucel, E.K. Cardiology patient pages. Computed tomography scan and magnetic resonance imaging. Circulation 2003, 108, e104–e106. [Google Scholar] [CrossRef] [PubMed]
- Pichler, B.J.; Judenhofer, M.S.; Pfannenberg, C. Multimodal imaging approaches: PET/CT and PET/MRI. Handb. Exp. Pharmacol. 2008, 109–132. [Google Scholar] [CrossRef]
- Tirziu, D.; Moodie, K.L.; Zhuang, Z.W.; Singer, K.; Helisch, A.; Dunn, J.F.; Li, W.; Singh, J.; Simons, M. Delayed arteriogenesis in hypercholesterolemic mice. Circulation 2005, 112, 2501–2509. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.K.; Anderson, R.E.; Sundt, T.M., Jr. A model of the pathophysiology of cerebral arteriovenous malformations by a carotid-jugular fistula in the rat. Brain Res. 1989, 496, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Abruzzo, T.; Tumialan, L.; Chaalala, C.; Kim, S.; Guldberg, R.E.; Lin, A.; Leach, J.; Khoury, J.C.; Morgan, A.E.; Cawley, C.M., 3rd. Microscopic computed tomography imaging of the cerebral circulation in mice: Feasibility and pitfalls. Synapse 2008, 62, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Jumabay, M.; Wang, A.; Bostrom, K.I. Matrix Gla protein deficiency causes arteriovenous malformations in mice. J. Clin. Investig. 2011, 121, 2993–3004. [Google Scholar] [CrossRef] [PubMed]
- Schambach, S.J.; Bag, S.; Schilling, L.; Groden, C.; Brockmann, M.A. Application of micro-CT in small animal imaging. Methods 2010, 50, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Vandeghinste, B.; Trachet, B.; Renard, M.; Casteleyn, C.; Staelens, S.; Loeys, B.; Segers, P.; Vandenberghe, S. Replacing vascular corrosion casting by in vivo micro-CT imaging for building 3D cardiovascular models in mice. Mol. Imaging Biol. 2011, 13, 78–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staub, D.; Partovi, S.; Imfeld, S.; Uthoff, H.; Baldi, T.; Aschwanden, M.; Jaeger, K. Novel applications of contrast-enhanced ultrasound imaging in vascular medicine. VASA. Z. Gefasskrankh. 2013, 42, 17–31. [Google Scholar] [CrossRef]
- Shim, C.Y.; Lindner, J.R. Cardiovascular molecular imaging with contrast ultrasound: Principles and applications. Korean Circ. J. 2014, 44, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bondke, A.; Hillmeister, P.; Buschmann, I.R. Exact assessment of perfusion and collateral vessel proliferation in small animal models. Circ. Res. 2007, 100, e82–e83. [Google Scholar] [CrossRef] [PubMed]
- Chin, M.S.; Freniere, B.B.; Lo, Y.-C.; Saleeby, J.H.; Baker, S.P.; Strom, H.M.; Ignotz, R.A.; Lalikos, J.F.; Fitzgerald, T.J. Hyperspectral imaging for early detection of oxygenation and perfusion changes in irradiated skin. J. Biomed. Opt. 2012, 17, 0260101–0260105. [Google Scholar] [CrossRef]
- Yudovsky, D.; Nouvong, A.; Pilon, L. Hyperspectral imaging in diabetic foot wound care. J. Diabetes Sci. Technol. 2010, 4, 1099–1113. [Google Scholar] [CrossRef] [PubMed]
- Frangioni, J.V. In vivo near-infrared fluorescence imaging. Curr. Opin. Chem. Biol. 2003, 7, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Herrero, P.; Sharp, T.; Laforest, R.; Rowland, D.J.; Tai, Y.C.; Lewis, J.S.; Welch, M.J. Minimally invasive method of determining blood input function from pet images in rodents. J. Nucl. Med. 2006, 47, 330–336. [Google Scholar] [PubMed]
- Yoo, H.; Kim, J.W.; Shishkov, M.; Namati, E.; Morse, T.; Shubochkin, R.; McCarthy, J.R.; Ntziachristos, V.; Bouma, B.E.; Jaffer, F.A.; et al. Intra-arterial catheter for simultaneous microstructural and molecular imaging in vivo. Nat. Med. 2011, 17, 1680–1684. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, J.; Jing, J.; Ma, T.; Liang, S.; Zhang, J.; Mohar, D.; Raney, A.; Mahon, S.; Brenner, M.; et al. Integrated ivus-oct imaging for atherosclerotic plaque characterization. IEEE J. Sel. Top. Quantum Electron. 2014, 20. [Google Scholar] [CrossRef]
- Wang, P.; Ma, T.; Slipchenko, M.N.; Liang, S.; Hui, J.; Shung, K.K.; Roy, S.; Sturek, M.; Zhou, Q.; Chen, Z.; et al. High-speed intravascular photoacoustic imaging of lipid-laden atherosclerotic plaque enabled by a 2-kHz barium nitrite raman laser. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tropea, B.I.; Schwarzacher, S.P.; Chang, A.; Asvar, C.; Huie, P.; Sibley, R.K.; Zarins, C.K. Reduction of aortic wall motion inhibits hypertension-mediated experimental atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 2127–2133. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.S.; Virag, L.; Di Achille, P.; Karsaj, I.; Humphrey, J.D. Biochemomechanics of intraluminal thrombus in abdominal aortic aneurysms. J. Biomech. Eng. 2013, 135. [Google Scholar] [CrossRef] [Green Version]
- Markl, M.; Wu, C.; Hurley, M.C.; Ansari, S.A.; Carroll, T.J.; Rahme, R.J.; Aoun, S.G.; Carr, J.; Batjer, H.; Bendok, B.R. Cerebral arteriovenous malformation: Complex 3D hemodynamics and 3D blood flow alterations during staged embolization. J. Magn. Reson. Imaging 2013, 38, 946–950. [Google Scholar] [CrossRef] [PubMed]
- Raut, S.S.; Chandra, S.; Shum, J.; Finol, E.A. The role of geometric and biomechanical factors in abdominal aortic aneurysm rupture risk assessment. Ann. Biomed. Eng. 2013, 41, 1459–1477. [Google Scholar] [CrossRef] [PubMed]
- Willmann, J.K.; Chen, K.; Wang, H.; Paulmurugan, R.; Rollins, M.; Cai, W.; Wang, D.S.; Chen, I.Y.; Gheysens, O.; Rodriguez-Porcel, M.; et al. Monitoring of the biological response to murine hindlimb ischemia with 64Cu-labeled vascular endothelial growth factor-121 positron emission tomography. Circulation 2008, 117, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Makowski, M.R.; Preissel, A.; von Bary, C.; Warley, A.; Schachoff, S.; Keithan, A.; Cesati, R.R.; Onthank, D.C.; Schwaiger, M.; Robinson, S.P.; et al. Three-dimensional imaging of the aortic vessel wall using an elastin-specific magnetic resonance contrast agent. Investig. Radiol. 2012, 47, 438–444. [Google Scholar] [CrossRef]
- Starmans, L.W.; van Duijnhoven, S.M.; Rossin, R.; Aime, S.; Daemen, M.J.; Nicolay, K.; Grull, H. Spect imaging of fibrin using fibrin-binding peptides. Contrast Media Mol. Imaging 2013, 8, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Maegdefessel, L.; Dalman, R.L.; Tsao, P.S. Pathogenesis of abdominal aortic aneurysms: Micrornas, proteases, genetic associations. Annu. Rev. Med. 2014, 65, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Daugherty, A.; Cassis, L.A. Mouse models of abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, D. The LDL modification hypothesis of atherogenesis: An update. J. Lipid Res. 2009, 50, S376–S381. [Google Scholar] [CrossRef] [PubMed]
- Chiou, A.C.; Chiu, B.; Pearce, W.H. Murine aortic aneurysm produced by periarterial application of calcium chloride. J. Surg. Res. 2001, 99, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Anidjar, S.; Salzmann, J.L.; Gentric, D.; Lagneau, P.; Camilleri, J.P.; Michel, J.B. Elastase-induced experimental aneurysms in rats. Circulation 1990, 82, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Krishna, S.; Golledge, J. The calcium chloride-induced rodent model of abdominal aortic aneurysm. Atherosclerosis 2013, 226, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Pyo, R.; Lee, J.K.; Shipley, J.M.; Curci, J.A.; Mao, D.; Ziporin, S.J.; Ennis, T.L.; Shapiro, S.D.; Senior, R.M.; Thompson, R.W. Targeted gene disruption of matrix metalloproteinase-9 (gelatinase B) suppresses development of experimental abdominal aortic aneurysms. J. Clin. Investig. 2000, 105, 1641–1649. [Google Scholar] [CrossRef] [PubMed]
- Carsten, C.G., 3rd; Calton, W.C.; Johanning, J.M.; Armstrong, P.J.; Franklin, D.P.; Carey, D.J.; Elmore, J.R. Elastase is not sufficient to induce experimental abdominal aortic aneurysms. J. Vasc. Surg. 2001, 33, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Bhamidipati, C.M.; Mehta, G.S.; Lu, G.Y.; Moehle, C.W.; Barbery, C.; DiMusto, P.D.; Laser, A.; Kron, I.L.; Upchurch, G.R.; Ailawadi, G. Development of a novel murine model of aortic aneurysms using peri-adventitial elastase. Surgery 2012, 152, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Plump, A.S.; Smith, J.D.; Hayek, T.; Aalto-Setala, K.; Walsh, A.; Verstuyft, J.G.; Rubin, E.M.; Breslow, J.L.; et al. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in es cells. Cell 1992, 71, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.H.; Reddick, R.L.; Piedrahita, J.A.; Maeda, N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein e. Science 1992, 258, 468–471. [Google Scholar] [CrossRef] [PubMed]
- Daugherty, A.; Cassis, L. Chronic angiotensin II infusion promotes atherogenesis in low density lipoprotein receptor −/− mice. Ann. N. Y. Acad. Sci. 1999, 892, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Meir, K.S.; Leitersdorf, E. Atherosclerosis in the apolipoprotein-e-deficient mouse: A decade of progress. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Upmacis, R.K.; Crabtree, M.J.; Deeb, R.S.; Shen, H.; Lane, P.B.; Benguigui, L.E.; Maeda, N.; Hajjar, D.P.; Gross, S.S. Profound biopterin oxidation and protein tyrosine nitration in tissues of apoe-null mice on an atherogenic diet: Contribution of inducible nitric oxide synthase. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H2878–H2887. [Google Scholar] [CrossRef] [PubMed]
- Daugherty, A.; Manning, M.W.; Cassis, L.A. Angiotensin ii promotes atherosclerotic lesions and aneurysms in apolipoprotein e-deficient mice. J. Clin. Investig. 2000, 105, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Saraff, K.; Babamusta, F.; Cassis, L.A.; Daugherty, A. Aortic dissection precedes formation of aneurysms and atherosclerosis in angiotensin II-infused, apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasic. 2003, 23, 1621–1626. [Google Scholar] [CrossRef]
- Davis, F.M.; Rateri, D.L.; Balakrishnan, A.; Howatt, D.A.; Strickland, D.K.; Muratoglu, S.C.; Haggerty, C.M.; Fornwalt, B.K.; Cassis, L.A.; Daugherty, A. Smooth muscle cell deletion of low-density lipoprotein receptor-related protein 1 augments angiotensin II-induced superior mesenteric arterial and ascending aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Kaijzel, E.L.; van Heijningen, P.M.; Wielopolski, P.A.; Vermeij, M.; Koning, G.A.; van Cappellen, W.A.; Que, I.; Chan, A.; Dijkstra, J.; Ramnath, N.W.; et al. Multimodality imaging reveals a gradual increase in matrix metalloproteinase activity at aneurysmal lesions in live fibulin-4 mice. Circ. Cardiovasc. Imaging 2010, 3, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, A.K.; Hamilton, M., 2nd; Joshi, R.V.; Kline, B.P.; Li, R.; Wang, P.; Goergen, C.J. Molecular imaging of experimental abdominal aortic aneurysms. Sci. World J. 2013, 2013. [Google Scholar] [CrossRef]
- Golledge, J.; Norman, P.E. Atherosclerosis and abdominal aortic aneurysm: Cause, response, or common risk factors? Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1075–1077. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, A.T.; Haskal, Z.J.; Hertzer, N.R.; Bakal, C.W.; Creager, M.A.; Halperin, J.L.; Hiratzka, L.F.; Murphy, W.R.; Olin, J.W.; Puschett, J.B.; et al. ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): A collaborative report from the american association for vascular surgery/society for vascular surgery, society for cardiovascular angiography and interventions, society for vascular medicine and biology, society of interventional radiology, and the acc/aha task force on practice guidelines (writing committee to develop guidelines for the management of patients with peripheral arterial disease): Endorsed by the american association of cardiovascular and pulmonary rehabilitation; national heart, lung, and blood institute; society for vascular nursing; transatlantic inter-society consensus; and vascular disease foundation. Circulation 2006, 113, e463–e654. [Google Scholar] [CrossRef] [PubMed]
- Lederle, F.A.; Wilson, S.E.; Johnson, G.R.; Reinke, D.B.; Littooy, F.N.; Acher, C.W.; Messina, L.M.; Ballard, D.J.; Ansel, H.J. Variability in measurement of abdominal aortic aneurysms. Abdominal aortic aneurysm detection and management veterans administration cooperative study group. J. Vasc. Surg. 1995, 21, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Sprouse, L.R., 2nd; Meier, G.H., 3rd; Parent, F.N.; DeMasi, R.J.; Glickman, M.H.; Barber, G.A. Is ultrasound more accurate than axial computed tomography for determination of maximal abdominal aortic aneurysm diameter? Eur. J. Vasc. Endovasc. Surg. 2004, 28, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Jaakkola, P.; Hippelainen, M.; Farin, P.; Rytkonen, H.; Kainulainen, S.; Partanen, K. Interobserver variability in measuring the dimensions of the abdominal aorta: Comparison of ultrasound and computed tomography. Eur. J. Vasc. Endovasc. Surg. 1996, 12, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Moxon, J.V.; Parr, A.; Emeto, T.I.; Walker, P.; Norman, P.E.; Golledge, J. Diagnosis and monitoring of abdominal aortic aneurysm: Current status and future prospects. Curr. Probl. Cardiol. 2010, 35, 512–548. [Google Scholar] [CrossRef] [PubMed]
- Turner, G.H.; Olzinski, A.R.; Bernard, R.E.; Aravindhan, K.; Karr, H.W.; Mirabile, R.C.; Willette, R.N.; Gough, P.J.; Jucker, B.M. In vivo serial assessment of aortic aneurysm formation in apolipoprotein E-deficient mice via MRI. Circ. Cardiovasc. Imaging 2008, 1, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Goergen, C.J.; Barr, K.N.; Huynh, D.T.; Eastham-Anderson, J.R.; Choi, G.; Hedehus, M.; Dalman, R.L.; Connolly, A.J.; Taylor, C.A.; Tsao, P.S.; et al. In vivo quantification of murine aortic cyclic strain, motion, and curvature: Implications for abdominal aortic aneurysm growth. J. Magn. Reson. Imaging JMRI 2010, 32, 847–858. [Google Scholar] [CrossRef]
- Trachet, B.; Renard, M.; de Santis, G.; Staelens, S.; De Backer, J.; Antiga, L.; Loeys, B.; Segers, P. An integrated framework to quantitatively link mouse-specific hemodynamics to aneurysm formation in angiotensin II-infused apoE−/− mice. Ann. Biomed. Eng. 2011, 39, 2430–2444. [Google Scholar] [CrossRef] [PubMed]
- Goergen, C.J.; Johnson, B.L.; Greve, J.M.; Taylor, C.A.; Zarins, C.K. Increased anterior abdominal aortic wall motion: Possible role in aneurysm pathogenesis and design of endovascular devices. J. Endovasc. Ther. 2007, 14, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Favreau, J.T.; Nguyen, B.T.; Gao, I.; Yu, P.; Tao, M.; Schneiderman, J.; Gaudette, G.R.; Ozaki, C.K. Murine ultrasound imaging for circumferential strain analyses in the angiotensin II abdominal aortic aneurysm model. J. Vasc. Surg. 2012, 56, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Phillips, E.H.; Yrineo, A.A.; Schroeder, H.D.; Wilson, K.E.; Cheng, J.-X.; Goergen, C.J. Morphological and biomechanical differences in the elastase and AngII apoE−/− rodent models of abdominal aortic aneurysms. BioMed. Res. Int. 2015, in press. [Google Scholar]
- Luo, J.; Konofagou, E.E. Imaging of wall motion coupled with blood flow velocity in the heart and vessels in vivo: A feasibility study. Ultrasound Med. Biol. 2011, 37, 980–995. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, J.D.; Taylor, C.A. Intracranial and abdominal aortic aneurysms: Similarities, differences, and need for a new class of computational models. Annu. Rev. Biomed. Eng. 2008, 10, 221–246. [Google Scholar] [CrossRef] [PubMed]
- Bersi, M.R.; Collins, M.J.; Wilson, E.; Humphrey, J.D. Disparate changes in the mechanical properties of murine carotid arteries and aorta in response to chronic infusion of angiotensin-II. Int. J. Adv. Eng. Sci. Appl. Math. 2013, 4, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Trachet, B.; Bols, J.; de Santis, G.; Vandenberghe, S.; Loeys, B.; Segers, P. The impact of simplified boundary conditions and aortic arch inclusion on CFD simulations in the mouse aorta: A comparison with mouse-specific reference data. J. Biomech. Eng. 2011, 133. [Google Scholar] [CrossRef]
- Assemat, P.; Siu, K.K.; Armitage, J.A.; Hokke, S.N.; Dart, A.; Chin-Dusting, J.; Hourigan, K. Haemodynamical stress in mouse aortic arch with atherosclerotic plaques: Preliminary study of plaque progression. Comput. Struct. Biotechnol. J. 2014, 10, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.Y.; Amand, T.; Ford, M.D.; Piomelli, U.; Funk, C.D. The murine angiotensin II-induced abdominal aortic aneurysm model: Rupture risk and inflammatory progression patterns. Front. Pharmacol. 2010, 1. [Google Scholar] [CrossRef]
- Ford, M.D.; Black, A.T.; Cao, R.Y.; Funk, C.D.; Piomelli, U. Hemodynamics of the mouse abdominal aortic aneurysm. J. Biomech. Eng. 2011, 133. [Google Scholar] [CrossRef]
- Truijers, M.; Kurvers, H.A.; Bredie, S.J.; Oyen, W.J.; Blankensteijn, J.D. In vivo imaging of abdominal aortic aneurysms: Increased FDG uptake suggests inflammation in the aneurysm wall. J. Endovasc. Ther. 2008, 15, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Courtois, A.; Nusgens, B.V.; Hustinx, R.; Namur, G.; Gomez, P.; Somja, J.; Defraigne, J.O.; Delvenne, P.; Michel, J.B.; Colige, A.C.; et al. 18F-FDG uptake assessed by PET/CT in abdominal aortic aneurysms is associated with cellular and molecular alterations prefacing wall deterioration and rupture. J. Nucl. Med. 2013, 54, 1740–1747. [Google Scholar] [CrossRef] [PubMed]
- Reeps, C.; Bundschuh, R.A.; Pellisek, J.; Herz, M.; van Marwick, S.; Schwaiger, M.; Eckstein, H.H.; Nekolla, S.G.; Essler, M. Quantitative assessment of glucose metabolism in the vessel wall of abdominal aortic aneurysms: Correlation with histology and role of partial volume correction. Int. J. Cardiovasc. Imaging 2013, 29, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Sheikine, Y.; Akram, K. FDG-PET imaging of atherosclerosis: Do we know what we see? Atherosclerosis 2010, 211, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Kotze, C.W.; Groves, A.M.; Menezes, L.J.; Harvey, R.; Endozo, R.; Kayani, I.A.; Ell, P.J.; Yusuf, S.W. What is the relationship between 18F-FDG aortic aneurysm uptake on pet/ct and future growth rate? Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1493–1499. [Google Scholar] [CrossRef] [PubMed]
- Palombo, D.; Morbelli, S.; Spinella, G.; Pane, B.; Marini, C.; Rousas, N.; Massollo, M.; Cittadini, G.; Camellino, D.; Sambuceti, G. A positron emission tomography/computed tomography (PET/CT) evaluation of asymptomatic abdominal aortic aneurysms: Another point of view. Ann. Vasc. Surg. 2012, 26, 491–499. [Google Scholar] [CrossRef] [PubMed]
- English, S.J.; Piert, M.R.; Diaz, J.A.; Gordon, D.; Ghosh, A.; D’Alecy, L.G.; Whitesall, S.E.; Sharma, A.K.; DeRoo, E.P.; Watt, T.; et al. Increased 18F-FDG uptake is predictive of rupture in a novel rat abdominal aortic aneurysm rupture model. Ann. Surg. 2015, 261, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ait-Oufella, H.; Herbin, O.; Bonnin, P.; Ramkhelawon, B.; Taleb, S.; Huang, J.; Offenstadt, G.; Combadiere, C.; Renia, L.; et al. TGF-β activity protects against inflammatory aortic aneurysm progression and complications in angiotensin II-infused mice. J. Clin. Investig. 2010, 120, 422–432. [Google Scholar] [CrossRef] [PubMed]
- Sarda-Mantel, L.; Coutard, M.; Rouzet, F.; Raguin, O.; Vrigneaud, J.M.; Hervatin, F.; Martet, G.; Touat, Z.; Merlet, P.; Le Guludec, D.; et al. 99m Tc-annexin-V functional imaging of luminal thrombus activity in abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2153–2159. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, T.; Kosuge, H.; Chang, E.; James, M.L.; Yamamoto, T.; Shen, B.; Chin, F.T.; Gambhir, S.S.; Dalman, R.L.; McConnell, M.V. Integrin-targeted molecular imaging of experimental abdominal aortic aneurysms by 18F-labeled Arg-Gly-Asp positron-emission tomography. Circ. Cardiovasc. Imaging 2013, 6, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Nahrendorf, M.; Keliher, E.; Marinelli, B.; Leuschner, F.; Robbins, C.S.; Gerszten, R.E.; Pittet, M.J.; Swirski, F.K.; Weissleder, R. Detection of macrophages in aortic aneurysms by nanoparticle positron emission tomography-computed tomography. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Sheth, R.A.; Maricevich, M.; Mahmood, U. In vivo optical molecular imaging of matrix metalloproteinase activity in abdominal aortic aneurysms correlates with treatment effects on growth rate. Atherosclerosis 2010, 212, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Sigovan, M.; Bessaad, A.; Alsaid, H.; Lancelot, E.; Corot, C.; Neyran, B.; Provost, N.; Majd, Z.; Breisse, M.; Canet-Soulas, E. Assessment of age modulated vascular inflammation in apoE−/− mice by uspio-enhanced magnetic resonance imaging. Investig. Radiol. 2010, 45, 702–707. [Google Scholar] [CrossRef]
- Hyafil, F.; Vucic, E.; Cornily, J.C.; Sharma, R.; Amirbekian, V.; Blackwell, F.; Lancelot, E.; Corot, C.; Fuster, V.; Galis, Z.S.; et al. Monitoring of arterial wall remodelling in atherosclerotic rabbits with a magnetic resonance imaging contrast agent binding to matrix metalloproteinases. Eur. Heart J. 2011, 32, 1561–1571. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wei, J.; Shao, Q.; Tang, Y.; Huang, Y.; Zhang, H.; Yang, W.; Jing, Z. Assessment of atherosclerotic plaques in the rabbit abdominal aorta with interleukin-8 monoclonal antibody-targeted ultrasound microbubbles. Mol. Biol. Rep. 2013, 40, 3083–3092. [Google Scholar] [CrossRef] [PubMed]
- Kee, P.; Bagalkot, V.; Johnson, E.; Danila, D. Noninvasive detection of macrophages in atheroma using a radiocontrast-loaded phosphatidylserine-containing liposomal contrast agent for computed tomography. Mol. Imaging Biol. 2014, in press. [Google Scholar]
- Lobatto, M.E.; Calcagno, C.; Millon, A.; Senders, M.L.; Fay, F.; Robson, P.M.; Ramachandran, S.; Binderup, T.; Paridaans, M.P.; Sensarn, S.; et al. Atherosclerotic plaque targeting mechanism of long-circulating nanoparticles established by multimodal imaging. ACS Nano 2015, 24, 1837–1847. [Google Scholar] [CrossRef]
- Mateo, J.; Izquierdo-Garcia, D.; Badimon, J.J.; Fayad, Z.A.; Fuster, V. Noninvasive assessment of hypoxia in rabbit advanced atherosclerosis using 18F-fluoromisonidazole positron emission tomographic imaging. Circ. Cardiovasc. Imaging 2014, 7, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, S.; Petrov, A.; Fujimoto, S.; Zhou, J.; Azure, M.; Edwards, D.S.; Murohara, T.; Narula, N.; Tsimikas, S.; Narula, J. Molecular imaging of matrix metalloproteinase expression in atherosclerotic plaques of mice deficient in apolipoprotein e or low-density-lipoprotein receptor. J. Nucl. Med. 2009, 50, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Phinikaridou, A.; Andia, M.E.; Indermuehle, A.; Onthank, D.C.; Cesati, R.R.; Smith, A.; Robinson, S.P.; Saha, P.; Botnar, R.M. Vascular remodeling and plaque vulnerability in a rabbit model of atherosclerosis: Comparison of delayed-enhancement mr imaging with an elastin-specific contrast agent and unenhanced black-blood MR imaging. Radiology 2014, 271, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Maegdefessel, L.; Azuma, J.; Toh, R.; Merk, D.R.; Deng, A.; Chin, J.T.; Raaz, U.; Schoelmerich, A.M.; Raiesdana, A.; Leeper, N.J.; et al. Inhibition of microRNA-29b reduces murine abdominal aortic aneurysm development. J. Clin. Investig. 2012, 122, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Pantoni, L. Cerebral small vessel disease: From pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010, 9, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Westrick, R.J.; Winn, M.E.; Eitzman, D.T. Murine models of vascular thrombosis (eitzman series). Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2079–2093. [Google Scholar] [CrossRef] [PubMed]
- Kurz, K.D.; Main, B.W.; Sandusky, G.E. Rat model of arterial thrombosis induced by ferric chloride. Thromb. Res. 1990, 60, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Kusada, A.; Isogai, N.; Cooley, B.C. Electric injury model of murine arterial thrombosis. Thromb. Res. 2007, 121, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Yeh, J.C.; Huang, K.L.; Hsiao, Y.C.; Hsu, Y.H.; Lin, Y.H.; Lou, S.L.; Lee, T.H. A rat model of thrombosis in common carotid artery induced by implantable wireless light-emitting diode device. BioMed. Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Longa, E.Z.; Weinstein, P.R.; Carlson, S.; Cummins, R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke J. Cereb. Circ. 1989, 20, 84–91. [Google Scholar] [CrossRef]
- Koizumi, J.; Yoshida, Y.; Nakazawa, T.; Ooneda, G. Experimental studies of ischemic brain edema, I: A new experimental model of cerebral embolism in rats in which recirculation can be introduced in the ischemic area. Jpn. J. Stroke 1986, 8, 1–8. [Google Scholar] [CrossRef]
- Schunke, K.J.; Toung, T.K.; Zhang, J.; Pathak, A.P.; Xu, J.; Zhang, J.; Koehler, R.C.; Faraday, N. A novel atherothrombotic model of ischemic stroke induced by injection of collagen into the cerebral vasculature. J. Neurosci. Methods 2015, 239, 65–74. [Google Scholar] [CrossRef] [PubMed]
- De Lange, F.; Dieleman, J.M.; Blezer, E.L.; Houston, R.J.; Kalkman, C.J.; Nijsen, J.F. Unilateral intracarotid injection of holmium microspheres to induce bilateral MRI-validated cerebral embolization in rats. J. Neurosci. Methods 2009, 176, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Kitamura, A.; Nagatsuka, K.; Ihara, M. A novel mouse model of ischemic carotid artery disease. PLoS ONE 2014, 9, e100257. [Google Scholar] [CrossRef] [PubMed]
- Tao, M.; Mauro, C.R.; Yu, P.; Favreau, J.T.; Nguyen, B.; Gaudette, G.R.; Ozaki, C.K. A simplified murine intimal hyperplasia model founded on a focal carotid stenosis. Am. J. Pathol. 2013, 182, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Hilger, T.; Niessen, F.; Diedenhofen, M.; Hossmann, K.A.; Hoehn, M. Magnetic resonance angiography of thromboembolic stroke in rats: Indicator of recanalization probability and tissue survival after recombinant tissue plasminogen activator treatment. J. Cereb. Blood Flow Metab. 2002, 22, 652–662. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Baran, U.; Wang, R.K. Application of thinned-skull cranial window to mouse cerebral blood flow imaging using optical microangiography. PLoS ONE 2014, 9, e113658. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Menon, P.; Kowalski, W.; Pekkan, K. Time-resolved oct-μpiv: A new microscopic piv technique for noninvasive depth-resolved pulsatile flow profile acquisition. Exp. Fluids 2012, 54, 1–9. [Google Scholar]
- Lam, C.K.; Yoo, T.; Hiner, B.; Liu, Z.; Grutzendler, J. Embolus extravasation is an alternative mechanism for cerebral microvascular recanalization. Nature 2010, 465, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Yeom, E.; Nam, K.H.; Jin, C.; Paeng, D.G.; Lee, S.J. 3D reconstruction of a carotid bifurcation from 2D transversal ultrasound images. Ultrasonics 2014, 54, 2184–2192. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, H.; Zheng, H.; Jiang, Y. Hepatic inflammation scores correlate with common carotid intima-media thickness in rats with nafld induced by a high-fat diet. BMC Vet. Res. 2014, 10. [Google Scholar] [CrossRef] [PubMed]
- Starmans, L.W.; van Duijnhoven, S.M.; Rossin, R.; Berben, M.; Aime, S.; Daemen, M.J.; Nicolay, K.; Grull, H. Evaluation of 111in-labeled epep and fibpep as tracers for fibrin spect imaging. Mol. Pharm. 2013, 10, 4309–4321. [Google Scholar] [CrossRef] [PubMed]
- Wenning, C.; Kloth, C.; Kuhlmann, M.T.; Jacobs, A.H.; Schober, O.; Hermann, S.; Schafers, M.A. Serial F-18-Fdg PET/CT distinguishes inflamed from stable plaque phenotypes in shear-stress induced murine atherosclerosis. Atherosclerosis 2014, 234, 276–282. [Google Scholar] [CrossRef]
- Keedy, A. An overview of intracranial aneurysms. McGill J. Med. 2006, 9, 141–146. [Google Scholar] [PubMed]
- Foutrakis, G.N.; Yonas, H.; Sclabassi, R.J. Saccular aneurysm formation in curved and bifurcating arteries. Am. J. Neuroradiol. 1999, 20, 1309–1317. [Google Scholar] [PubMed]
- Wang, Y.; Emeto, T.I.; Lee, J.; Marshman, L.; Moran, C.; Seto, S.W.; Golledge, J. Mouse models of intracranial aneurysm. Brain Pathol. 2014. [Google Scholar] [CrossRef]
- Short, J.G.; Fujiwara, N.H.; Marx, W.F.; Helm, G.A.; Cloft, H.J.; Kallmes, D.F. Elastase-induced saccular aneurysms in rabbits: Comparison of geometric features with those of human aneurysms. Am. J. Neuroradiol. 2001, 22, 1833–1837. [Google Scholar] [PubMed]
- Kondo, S.; Hashimoto, N.; Kikuchi, H.; Hazama, F.; Nagata, I.; Kataoka, H. Apoptosis of medial smooth muscle cells in the development of saccular cerebral aneurysms in rats. Stroke J. Cereb. Circ. 1998, 29, 181–188. [Google Scholar] [CrossRef]
- Nagata, I.; Handa, H.; Hashimoto, N.; Hazama, F. Experimentally induced cerebral aneurysms in rats: Part VI. Hypertension. Surg. Neurol. 1980, 14, 477–479. [Google Scholar] [PubMed]
- Kirse, D.J.; Flock, S.; Teo, C.; Rahman, S.; Mrak, R. Construction of a vein-pouch aneurysm at a surgically created carotid bifurcation in the rat. Microsurgery 1996, 17, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Bouzeghrane, F.; Naggara, O.; Kallmes, D.F.; Berenstein, A.; Raymond, J.; International Consortium of Neuroendovascular, C. In vivo experimental intracranial aneurysm models: A systematic review. AJNR. Am. J. Neuroradiol. 2010, 31, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.K.; Kottke, R.; Andereggen, L.; Weisstanner, C.; Zubler, C.; Gralla, J.; Kiefer, C.; Slotboom, J.; Wiest, R.; Schroth, G.; et al. Detecting subarachnoid hemorrhage: Comparison of combined flair/swi versus ct. Eur. J. Radiol. 2013, 82, 1539–1545. [Google Scholar] [CrossRef] [PubMed]
- Busch, E.; Beaulieu, C.; de Crespigny, A.; Moseley, M.E. Diffusion mr imaging during acute subarachnoid hemorrhage in rats. Stroke J. Cereb. Circ. 1998, 29, 2155–2161. [Google Scholar] [CrossRef]
- McCormick, P.W.; McCormick, J.; Zabramski, J.M.; Spetzler, R.F. Hemodynamics of subarachnoid hemorrhage arrest. J. Neurosurg. 1994, 80, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Stapf, C.; Mohr, J.P.; Choi, J.H.; Hartmann, A.; Mast, H. Invasive treatment of unruptured brain arteriovenous malformations is experimental therapy. Curr. Opin. Neurol. 2006, 19, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Hanes, M.A.; Dickens, T.; Porteous, M.E.; Oh, S.P.; Hale, L.P.; Marchuk, D.A. A mouse model for hereditary hemorrhagic telangiectasia (HHT) type 2. Hum. Mol. Genet. 2003, 12, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Wu, Y.Q.; Huey, M.; Arthur, H.M.; Marchuk, D.A.; Hashimoto, T.; Young, W.L.; Yang, G.Y. Vascular endothelial growth factor induces abnormal microvasculature in the endoglin heterozygous mouse brain. J. Cereb. Blood Flow Metab. 2004, 24, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Walker, E.J.; Su, H.; Shen, F.; Choi, E.J.; Oh, S.P.; Chen, G.; Lawton, M.T.; Kim, H.; Chen, Y.; Chen, W.; et al. Arteriovenous malformation in the adult mouse brain resembling the human disease. Ann. Neurol. 2011, 69, 954–962. [Google Scholar] [CrossRef] [PubMed]
- Satomi, J.; Mount, R.J.; Toporsian, M.; Paterson, A.D.; Wallace, M.C.; Harrison, R.V.; Letarte, M. Cerebral vascular abnormalities in a murine model of hereditary hemorrhagic telangiectasia. Stroke J. Cereb. Circ. 2003, 34, 783–789. [Google Scholar] [CrossRef]
- Milton, I.; Ouyang, D.; Allen, C.J.; Yanasak, N.E.; Gossage, J.R.; Alleyne, C.H., Jr.; Seki, T. Age-dependent lethality in novel transgenic mouse models of central nervous system arteriovenous malformations. Stroke J. Cereb. Circ. 2012, 43, 1432–1435. [Google Scholar] [CrossRef]
- Choi, E.J.; Chen, W.; Jun, K.; Arthur, H.M.; Young, W.L.; Su, H. Novel brain arteriovenous malformation mouse models for type 1 hereditary hemorrhagic telangiectasia. PLoS ONE 2014, 9, e88511. [Google Scholar] [CrossRef] [PubMed]
- Park, S.O.; Wankhede, M.; Lee, Y.J.; Choi, E.J.; Fliess, N.; Choe, S.W.; Oh, S.H.; Walter, G.; Raizada, M.K.; Sorg, B.S.; et al. Real-time imaging of de novo arteriovenous malformation in a mouse model of hereditary hemorrhagic telangiectasia. J. Clin. Investig. 2009, 119, 3487–3496. [Google Scholar] [PubMed]
- Murphy, P.A.; Kim, T.N.; Lu, G.; Bollen, A.W.; Schaffer, C.B.; Wang, R.A. Notch4 normalization reduces blood vessel size in arteriovenous malformations. Sci. Transl. Med. 2012, 4. [Google Scholar] [CrossRef]
- Murphy, P.A.; Lu, G.; Shiah, S.; Bollen, A.W.; Wang, R.A. Endothelial notch signaling is upregulated in human brain arteriovenous malformations and a mouse model of the disease. Lab. Investig. J. Tech. Methods Pathol. 2009, 89, 971–982. [Google Scholar] [CrossRef]
- Murphy, P.A.; Lam, M.T.; Wu, X.; Kim, T.N.; Vartanian, S.M.; Bollen, A.W.; Carlson, T.R.; Wang, R.A. Endothelial notch4 signaling induces hallmarks of brain arteriovenous malformations in mice. Proc. Natl. Acad. Sci. USA 2008, 105, 10901–10906. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Su, H.; Weinsheimer, S.; Pawlikowska, L.; Young, W.L. Brain arteriovenous malformation pathogenesis: A response-to-injury paradigm. Acta Neurochir. Suppl. 2011, 111, 83–92. [Google Scholar] [PubMed]
- Braverman, I.M.; Keh, A.; Jacobson, B.S. Ultrastructure and three-dimensional organization of the telangiectases of hereditary hemorrhagic telangiectasia. J. Investig. Dermatol. 1990, 95, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Ouriel, K. Peripheral arterial disease. Lancet 2001, 358, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Vasculogenesis, angiogenesis, and arteriogenesis: Mechanisms of blood vessel formation and remodeling. J. Cell. Biochem. 2007, 102, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Baltgalvis, K.A.; White, K.; Li, W.; Claypool, M.D.; Lang, W.; Alcantara, R.; Singh, B.K.; Friera, A.M.; McLaughlin, J.; Hansen, D.; et al. Exercise performance and peripheral vascular insufficiency improve with ampk activation in high-fat diet-fed mice. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H1128–H1145. [Google Scholar] [CrossRef] [PubMed]
- Niiyama, H.; Huang, N.F.; Rollins, M.D.; Cooke, J.P. Murine model of hindlimb ischemia. J. Vis. Exp. 2009, 23. [Google Scholar] [CrossRef] [PubMed]
- Van Weel, V.; Toes, R.E.; Seghers, L.; Deckers, M.M.; de Vries, M.R.; Eilers, P.H.; Sipkens, J.; Schepers, A.; Eefting, D.; van Hinsbergh, V.W.; et al. Natural killer cells and CD4+ T-cells modulate collateral artery development. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2310–2318. [Google Scholar] [CrossRef] [PubMed]
- Couffinhal, T.; Silver, M.; Zheng, L.P.; Kearney, M.; Witzenbichler, B.; Isner, J.M. Mouse model of angiogenesis. Am. J. Pathol. 1998, 152, 1667–1679. [Google Scholar] [PubMed]
- Brenes, R.A.; Jadlowiec, C.C.; Bear, M.; Hashim, P.; Protack, C.D.; Li, X.; Lv, W.; Collins, M.J.; Dardik, A. Toward a mouse model of hind limb ischemia to test therapeutic angiogenesis. J. Vasc. Surg. 2012, 56, 1669–1679. [Google Scholar] [CrossRef] [PubMed]
- Behm, C.Z.; Kaufmann, B.A.; Carr, C.; Lankford, M.; Sanders, J.M.; Rose, C.E.; Kaul, S.; Lindner, J.R. Molecular imaging of endothelial vascular cell adhesion molecule-1 expression and inflammatory cell recruitment during vasculogenesis and ischemia-mediated arteriogenesis. Circulation 2008, 117, 2902–2911. [Google Scholar] [CrossRef] [PubMed]
- Hellingman, A.A.; Bastiaansen, A.; de Vries, M.R.; Seghers, L.; Lijkwan, M.A.; Löwik, C.W.; Hamming, J.F.; Quax, P.H.A. Variations in surgical procedures for hind limb ischaemia mouse models result in differences in collateral formation. Eur. J. Vasc. Endovasc. Surg. 2010, 40, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Shireman, P.K.; Quinones, M.P. Differential necrosis despite similar perfusion in mouse strains after ischemia1. J. Surg. Res. 2005, 129, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Lawall, H.; Bramlage, P.; Amann, B. Stem cell and progenitor cell therapy in peripheral artery disease. A critical appraisal. Thromb. Haemost. 2010, 103, 696–709. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.L.; Chang, D.S.; Sarkar, R.; Wang, R.; Messina, L.M. The effect of gradual or acute arterial occlusion on skeletal muscle blood flow, arteriogenesis, and inflammation in rat hindlimb ischemia. J. Vasc. Surg. 2005, 41, 312–320. [Google Scholar] [CrossRef] [PubMed]
- McGuigan, M.R.; Bronks, R.; Newton, R.U.; Sharman, M.J.; Graham, J.C.; Cody, D.V.; Kraemer, W.J. Muscle fiber characteristics in patients with peripheral arterial disease. Med. Sci. Sports Exerc. 2001, 33, 2016–2021. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Tang, G.; Yan, J.; Park, B.; Hoffman, A.; Tie, G.; Wang, R.; Messina, L.M. Cellular and molecular mechanism regulating blood flow recovery in acute versus gradual femoral artery occlusion are distinct in the mouse. J. Vasc. Surg. 2008, 48, 1546–1558. [Google Scholar] [CrossRef] [PubMed]
- Baffour, R.; Garb, J.L.; Kaufman, J.; Berman, J.; Rhee, S.W.; Norris, M.A.; Friedmann, P. Angiogenic therapy for the chronically ischemic lower limb in a rabbit model. J. Surg. Res. 2000, 93, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hazarika, S.; Xie, D.; Pippen, A.M.; Kontos, C.D.; Annex, B.H. In mice with type 2 diabetes, a vascular endothelial growth factor (VEGF)-activating transcription factor modulates signaling and induces therapeutic angiogenesis after hindlimb ischemia. Diabetes 2007, 56, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Hazarika, S.; Dokun, A.O.; Li, Y.; Popel, A.S.; Kontos, C.D.; Annex, B.H. Impaired angiogenesis after hindlimb ischemia in type 2 diabetes mellitus differential regulation of vascular endothelial growth factor receptor 1 and soluble vascular endothelial growth factor receptor 1. Circ. Res. 2007, 101, 948–956. [Google Scholar] [CrossRef] [PubMed]
- van Weel, V.; de Vries, M.; Voshol, P.J.; Verloop, R.E.; Eilers, P.H.C.; van Hinsbergh, V.W.M.; van Bockel, J.H.; Quax, P.H.A. Hypercholesterolemia reduces collateral artery growth more dominantly than hyperglycemia or insulin resistance in mice. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1383–1390. [Google Scholar] [CrossRef] [PubMed]
- Shimada, T.; Takeshita, Y.; Murohara, T.; Sasaki, K.; Egami, K.; Shintani, S.; Katsuda, Y.; Ikeda, H.; Nabeshima, Y.; Imaizumi, T. Angiogenesis and vasculogenesis are impaired in the precocious-aging klotho mouse. Circulation 2004, 110, 1148–1155. [Google Scholar] [CrossRef] [PubMed]
- Faber, J.E.; Zhang, H.; Lassance-Soares, R.M.; Prabhakar, P.; Najafi, A.H.; Burnett, M.S.; Epstein, S.E. Aging causes collateral rarefaction and increased severity of ischemic injury in multiple tissues. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1748–1756. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Wang, J.; Lassance-Soares, R.M.; Najafi, A.H.; Sood, S.; Aghili, N.; Alderman, L.O.; Panza, J.A.; Faber, J.E.; Wang, S.; et al. Gender differences affect blood flow recovery in a mouse model of hindlimb ischemia. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H2027–H2034. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Ferreira, V.; Breier, G.; Pollefeyt, S.; Kieckens, L.; Gertsenstein, M.; Fahrig, M.; Vandenhoeck, A.; Harpal, K.; Eberhardt, C.; et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 1996, 380, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Greve, J.M.; Chico, T.J.; Goldman, H.; Bunting, S.; Peale, F.V.; Daugherty, A.; van Bruggen, N.; Williams, S.P. Magnetic resonance angiography reveals therapeutic enlargement of collateral vessels induced by VEGF in a murine model of peripheral arterial disease. J. Magn. Reson. Imaging 2006, 24, 1124–1132. [Google Scholar] [CrossRef] [PubMed]
- Murohara, T.; Asahara, T.; Silver, M.; Bauters, C.; Masuda, H.; Kalka, C.; Kearney, M.; Chen, D.; Symes, J.F.; Fishman, M.C.; et al. Nitric oxide synthase modulates angiogenesis in response to tissue ischemia. J. Clin. Investig. 1998, 101, 2567–2578. [Google Scholar] [CrossRef] [PubMed]
- Park, B.; Hoffman, A.; Yang, Y.; Yan, J.; Tie, G.; Bagshahi, H.; Nowicki, P.T.; Messina, L.M. Enos affects both early and late collateral arterial adaptation and blood flow recovery after induction of hindlimb ischemia in mice. J. Vasc. Surg. 2010, 51, 165. [Google Scholar] [CrossRef] [PubMed]
- Hendgen-Cotta, U.B.; Luedike, P.; Totzeck, M.; Kropp, M.; Schicho, A.; Stock, P.; Rammos, C.; Niessen, M.; Heiss, C.; Lundberg, J.O.; et al. Dietary nitrate supplementation improves revascularization in chronic ischemia. Circulation 2012, 126, 1983–1992. [Google Scholar] [CrossRef] [PubMed]
- Mishima, T.; Ito, Y.; Hosono, K.; Tamura, Y.; Uchida, Y.; Hirata, M.; Suzsuki, T.; Amano, H.; Kato, S.; Kurihara, Y.; et al. Calcitonin gene-related peptide facilitates revascularization during hindlimb ischemia in mice. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H431–H439. [Google Scholar] [CrossRef] [PubMed]
- Cochain, C.; Rodero, M.P.; Vilar, J.; Recalde, A.; Richart, A.L.; Loinard, C.; Zouggari, Y.; Guerin, C.; Duriez, M.; Combadiere, B.; et al. Regulation of monocyte subset systemic levels by distinct chemokine receptors controls post-ischaemic neovascularization. Cardiovasc. Res. 2010, 88, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, P.G.; Prior, B.M.; Li, H.; Yang, H.T.; Terjung, R.L. VEGF receptor antagonism blocks arteriogenesis, but only partially inhibits angiogenesis, in skeletal muscle of exercise-trained rats. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H759–H768. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.T.; Laughlin, M.H.; Terjung, R.L. Prior exercise training increases collateral-dependent blood flow in rats after acute femoral artery occlusion. Am. J. Physiol. Heart Circ. Physiol. 2000, 279, H1890–H1897. [Google Scholar] [PubMed]
- Copp, S.W.; Hirai, D.M.; Schwagerl, P.J.; Musch, T.I.; Poole, D.C. Effects of neuronal nitric oxide synthase inhibition on resting and exercising hindlimb muscle blood flow in the rat. J. Physiol. 2010, 588, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.W.; Kuzuya, M.; Kim, W.; Song, H.; Hu, L.; Inoue, A.; Nakamura, K.; Di, Q.; Sasaki, T.; Tsuzuki, M.; et al. Exercise training stimulates ischemia-induced neovascularization via phosphatidylinositol 3-kinase/Akt-dependent hypoxia-induced factor-1 α reactivation in mice of advanced age. Circulation 2010, 122, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Porcel, M.; Cai, W.; Gheysens, O.; Willmann, J.K.; Chen, K.; Wang, H.; Chen, I.Y.; He, L.; Wu, J.C.; Li, Z.-B. Imaging of vegf receptor in a rat myocardial infarction model using pet. J. Nucl. Med. 2008, 49, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Leong-Poi, H.; Christiansen, J.; Heppner, P.; Lewis, C.W.; Klibanov, A.L.; Kaul, S.; Lindner, J.R. Assessment of endogenous and therapeutic arteriogenesis by contrast ultrasound molecular imaging of integrin expression. Circulation 2005, 111, 3248–3254. [Google Scholar] [CrossRef] [PubMed]
- Skajaa, T.; Cormode, D.P.; Falk, E.; Mulder, W.J.; Fisher, E.A.; Fayad, Z.A. High-density lipoprotein-based contrast agents for multimodal imaging of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Mulder, W.J.; Strijkers, G.J.; Briley-Saboe, K.C.; Frias, J.C.; Aguinaldo, J.G.; Vucic, E.; Amirbekian, V.; Tang, C.; Chin, P.T.; Nicolay, K.; et al. Molecular imaging of macrophages in atherosclerotic plaques using bimodal PEG-micelles. Magn. Reson. Med. 2007, 58, 1164–1170. [Google Scholar] [CrossRef] [PubMed]
- Majmudar, M.D.; Yoo, J.; Keliher, E.J.; Truelove, J.J.; Iwamoto, Y.; Sena, B.; Dutta, P.; Borodovsky, A.; Fitzgerald, K.; di Carli, M.F.; et al. Polymeric nanoparticle PET/MR imaging allows macrophage detection in atherosclerotic plaques. Circ. Res. 2013, 112, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Tearney, G.J.; Yabushita, H.; Houser, S.L.; Aretz, H.T.; Jang, I.K.; Schlendorf, K.H.; Kauffman, C.R.; Shishkov, M.; Halpern, E.F.; Bouma, B.E. Quantification of macrophage content in atherosclerotic plaques by optical coherence tomography. Circulation 2003, 107, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Douma, K.; Prinzen, L.; Slaaf, D.W.; Reutelingsperger, C.P.; Biessen, E.A.; Hackeng, T.M.; Post, M.J.; van Zandvoort, M.A. Nanoparticles for optical molecular imaging of atherosclerosis. Small 2009, 5, 544–557. [Google Scholar] [CrossRef] [PubMed]
- Duivenvoorden, R.; Tang, J.; Cormode, D.P.; Mieszawska, A.J.; Izquierdo-Garcia, D.; Ozcan, C.; Otten, M.J.; Zaidi, N.; Lobatto, M.E.; van Rijs, S.M.; et al. A statin-loaded reconstituted high-density lipoprotein nanoparticle inhibits atherosclerotic plaque inflammation. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Kukreja, A.; Lim, E.K.; Kang, B.; Choi, Y.; Lee, T.; Suh, J.S.; Huh, Y.M.; Haam, S. One-pot synthesis of magnetic nanoclusters enabling atherosclerosis-targeted magnetic resonance imaging. Int. J. Nanomed. 2014, 9, 2489–2498. [Google Scholar]
- Kim, M.H.; Kim, B.; Lim, E.K.; Choi, Y.; Choi, J.; Kim, E.; Jang, E.; Park, H.S.; Suh, J.S.; Huh, Y.M.; et al. Magnetic nanoclusters engineered by polymer-controlled self-assembly for the accurate diagnosis of atherosclerotic plaques via magnetic resonance imaging. Macromol. Biosci. 2014, 14, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Burtea, C.; Ballet, S.; Laurent, S.; Rousseaux, O.; Dencausse, A.; Gonzalez, W.; Port, M.; Corot, C.; Vander Elst, L.; Muller, R.N. Development of a magnetic resonance imaging protocol for the characterization of atherosclerotic plaque by using vascular cell adhesion molecule-1 and apoptosis-targeted ultrasmall superparamagnetic iron oxide derivatives. Arterioscler. Thromb. Vasc. Biol. 2012, 32, e36–e48. [Google Scholar] [CrossRef] [PubMed]
- Laing, S.T.; Moody, M.; Smulevitz, B.; Kim, H.; Kee, P.; Huang, S.; Holland, C.K.; McPherson, D.D. Ultrasound-enhanced thrombolytic effect of tissue plasminogen activator-loaded echogenic liposomes in an in vivo rabbit aorta thrombus model—Brief report. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1357–1359. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Britton, G.L.; Peng, T.; Holland, C.K.; McPherson, D.D.; Huang, S.L. Nitric oxide-loaded echogenic liposomes for treatment of vasospasm following subarachnoid hemorrhage. Int. J. Nanomed. 2014, 9, 155–165. [Google Scholar]
- Almutairi, A.; Rossin, R.; Shokeen, M.; Hagooly, A.; Ananth, A.; Capoccia, B.; Guillaudeu, S.; Abendschein, D.; Anderson, C.J.; Welch, M.J. Biodegradable dendritic positron-emitting nanoprobes for the noninvasive imaging of angiogenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Mulder, W.J.; Jaffer, F.A.; Fayad, Z.A.; Nahrendorf, M. Imaging and nanomedicine in inflammatory atherosclerosis. Sci. Transl. Med. 2014, 6, 239sr231. [Google Scholar] [CrossRef]
- Press, M.C.; Jaffer, F.A. Molecular intravascular imaging approaches for atherosclerosis. Curr. Cardiovasc. Imaging Rep. 2014, 7, 9293. [Google Scholar] [CrossRef] [PubMed]
- Solomon, M.; Nothdruft, R.E.; Akers, W.; Edwards, W.B.; Liang, K.; Xu, B.; Suddlow, G.P.; Deghani, H.; Tai, Y.C.; Eggebrecht, A.T.; et al. Multimodal fluorescence-mediated tomography and spect/ct for small-animal imaging. J. Nucl. Med. 2013, 54, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Poole, K.M.; Patil, C.A.; Nelson, C.E.; McCormack, D.R.; Madonna, M.C.; Duvall, C.L.; Skala, M.C. Longitudinal study of arteriogenesis with swept source optical coherence tomography and hyperspectral imaging. In Proceedings of the SPIE BiOS, International Society for Optics and Photonics, San Francisco, CA, USA, 4 March 2014.
- Bouccara, S.; Sitbon, G.; Fragola, A.; Loriette, V.; Lequeux, N.; Pons, T. Enhancing fluorescence in vivo imaging using inorganic nanoprobes. Curr. Opin. Biotechnol. 2015, 34, 65–72. [Google Scholar] [CrossRef]
- Hong, G.; Lee, J.C.; Jha, A.; Diao, S.; Nakayama, K.H.; Hou, L.; Doyle, T.C.; Robinson, J.T.; Antaris, A.L.; Dai, H.; et al. Near-infrared ii fluorescence for imaging hindlimb vessel regeneration with dynamic tissue perfusion measurement. Circ. Cardiovasc. Imaging 2014, 7, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.; Lee, J.C.; Robinson, J.T.; Raaz, U.; Xie, L.; Huang, N.F.; Cooke, J.P.; Dai, H. Multifunctional in vivo vascular imaging using near-infrared ii fluorescence. Nat. Med. 2012, 18, 1841–1846. [Google Scholar] [CrossRef] [PubMed]
- Haka, A.S.; Potteaux, S.; Fraser, H.; Randolph, G.J.; Maxfield, F.R. Quantitative analysis of monocyte subpopulations in murine atherosclerotic plaques by multiphoton microscopy. PLoS ONE 2012, 7, e44823. [Google Scholar] [CrossRef] [PubMed]
- Engelbrecht, C.J.; Johnston, R.S.; Seibel, E.J.; Helmchen, F. Ultra-compact fiber-optic two-photon microscope for functional fluorescence imaging in vivo. Opt. Express 2008, 16, 5556–5564. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wang, L.V. Photoacoustic imaging in biomedicine. Rev. Sci. Instrum. 2006, 77. [Google Scholar] [CrossRef]
- Wang, L.V.; Hu, S. Photoacoustic tomography: In vivo imaging from organelles to organs. Science 2012, 335, 1458–1462. [Google Scholar] [CrossRef] [PubMed]
- Maslov, K.; Zhang, H.F.; Hu, S.; Wang, L.V. Optical-resolution photoacoustic microscopy for in vivo imaging of single capillaries. Opt. Lett. 2008, 33, 929–931. [Google Scholar] [CrossRef] [PubMed]
- Gamelin, J.; Maurudis, A.; Aguirre, A.; Huang, F.; Guo, P.; Wang, L.V.; Zhu, Q. A real-time photoacoustic tomography system for small animals. Opt. Express 2009, 17, 10489–10498. [Google Scholar] [CrossRef] [PubMed]
- Akers, W.J.; Kim, C.; Berezin, M.; Guo, K.; Fuhrhop, R.; Lanza, G.M.; Fischer, G.M.; Daltrozzo, E.; Zumbusch, A.; Cai, X.; et al. Noninvasive photoacoustic and fluorescence sentinel lymph node identification using dye-loaded perfluorocarbon nanoparticles. ACS Nano 2011, 5, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Beard, P. Biomedical photoacoustic imaging. Interface Focus 2011, 1, 602–631. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.F.; Maslov, K.; Stoica, G.; Wang, L.V. Functional photoacoustic microscopy for high-resolution and noninvasive in vivo imaging. Nat. Biotechnol. 2006, 24, 848–851. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Su, J.L.; Amirian, J.; Litovsky, S.H.; Smalling, R.; Emelianov, S. Detection of lipid in atherosclerotic vessels using ultrasound-guided spectroscopic intravascular photoacoustic imaging. Opt. Express 2010, 18, 4889–4897. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, V.; Schiano, C.; Casamassimi, A.; Zullo, A.; Soricelli, A.; Mancini, F.P.; Napoli, C. Imaging techniques to evaluate cell therapy in peripheral artery disease: State of the art and clinical trials. Clin. Physiol. Funct. Imaging 2014. [Google Scholar] [CrossRef]
- Silvestre, J.S.; Smadja, D.M.; Levy, B.I. Postischemic revascularization: From cellular and molecular mechanisms to clinical applications. Physiol. Rev. 2013, 93, 1743–1802. [Google Scholar] [CrossRef] [PubMed]
- Sneider, E.B.; Nowicki, P.T.; Messina, L.M. Regenerative medicine in the treatment of peripheral arterial disease. J. Cell. Biochem. 2009, 108, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Lu, G.; Jester, A.; Johnston, W.F.; Zhao, Y.; Hajzus, V.A.; Saadatzadeh, M.R.; Su, G.; Bhamidipati, C.M.; Mehta, G.S.; et al. Experimental abdominal aortic aneurysm formation is mediated by IL-17 and attenuated by mesenchymal stem cell treatment. Circulation 2012, 126, S38–S45. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.M.; Yamawaki-Ogata, A.; Oshima, H.; Ueda, Y.; Usui, A.; Narita, Y. Intravenous administration of mesenchymal stem cells prevents angiotensin II-induced aortic aneurysm formation in apolipoprotein E-deficient mouse. J. Transl. Med. 2013, 11, 175. [Google Scholar] [CrossRef] [PubMed]
- Yamawaki-Ogata, A.; Fu, X.; Hashizume, R.; Fujimoto, K.L.; Araki, Y.; Oshima, H.; Narita, Y.; Usui, A. Therapeutic potential of bone marrow-derived mesenchymal stem cells in formed aortic aneurysms of a mouse model. Eur. J. Cardiol. Thorac. Surg. 2014, 45, e156–e165. [Google Scholar] [CrossRef]
- Norgren, L.; Hiatt, W.R.; Dormandy, J.A.A.; Nehler, M.R.; Harris, K.A.; Fowkes, F.G.R.; Tasc, I.I.W.G. Inter-society consensus for the management of peripheral arterial disease (tasc II). Eur. J. Vasc. Endovasc. Surg. 2007, 33, S1–S75. [Google Scholar] [CrossRef] [PubMed]
- Isner, J.M.; Asahara, T. Angiogenesis and vasculogenesis as therapeutic strategies for postnatal neovascularization. J. Clin. Investig. 1999, 103. [Google Scholar] [CrossRef]
- Huang, N.F.; Niiyama, H.; Peter, C.; de, A.; Natkunam, Y.; Fleissner, F.; Li, Z.; Rollins, M.D.; Wu, J.C.; Gambhir, S.S. Embryonic stem cell-derived endothelial cells engraft into the ischemic hindlimb and restore perfusion. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Ishii, M.; Shibata, R.; Numaguchi, Y.; Kito, T.; Suzuki, H.; Shimizu, K.; Ito, A.; Honda, H.; Murohara, T. Enhanced angiogenesis by transplantation of mesenchymal stem cell sheet created by a novel magnetic tissue engineering method. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2210–2215. [Google Scholar] [CrossRef] [PubMed]
- Van der Bogt, K.E.A.; Hellingman, A.A.; Lijkwan, M.A.; Bos, E.-J.; de Vries, M.R.; van Rappard, J.R.M.; Fischbein, M.P.; Quax, P.H.; Robbins, R.C.; Hamming, J.F. Molecular imaging of bone marrow mononuclear cell survival and homing in murine peripheral artery disease. Cardiovasc. Imaging 2012, 5, 46–55. [Google Scholar] [CrossRef]
- Orbay, H.; Zhang, Y.; Hong, H.; Hacker, T.A.; Valdovinos, H.F.; Zagzebski, J.A.; Theuer, C.P.; Barnhart, T.E.; Cai, W. Positron emission tomography imaging of angiogenesis in a murine hindlimb ischemia model with 64Cu-labeled TRC105. Mol. Pharm. 2013, 10, 2749–2756. [Google Scholar] [CrossRef] [PubMed]
- Poole, K.M.; Tucker-Schwartz, J.M.; Sit, W.W.; Walsh, A.J.; Duvall, C.L.; Skala, M.C. Quantitative optical imaging of vascular response in vivo in a model of peripheral arterial disease. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H1168–H1180. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, J.B.; Phillips, E.H.; Riggins, T.E.; Sangha, G.S.; Chakraborty, S.; Lee, J.Y.; Lycke, R.J.; Hernandez, C.L.; Soepriatna, A.H.; Thorne, B.R.H.; et al. Imaging of Small Animal Peripheral Artery Disease Models: Recent Advancements and Translational Potential. Int. J. Mol. Sci. 2015, 16, 11131-11177. https://doi.org/10.3390/ijms160511131
Lin JB, Phillips EH, Riggins TE, Sangha GS, Chakraborty S, Lee JY, Lycke RJ, Hernandez CL, Soepriatna AH, Thorne BRH, et al. Imaging of Small Animal Peripheral Artery Disease Models: Recent Advancements and Translational Potential. International Journal of Molecular Sciences. 2015; 16(5):11131-11177. https://doi.org/10.3390/ijms160511131
Chicago/Turabian StyleLin, Jenny B., Evan H. Phillips, Ti'Air E. Riggins, Gurneet S. Sangha, Sreyashi Chakraborty, Janice Y. Lee, Roy J. Lycke, Clarissa L. Hernandez, Arvin H. Soepriatna, Bradford R. H. Thorne, and et al. 2015. "Imaging of Small Animal Peripheral Artery Disease Models: Recent Advancements and Translational Potential" International Journal of Molecular Sciences 16, no. 5: 11131-11177. https://doi.org/10.3390/ijms160511131